?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
We aimed to analyze the efficacy of cytarabine + aclarubicin + recombinant human granulocyte colony-stimulating factor (G-CSF, i.e. CAG) regimen combined with decitabine in the clinical treatment of adult acute myeloid leukemia (AML). In total, 68 patients with adult AML were randomized into observation and control groups by random sampling. The control group (n = 34) was treated with CAG regimen alone and the observation group (n = 34) with CAG regimen combined with decitabine; the two groups were compared in terms of effective rate, incidence of adverse reactions, hemoglobin level, and blood progenitor cell and white blood cell counts. The observation and control groups exhibited a total effective rate of 85.29% and 61.76% after treatment, respectively (P < 0.05). During follow-up, recurrence and survival rates were 11.76% and 85.29% in the observation group and 32.36% and 61.76% in the control group, respectively (P < 0.05), indicating better results in the former group. CAG regimen combined with decitabine was superior to CAG regimen alone in treating adult AML. However, our study is limited by a small sample size, short follow-up period, few study indices, and low sufficiently representative results, warranting further studies.
Introduction
Acute myeloid leukemia (AML) is a malignancy (Stein et al. Citation2018) of the hematopoietic system and has a higher incidence than other types of adult acute leukemia. The underlying cause of AML is malignant transformation and hematopoietic stem cell proliferation. Most leukemia cells exhibit unique manifestations such as antigenic deletion and excessive expression, which contribute to the diagnosis of leukemia and its differentiation from other types of leukemia (Sayad et al. Citation2017). Clinically, initial induction chemotherapy is considered for bone marrow remission, but it may sometimes fail or leukemia may recur during treatment in some patients because they are primarily drug resistant (Nair et al. Citation2013).
Due to poor prognosis, patients with AML require a treatment of higher intensity than the initial treatment if the disease recurs, resulting in more severe bone marrow toxicity and more obvious and severe non-hematological toxicity, because of which some patients may discontinue treatment and die of complications (Amatangelo et al. Citation2017). The current epigenetic research on AML is more profound. Studies on the pathogenesis of AML have shown not only cytogenetic changes but also epigenetic abnormalities, which are related to the occurrence of AML (Weinberg et al. Citation2017; Rastogi Citation2018).
Epigenetic variation refers to changes in genetic characteristics and gene functions, with the deoxyribonucleic acid (DNA) sequence of the gene remaining unchanged. It leads to changes in the phenotypes of biological cells and is associated with various mechanisms such as uncoded ribonucleic acid regulation, DNA methylation, chromatin remodeling, and histone modification (Robertson et al. Citation2017).
Chemotherapy and decitabine are used in the treatment of AML or some other hematological malignancies. The standard induction chemotherapy regimen is 3 + 7 regimen, which has been clinically proved to have favorable efficacy. However, some patients do not achieve remission even if they continue to use this regimen. Therefore, it is necessary to select other chemotherapy regimens. One such chemotherapy option is CAG regimen, which involves three drugs, i.e. cytarabine (C), aclarubicin (A), and recombinant human granulocyte colony-stimulating factor (G-CSF, G). Compared with 3 + 7 regimen, CAG regimen has a milder effect, higher efficiency, less primary drug resistance, and better chemotherapy tolerance. Moreover, studies have shown that CAG regimen can achieve high efficiency and satisfactory safety in the treatment of AML in the elderly. However, it has been found that aberrant DNA methylation not only results in malignant transformation of cells but also is a major cause of inactivation and expression loss in tumor suppressor genes, which are related to various bioprocesses such as cell cycle regulation, cell growth and differentiation, vascular formation, metastasis and invasion, and DNA mismatch repair (Bird Citation2002). Therefore, aberrant DNA methylation weakens the antitumor activity of tumor suppressor genes, thereafter continuing tumor development and tumor cell differentiation and hindering the effect of chemotherapy. Therefore, treatment with CGA regimen alone cannot achieve satisfactory results. Decitabine is a type of DNA methyltransferase inhibitor, which can induce demethylation as well as differentiation and formation of hematopoietic cells at low doses, but it results in obvious cytotoxic effect at high doses (Momparler et al. Citation2017).
Demethylating drugs (hypomethylating agents) can also be used as chemotherapy sensitizers to promote the recovery of the expression levels of tumor suppressor genes and improve the susceptibility to chemotherapy. Therefore, the application of CAG regimen combined with decitabine can have a synergistic anti-leukemia effect and ensure a higher clinical effect.
Previously, in the treatment of leukemia, decitabine and GAC regimen were used as a single application or in combination with other drugs, and there are few studies on the combined application of decitabine and GAC regimen. We specifically analyzed the efficiency of CAG regimen combined with decitabine in the treatment of adult AML by comparing CAG regimen alone and GAG regimen combined with decitabine to determine a more effective means for the clinical treatment of adult AML.
Materials and methods
Materials
In total, 68 patients with adult AML who were admitted to our hospital were enrolled in the study and randomized into observation (19 men and 15 women; age range: 20–59 years; mean age: 42.26 ± 7.34 years) and control (21 men and 13 women; age range: 21–59 years; mean age: 43.41 ± 7.43 years) groups by random sampling. The control group received CAG regimen and the observation group received CAG regimen combined with decitabine for the treatment of AML. Inclusion criteria included patients aged >18 years with compliance to AML diagnosis standards (Appelbaum et al. Citation2001) and the absence of cognitive disorders; signed informed consent was obtained from the included patients or their guardians. The study protocol was approved by the Ethics Committee of Shaanxi Provincial People’s Hospital. Exclusion criteria included patients who did not conform to the inclusion criteria and presence of severe disorders of the heart, liver, lungs, and kidneys; other diseases of the blood system; malignant tumors; and concurrent psychogenic disorders.
Methods
The control group received CAG regimen including 10 mg/m2 intravenous aclacinomycin drip (specification: 20 mg (20,000 units), approval document No.: GYZZ H20060196, manufacturer: Yangzhou Aosaikang Pharmaceutical Co., Ltd.) from 1 to 4 days, 10 mg/m2 subcutaneous cytarabine injection (specification: 0.1 × 1 bottle, approval document No.: H20100594, manufacturer: Actavis Italy SpA) from 3 to 9 days, and 200 µg/m2·day rhG-CSF injection (commodity name: GRAN®, specification: 0.6 ml and 150 µg, approval document No.: GYZZ S200100631, manufacturer: Kyowa Hakko Kirin Co., Ltd.). This treatment was administered for 4 weeks.
The observation group received a combined treatment with 15 mg/m2 decitabine (commodity name: Dacogen®, specification: 50 mg/bottle, approval document No.: H20080548 (No. of registration certificate), manufacturer: Pharmachemie B.V.) through an intravenous drip from 1 to 5 days and CAG regimen, at the same dose as administered to the control group. This treatment was administered for 4 weeks.
Observation indices
Effective rate: The judgment criteria for treatment efficacy were formulated based on the literature (Iris et al. Citation2013) and specific patient conditions. They included the following: ‘basic cure’ wherein all clinical symptoms disappeared, vital signs recovered, laboratory parameters returned to normal levels, and archaeocytes accounted for <5% cells in bone marrow after a 4-week treatment; ‘turn for the better’ wherein clinical symptoms were alleviated, vital signs and laboratory parameters returned to normal levels, and archaeocytes accounted for >5% cells in bone marrow but reduced by >50% after a 4-week treatment compared with the pretreatment conditions; and ‘invalid’ wherein symptoms persisted, vital signs worsened in some cases, and archaeocyte count in the bone marrow was less than half. The total effective rate of treatment was calculated as follows: the rate of basic cure + rate of turn for the better.
Incidence of adverse reactions: The incidence of liver damage, nausea and vomiting, bone marrow suppression, lung infection, and thrombocytopenia during and after treatment were compared between the two groups. The criteria for bone marrow suppression were as follows: white blood cell (WBC) count <4 × 109/L, granulocyte count <2 × 109/L, hemoglobin level <110 g/L, and blood platelet count <100 × 109/L.
Hemoglobin level and blood progenitor cell (BPC) and WBC counts: hemoglobin level and BPC and WBC counts were measured before and after 4-week treatment by collecting 0.1 ml fingertip blood sample in test tubes containing ethylenediaminetetraacetic acid.
NCCN risk stratification: Risk stratification was performed 1 year after the end of treatment in accordance with the NCCN (Citation2017) guidelines. Low risk: normal cytogenetic karyotype of the intermediate risk group without FLT3-ITD but with NPM1 mutation or a combination of isolated CBEPA allele mutation; intermediate risk: normal cytogenetic karyotype of the low risk group combined with C-kit mutation; and high risk: normal cytogenetic karyotype of the intermediate risk group with FLT3-ITD mutation.
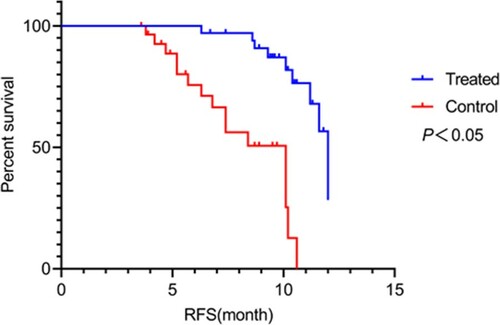
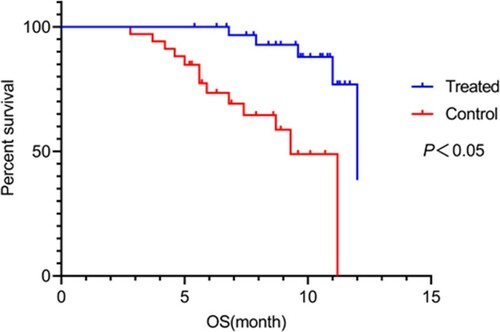
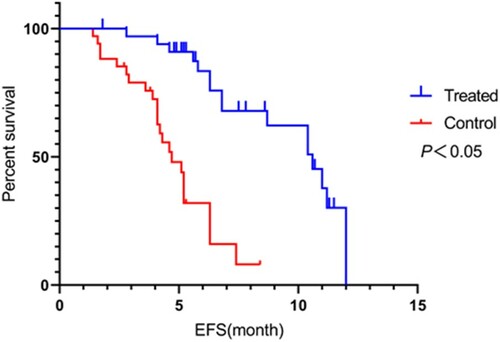
Recurrence and survival rates: After treatment, the two groups were followed up for 1 year to record recurrence and survival rates. Survival was analyzed from three aspects: overall survival (OS), event-free survival (EFS), and relapse-free survival (RFS). OS is the period between the start of randomization and death from any cause; EFS is the period between the start of treatment and onset of disease; and RFS is the period between the start of randomization and recurrence of disease.
Statistical analysis
Statistical analysis was performed using SPSS22.0. Numerical data are expressed as mean ± standard deviation, and comparison studies were conducted using independent samples t-test for normally distributed data and Mann–Whitney U-test for non-normally distributed data. A paired test was performed for pre-and-pro comparisons of the groups. In case of nominal data expressed as n (%), comparison studies were conducted using χ2 test for intergroup comparison. For all statistical comparisons, statistical significance was defined as P-value of <0.05.
Results
General characteristics
The proportions of men and women were 55.88% and 44.12% in the observation group and 61.76% and 38.24% in the control group, respectively. Furthermore, the proportions of M2, M4, M5, and M6 subtypes were 20.59%, 11.76%, 38.24%, and 29.41% in the observation group and 17.65%, 14.71%, 35.29%, and 32.35% in the control group, respectively. No between-group difference was found in terms of proportion of men and women; mean age, height, and body weight; proportion of French–American–British subtypes; and proportion of NCCN risk stratification (P > 0.05; Table ).
Table 1. Comparison of general characteristics between the observation and control groups [n (%)]/(
 ± s).
± s).
Effective rate
Twelve patients showed basic cure, 17 showed turn for the better, and 5 showed invalid findings in the observation group (total effective rate: 85.29%), whereas 9 showed basic cure, 12 showed turn for the better, and 13 showed invalid findings in the control group (total effective rate: 61.76%; P < 0.05; Table ).
Table 2. Comparison of total effective rate with different treatment regimens between the observation and control groups [n (%)].
Incidence of adverse reactions
Incidences of bone marrow suppression, lung infection, thrombocytopenia, nausea and vomiting, and liver damage during and after treatment were 14.71%, 11.76%, 8.82%, 23.53%, and 17.65% in the observation group and 11.76%, 17.65%, 11.76%, 26.47%, and 14.71% in the control group, respectively (P > 0.05; Table ).
Table 3. Comparison of the incidence of adverse reactions between the observation and control groups [n (%)].
Hemoglobin level and BPC and WBC counts
Before treatment, there was no significant between-group difference in terms of hemoglobin level and BPC and WBC counts (P > 0.05). After 4-week treatment, hemoglobin level, BPC count, and WBC count were 64.27 ± 6.87 g/L, 375.49 ± 52.92 × 109/L, and 5.88 ± 0.79 × 109/L in the observation group and 73.49 ± 7.34 g/L, 287.56 ± 48.93 × 109/L, and 3.89 ± 0.72 × 109/L in the control group, respectively. Both groups exhibited reduced levels of these parameters (P < 0.05), with the reduction from the pretreatment levels being significant in the observation group (P < 0.05; Table ).
Table 4. Comparison of hemoglobin level, BPC count, and WBC count between the observation and control groups (
 ± s).
± s).
NCCN risk stratification
The results of NCCN risk stratification in the two groups before treatment were used as reference. One year after the treatment, the low risk rate of the observation group was higher (P<0.05) and the high risk rate was lower (P<0.05) than those of the control group. There was no significant difference in the intermediate risk rate between the two groups (P > 0.05; Table ).
Table 5. Comparison of recurrence and survival rates during follow-up between the observation and control groups [n (%)].
Recurrence and survival rates
During the 1-year follow-up, recurrence rate, EFS, RFS, and OS were 4 (11.76%), 18 (52.94%), 24 (70.59%), and 29 (85.29%) in the observation group and 11 (32.36%), 10 (29.41), 15 (44.12%), and 21 (61.76%) in the control group, respectively. The observation group exhibited significantly lower recurrence rate (P = 0.041) and higher EFS, RFS, and OS (P = 0.049, 0.027, and 0.028, respectively) than the control group (P < 0.05; Figures –).
Figure 1. Comparison of event-free survival (EFS) between the two groups. Compared with the control group, EFS was significantly longer in the observation group (P < 0.05).
Multifactor analysis of remission rate and survival outcome
WBC count of >20 × 109/L, lactate dehydrogenase (LDH) level of >450 U/L, positive CD56 expression, and adverse karyotype were found to be single factors contributing to AML. Multivariate logistic regression analysis showed that WBC count was an independent risk factor for remission rate and survival outcome (P < 0.05), but LDH level of >450 U/L, positive CD56 expression, and adverse karyotype had no direct effect on remission rate and survival outcome (P > 0.05; Table ).
Table 6. Multifactor analysis of remission rate and survival outcome.
Discussion
CAG regimen is a commonly used chemotherapy option in the treatment of AML. G-CSF promotes the transformation of malignant cloned cells from G0 to G1 to increase cell exposure to chemotherapy drugs. Aclacinomycin and cytarabine can be combined to accelerate malignant cell apoptosis and terminate malignant cloned cell growth (Dou et al. Citation2013; Wang et al. Citation2016), with limited impact of resistance to various drugs and nonobvious cardiac toxicity (Zheng et al. Citation2014). Meanwhile, low-dose cytarabine, G-CSF, and aclacinomycin can induce cell differentiation (Basso et al. Citation2011). However, few studies have shown that satisfactory clinical efficacy cannot be achieved with CAG regimen alone because antioncogenes in patients with AML are highly methylated to promote basic inactivation and result in the development and progression of AML (Obara et al. Citation2013; Tabayashi et al. Citation2014). We showed that compared with CAG regimen alone, CAG regimen combined with decitabine could significantly improve efficiency, alleviate disease severity, obtain better survival benefits, and have better long-term efficacy.
In the present study, the observation group received CAG regimen combined with decitabine and achieved a total effective rate of 85.29%, which was higher than that of the control group (61.76%); this is attributable to decitabine, a demethylating drug with a distinct inhibitory effect on methyltransferase, which has an effect on some local highly demethylated CpG islands by inhibiting DNA methyltransferase to reactivate antioncogenes (Qiao et al. Citation2017; Xie et al. Citation2019). Moreover, its demethylation function does not obviously intervene with other genes that are hypomethylated and worsen the instability of genomes (Momparler et al. Citation2017).
In the present study, after treatment, the observation group showed a significant decrease in hemoglobin level and increase in BPC and WBC counts (P < 0.05). This indicates that CAG regimen combined with decitabine exerts better effects at the cellular level, improving BPC and WBC counts in patients with adult AML, and it plays a vital role in improving immunity. These findings are consistent with those reported by Momparler et al. (Citation2017) who also observed improved hemoglobin level and WBC and platelet counts in patients who received CAG regimen combined with decitabine compared with those who received CAG regimen (P < 0.05). This can be attributed to the fact that in CGA regimen, G-CSF can accelerate the entry of naive primitive cells into the S phase of proliferation, whereas decitabine can increase the sensitivity of cytarabine to leukemia cells under the action of G-CSF; therefore, it can achieve a higher effect through synergy. Furthermore, after treatment, both groups exhibited insignificant changes in the incidences of liver damage, nausea and vomiting, bone marrow suppression, lung infection, and thrombocytopenia. Additionally, the observation group exhibited a significant increase in EFS, RFS, and OS and decrease in recurrence rate after 1-year follow-up (P < 0.05). A long-term randomized study (Seelan et al. Citation2018) showed that the mean survival rate and OS were significantly extended in patients with AML who received with CAG regimen combined with decitabine compared within those who received with CAG regimen alone. Moreover, Stich et al. (Citation2017) reported that decitabine can effectively reduce AML recurrence, increase survival rate, and prolong survival time, clearly indicating that the combination of decitabine and CAG regimen can reduce disease recurrence rate after drug withdrawal and significantly increase the survival rate to improve the quality of life of patients on a long-term basis without overlooking the control of adverse reactions and safety. This can be attributed to the fact that phosphorylated decitabine can penetrate DNA, covalently bond with DNA methyltransferase, and irreversibly inhibits the activation of methyltransferase (Niitsu et al. Citation2015). Moreover, Ramos et al. (Citation2015) found that whether in vivo or in vitro, decitabine can exert demethylation effect to recover the expressions of deactivated genes.
In conclusion, CAG regimen compared with decitabine can markedly improve clinical efficacy in treating adult AML by improving hemoglobin level and BPC and WBC counts, with decreased recurrence and increased survival rate without hindering treatment safety. However, our study is limited by its small sample size, short duration and follow-up period, limited scope in adults, few study indices, and low sufficiently representative results. In the future, in-depth studies based on large sample size and longer follow-up will be required to provide more information on the clinical treatment of AML.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data is not available.
References
- Amatangelo MD, Quek L, Shih A, Stein EM, Roshal M, David MD, Marteyn B, Farnoud NR, de Botton S, Bernard OA, et al. 2017. Enasidenib induces acute myeloid leukemia cell differentiation to promote clinical response. Blood. 130(6):732–741. doi: https://doi.org/10.1182/blood-2017-04-779447
- Appelbaum FR, Rowe JM, Radich J, Dick JE. 2001. Acute myeloid leukemia. Hematology. 2001(1):62–86. doi: https://doi.org/10.1182/asheducation-2001.1.62
- Basso M, Modoni A, Spada D, Cassano A, Schinzari G, Lo Monaco M, Quaranta D, Tonali PA, Barone C. 2011. Polymorphism of CAG motif of SK3 gene is associated with acute oxaliplatin neurotoxicity. Cancer Chemother Pharmacol. 67(5):1179–1187. doi: https://doi.org/10.1007/s00280-010-1466-y
- Berger MJ, Ettinger DS, Aston J, Barbour S, Bergsbaken J, Bierman PJ, Brandt D, Dolan DE, Ellis G, Jeong Kim E, et al. 2017. NCCN guidelines insights: antiemesis, version 2.2017. J Natl Compr Canc Netw. 15(7):883–893. doi: https://doi.org/10.6004/jnccn.2017.0117
- Bird A. 2002. DNA methylation patterns and epigenetic memory. Genes Dev. 16(1):6–21. doi: https://doi.org/10.1101/gad.947102
- Dou LP, Jing Y, Wang QS, Mei JH, Yu L. 2013. Clinical efficacy of decitabine plus improved CAG chemotherapy and haplo-identical donor peripheral lymphocyte infusion regimen on elderly patients with high risk myelodysplastic syndrome and acute myeloid leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 21(3):662–666.
- Iris S, Matthew B, Veronika N, Chao W, Sara R, Nancy K, Fang-Yong L, Ehrenkranz RA, Rinder HM, Vineet B. 2013. Neutrophil CD64 with hematologic criteria for diagnosis of neonatal sepsis. Am J Perinatol. 31(1):021–030. doi: https://doi.org/10.1055/s-0033-1334453
- Momparler RL, Cote S, Momparler LF, Idaghdour Y. 2017. Inhibition of DNA and histone methylation by 5-aza-2'-deoxycytidine (decitabine) and 3-deazaneplanocin-A on antineoplastic action and gene expression in myeloid leukemic cells. Front Oncol. 7:19. doi: https://doi.org/10.3389/fonc.2017.00019
- Nair CK, Kumar M, Odayoth SM. 2013. Pleural effusion during acute myeloid leukemia induction chemotherapy: a perplexing case. J Cancer Res Ther. 9(2):290–291. doi: https://doi.org/10.4103/0973-1482.113390
- Niitsu N, Hayashi Y, Sugita K, Honma Y. 2015. Sensitization by 5-aza-2'-deoxycytidine of leukaemia cells with MLL abnormalities to induction of differentiation by all-trans retinoic acid and 1alpha,25-dihydroxyvitamin D3. Br J Haematol. 112(2):315–326. doi: https://doi.org/10.1046/j.1365-2141.2001.02523.x
- Obara M, Ara T, Shima K, Yasumoto A, Nakata M, Ota S, Imai K, Hirano T, Kiyama Y, Ogasawara M. 2013. Aclarubicin, low-dose cytarabine combined with G-CSF (CAG) regimen for patients previously treated or ineligible for intensive chemotherapy with acute myeloid leukemia and myelodysplastic syndrom: a single center experience. Blood. 122:1241–1241. doi: https://doi.org/10.1182/blood.V122.21.1241.1241
- Qiao X, Yin F, Ji Y, Li Y, Yan P, Lai J. 2017. 5-Aza-2'-deoxycytidine in the medial prefrontal cortex regulates alcohol-related behavior and Ntf3-TrkC expression in rats. PLoS One. 12(6):e0179469. doi: https://doi.org/10.1371/journal.pone.0179469
- Ramos MP, Wijetunga NA, McLellan AS, Suzuki M, Greally JM. 2015. DNA demethylation by 5-aza-2'-deoxycytidine is imprinted, targeted to euchromatin, and has limited transcriptional consequences. Epigenetics Chromatin. 8:11. doi: https://doi.org/10.1186/s13072-015-0004-x
- Rastogi N. 2018. Genetics of acute myeloid leukemia - a paradigm shift. Indian Pediatr. 55(6):465–468. doi: https://doi.org/10.1007/s13312-018-1334-0
- Robertson M, Schrey A, Shayter A, Moss CJ, Richards C. 2017. Genetic and epigenetic variation in Spartina alterniflora following the Deepwater Horizon oil spill. Evol Appl. 10(8):792–801. doi: https://doi.org/10.1111/eva.12482
- Sayad A, Hajifathali A, Hamidieh AA, Roshandel E, Taheri M. 2017. HOTAIR long noncoding RNA is not a biomarker for acute myeloid leukemia (AML) in Iranian patients. Asian Pac J Cancer Prev. 18(6):1581–1584.
- Seelan RS, Mukhopadhyay P, Pisano MM, Greene RM. 2018. Effects of 5-aza-2'-deoxycytidine (decitabine) on gene expression. Drug Metab Rev. 50(2):193–207. doi: https://doi.org/10.1080/03602532.2018.1437446
- Stein EM, Walter RB, Erba HP, Fathi AT, Advani AS, Lancet JE, Ravandi F, Kovacsovics T, DeAngelo DJ, Bixby D, et al. 2018. A phase 1 trial of vadastuximab talirine as monotherapy in patients with CD33-positive acute myeloid leukemia. Blood. 131(4):387–396. doi: https://doi.org/10.1182/blood-2017-06-789800
- Stich M, Ganss L, Puschhof J, Prigge ES, Reuschenbach M, Guiterrez A, Vinokurova S, Doeberitz MVK. 2017. 5-Aza-2′-deoxycytidine (DAC) treatment downregulates the HPV E6 and E7 oncogene expression and blocks neoplastic growth of HPV-associated cancer cells. Oncotarget. 8(32):52104–52117. doi: https://doi.org/10.18632/oncotarget.10631
- Tabayashi T, Takahashi Y, Kimura Y, Tomikawa T, Sagawa M, Anan T, Watanabe R, Tokuhira M, Mori S, Kizaki M. 2014. P1-21-1efficacy of low-dose cytarabine-based regimen(CAG)followed by azacitidine for elderly patients with MDS/AML. Ann Oncol. 25(suppl 5):v84–v84. doi: https://doi.org/10.1093/annonc/mdu436.42
- Wang J, Jiang B, Jiang Q, Lu J, Zhu HH, Yang SM, Zhao T, Wen L, Bao L, Huang XJ, et al. 2016. Comparison of efficacy and prognostic factors in elderly acute myelogenous patients with MA or CAG induction chemotherapy regimen. Zhonghua Xue Ye Xue Za Zhi. 37(3):194–200.
- Weinberg OK, Sohani AR, Bhargava P, Nardi V. 2017. Diagnostic work-up of acute myeloid leukemia. Am J Hematol. 92(3):317–321. doi: https://doi.org/10.1002/ajh.24648
- Xie MY, Yang Y, Liu P, Luo Y, Tang SB. 2019. 5-Aza-2'-deoxycytidine in the regulation of antioxidant enzymes in retinal endothelial cells and rat diabetic retina. Int J Ophthalmol. 12(1):1–7.
- Zheng C, Cai X, Wu S, Liu Z, Shi Y, Zhou W. 2014. Enhancing effect of β-elemene emulsion on chemotherapy with harringtonine, aclacinomycin, and Ara-c in treatment of refractory/relapsed acute myeloid leukemia. Pak J Med Sci. 30(6):1270–1272.