Abstract
Endometriosis (EM) is an estrogen-dependent, frequent benign gynecological disease, which reduces the quality of life in EM patients. Propofol regulates the apoptosis of EM. miR-9-5p is decreased in early secretory endometrium, TGFBI is increased in EM, however, their functions in EM remain unclear. This study aimed to investigate the mechanisms underlying the effects of propofol on the proliferation and invasion of EM by regulating miR-9-5p/TGFBI. The proliferation and invasion of ectopic endometrial stromal cells (ESCs) were determined by MTT assay and Transwell assay, respectively. Real-time PCR was applied for the evaluation of miR-9-5p and transforming growth factor-β (TGF-β)-induced gene (TGFBI) mRNA level in ESCs. Western blot was used to examine the protein level of TGFBI protein (TGFBIp) in ESCs. The relation between miR-9-5p and TGFBI was predicted by TargetScan 7.1 and verified by dual luciferase reporter assay. Propofol reduced cell proliferation and invasion, while induced miR-9-5p level and reduced TGFBI/TGFBIp level in ectopic ESCs. Additionally, in ectopic ESCs from patients with EM, miR-9-5p was lower, while TGFBI and TGFBIp were higher than those from eutopic endometrium and normal endometrium from patients with hysteromyoma. Moreover, propofol reduced proliferation and invasion in ectopic ESCs, which was realized by the regulation of miR-9-5p/TGFBI.
KEYWORDS:
Background
Endometriosis, which is a kind of frequent gynecological disorder, is resulted from the appearance of active endometrium at outer uterine cavity (Nyholt et al. Citation2012). Generally, EM is observed in females at reproductive age (25–45 years old) (Maheshwari et al. Citation2012); and the morbidity of EM is approximately 10% (Schrager et al. Citation2013). Endometriosis reduces the quality of life in patients with EM due to the symptoms of pelvic pain and subfertility (Painter et al. Citation2011). Despite the benign characteristic of EM, ectopic endometrial stromal cells (ESCs) are characterized with migratory and invasive ability (Ahn et al. Citation2015). If suspension happens to the current treatments, the mitigatory symptoms possibly recur, the health-care resources and persistent treatments are indispensable for patients with EM (Lindsay et al. Citation2015).
Propofol, an intravenous anesthetic hypnotic agent, is frequently applied for surgeries on account of the characteristics of short effect as well as rapid recovery; besides, propofol also is characterized with the antioxidant, immunomodulatory, anxiolytic, neuroprotective effects, etc. (Vasileiou et al. Citation2009). Mounting reports demonstrate that propofol represses the recurrence/metastasis risk after cancer surgery (Kim Citation2018; Freeman et al. Citation2019); in addition, the anti-cancer property of propofol has also been verified, for instance, propofol reduces cell proliferation, migration and invasion in non-small cell lung cancer A549 cells (Sun and Gao Citation2018). Whereas the effects of propofol in the ectopic lesions of EM are left to be investigated.
MicroRNAs (20–22 nucleotides), which are known as small and noncoding RNAs, post-transcriptionally modulate the expression of target mRNAs by binding to their 3’untranlational region (UTR) (Bartel Citation2004). Dysregulation of miRNAs has been found to be correlated with the progression of EM (Nothnick Citation2017), for instance, miR-199a relieves ESC invasiveness by inactivation of IKKβ/NF-κB pathway (Dai et al. Citation2012), miR-126 inhibits the progression of EM by regulating Crk (Liu et al. Citation2012), and miR-183 induces ESC invasiveness by regulating ITGB1P (Chen et al. Citation2015). From an array-based and global miRNA profiling, miR-9-5p was observed to be the most significantly decreased miRNA in early secretory endometrium (Liu et al. Citation2017). Therefore, we further investigated the function of miR-9-5p in EM as well as the interaction between propofol and miR-9-5p in EM currently.
A particular miRNA affects numerous mRNA targets (Nothnick Citation2017). mRNA targets that was increased in EM attract our attention. As acknowledged, transforming growth factor-β (TGF-β) signaling plays a crucial function in cancer metastasis (Xie et al. Citation2018) and cancer treatment (Fabregat et al. Citation2014). As an important factor of TGF-β family which is related with the invasion of cancer cells, TGF-β-induced gene (TGFBI) played a promotional role in the growth and invasion of numerous cancer types, e.g. TGFBI promotes cell migration in renal cell carcinoma (Shang et al. Citation2012), TGFBI increases cell proliferation and migration in glioma (Guo et al. Citation2018), TGFBI induces tumor growth of oral squamous cell carcinoma (Wang et al. Citation2019). As for the relation between TGFBI and EM, there was only one report which performed a cDNA microarray with 23,040 genes, demonstrating the elevated TGFBI in EM tissues (Arimoto et al. Citation2003). As for the relation between TGFBI and miR-9-5p, there was only one report which indicated it in Alzheimer’s disease (Schonrock et al. Citation2012). However, the role of TGFBI in EM, the relation between miR-9-5p and TGFBI in EM, or the effects of propofol on miR-9-5p/TGFBI expression in EM has not been indicated, which will be investigated in the present study.
Results
Propofol repressed ectopic ESCs proliferation and invasion
The ectopic ESCs were stimulated by increasing concentrations of propofol (1, 5 or 10 µg/ml) for 24, 48 and 72 h, respectively. Cell proliferation was determined by the MTT assay, results exhibited that the proliferative ability of ectopic ESCs was significantly reduced by propofol dose- and time dependently (Figure A). Afterwards, ectopic ESCs which were stimulated by 5 µg/ml propofol for 48 h was used in the subsequent experiments.
Figure 1. The effects of propofol on ectopic ESCs proliferation and invasion. Cell proliferation of ectopic ESCs was determined by MTT assay (A). Cell invasion of ectopic ESCs was determined by Transwell assay (B and C). * p<0.05, ** p<0.01, propofol vs. DMSO.
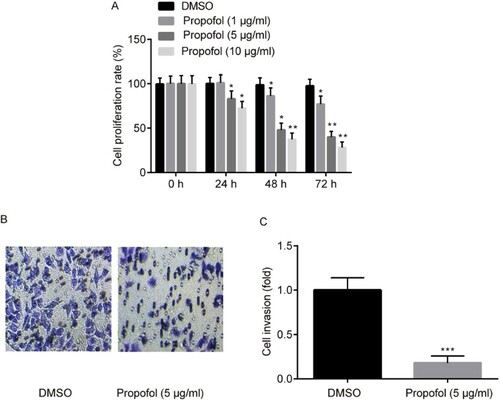
Cell invasion was determined by Transwell assay, results exerted that the invasive ability of ectopic ESCs was predominantly reduced by propofol (Figure B and C).
In a word, propofol indeed exerted an inhibitory effect on the proliferation and ectopic lesions of EM.
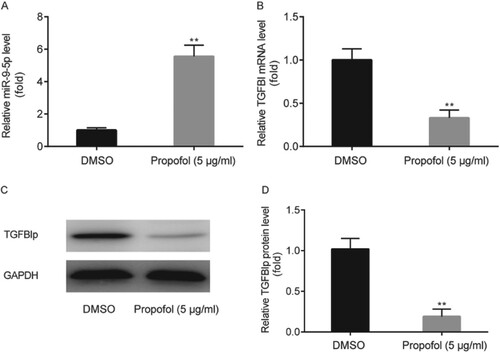
Propofol induced miR-9-5p expression and reduced TGFBI/TGFBIp expression in ectopic ESCs
The effect of propofol on miR-9-5p and TGFBI/TGFBIp expression in ectopic ESCs was examined by RT-qPCR and western blot, respectively. Results showed that miR-9-5p level in ectopic ESCs was significantly increased by propofol administration (Figure A), while TGFBI and TGFBIp levels in ectopic ESCs were significantly decreased by propofol (Figure B–D).
miR-9-5p expression was decreased and TGFBI/TGFBIp expression was increased in ectopic ESCs
miR-9-5p level in ectopic ESCs was significantly reduced compared to eutopic ESCs from patients with EM and normal ESCs from patients with hysteromyoma (Figure A). Whereas TGFBI and TGFBIp levels in ectopic ESCs were significantly elevated compared to eutopic ESCs from patients with EM and normal ESCs from patients with hysteromyoma (Figure B–D).
Figure 3. The expression profile of miR-9-5p and TGFBI/TGFBIp in ectopic ESCs. miR-9-5p level in ectopic ESCs, eutopic ESCs from patients with EM and normal ESCs from patients with hysteromyoma was determined by RT-qPCR (A). TGFBI and TGFBIp levels in ectopic ESCs, eutopic ESCs from patients with EM and normal ESCs from patients with hysteromyoma were determined by RT-qPCR and western blot, respectively (B–D). ** p < 0.01, *** p < 0.001, ectopic ESCs vs. eutopic ESCs and normal ESCs.
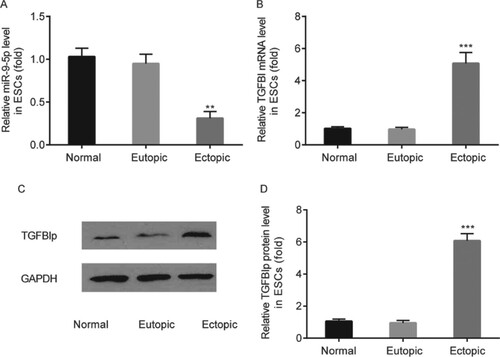
Taken together, miR-9-5p and TGFBI/TGFBIp played a role in EM, which also could be regulated by propofol in EM.
miR-9-5p inhibitor and siRNA-TGFBI influence the effects of propofol on ectopic ESCs proliferation and invasion
To further study the effects of miR-9-5p in EM, we transfected the miR-9-5p inhibitor and/or siRNA-TGFBI into ectopic ESCs and then evaluated the proliferation and migration of ESCs by MTT and Transwell assay, respectively.
Meanwhile, in ectopic ESCs, miR-9-5p inhibitor significantly decreased miR-9-5p level when compared with miR-NC inhibitor (Figure A). Meanwhile, TGFBI and TGFBIp levels in siRNA-TGFBI were significantly decreased compared to siRNA-NC (Figure B–D).
Figure 4. The effects of miR-9-5p inhibitor and siRNA-TGFBI on ectopic ESCs proliferation. In ectopic ESCs, the transfection efficacy of miR-9-5p inhibitor was tested by RT-qPCR (A); the transfection efficacy of siRNA-TGFBI was tested by RT-qPCR and western blot, respectively (B–D). Cell proliferation of ectopic ESCs was determined by MTT assay (E). ** p < 0.01, miR-9-5p inhibitor vs. miR-NC inhibitor; siRNA-TGFBI vs. siRNA-NC; propofol+ miR-9-5p inhibitor vs. propofol + miR-NC inhibitor. #p < 0.05, propofol + miR-9-5p inhibitor + siRNA-TGFBI vs. propofol+ miR-9-5p inhibitor.
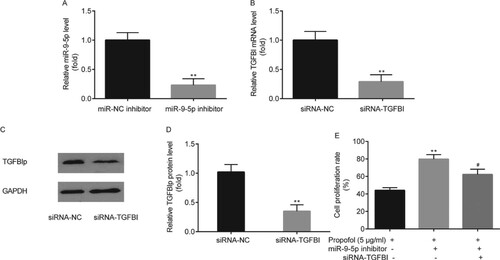
The results of MTT assay indicated that in comparison with control group (propofol + miR-NC inhibitor), a significant increase in cell proliferation rate of ectopic ESCs was observed in miR-9-5p inhibitor group (propofol+ miR-9-5p inhibitor), whereas, it was significantly reversed by the administration of siRNA-TGFBI (propofol + miR-9-5p inhibitor + siRNA-TGFBI) (Figure E).
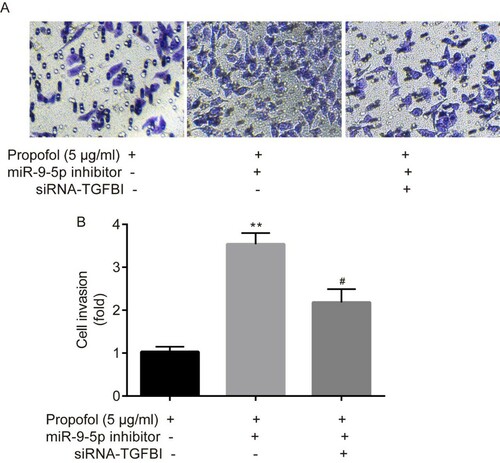
Transwell assay indicated that in comparison with the control group (propofol + miR-NC inhibitor), a significant increase in cell invasion rate of ectopic ESCs was observed in miR-9-5p inhibitor group (propofol+ miR-9-5p inhibitor), whereas it was significantly reversed by the administration of siRNA-TGFBI (propofol + miR-9-5p inhibitor + siRNA-TGFBI) (Figure A–B).
Figure 5. The effects of miR-9-5p inhibitor and siRNA-TGFBI on ectopic ESCs invasion. Cell invasion of ectopic ESCs was determined by Transwell assay (A-B). **p < 0.01, propofol + miR-9-5p inhibitor vs. propofol + miR-NC inhibitor. #p < 0.05, propofol + miR-9-5p inhibitor + siRNA-TGFBI vs. propofol+ miR-9-5p inhibitor.
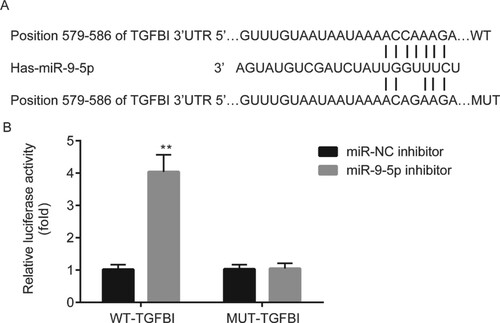
miR-9-5p targeted TGFBI in ectopic ESCs
TargetScan 7.1 revealed that miR-9-5p was complementary to the 3’UTR of TGFBI (Figure A). Dual luciferase reporter assay demonstrated that miR-9-5p inhibitor significantly increased the luciferase activity of ectopic ESCs which were transfected with WT-TGFBI, however, there was no significance of luciferase activity between miR-NC inhibitor group and miR-9-5p inhibitor group in ectopic ESCs which were transfected with MUT-TGFBI (Figure B).
Figure 6. The relation between miR-9-5p and TGFBI in ectopic ESCs. The binding sites between miR-9-5p and 3’UTR of TGFBI were predicted by TargetScan 7.1 (A). The relation between miR-9-5p and 3’UTR of TGFBI was verified by dual luciferase reporter assay (B). **p < 0.01, miR-9-5p inhibitor vs. miR-NC inhibitor.
Discussion
Our data suggested that propofol inhibited the growth and ectopic lesions of EM by regulating miR-9-5p/TGFBI axis.
Up to now, the outcomes of the current treatments in patients with EM have remained unsatisfactory, meanwhile, the pathogeneses of EM have not yet been clearly illuminated (Vercellini et al. Citation2014). The mechanisms potentially involved in invasion and migration of endometriotic cells have attracted more and more attention from increasing researchers. For instance, emodin represses the migration and invasion of human ESCs by regulating ILK (Zheng et al. Citation2016); chloride channel-3 overexpression promotes the migration and invasion of human ESCs from patients with EM (Guan et al. Citation2016). Blocking of Wnt/β-catenin signaling reduced the expression of matrix metalloproteinase-7 and vascular endothelial growth factor in EM (Xu et al. Citation2016). Consequently, it is urgent to explore the methods for relieving ectopic lesions progression in patients with EM.
Propofol has been found to inhibit the metastasis of numerous cancers. For instance, propofol protects cells from IL-13-stimulated cell migration and invasion in human colorectal cancer (Xu et al. Citation2018); propofol prevents LPS-induced cell migration and invasion in non-small cell lung cancer cells (Yang et al. Citation2017); perioperative anesthesia with propofol represses the metastasis of breast cancer cells (Chen et al. Citation2015). However, the function of propofol in the ectopic lesions of EM has not been investigated, which will be explored in the current study. More interestingly, during the conduction of our study, propofol is found to inhibit cell viability and induce cell apoptosis of EM (Feng and Sun Citation2018), which further indicated the inhibitory role of propofol in the progression of EM. Consistently, we found the inhibitory effects of propofol in EM, evidenced by the decreased cell proliferation and invasion of ESCs after propofol administration. Whereas the molecules which might be responsible for its function are left to be explored.
Propofol can regulate multiple miRNAs in different diseases, including inflammation, ischemia/reperfusion injury,cancer, etc. The representative reports are as follows: propofol relieves LPS-induced inflammation in microglia cells by regulating the level of miR-155 (Zheng et al. Citation2018); propofol prevents hepatic ischemia/reperfusion injury by regulating the expression of miR-133a-5p (Hao et al. Citation2017); propofol reduces the cell invasion and proliferation in ovarian cancer by regulating the level of miR-9-5p (Huang et al. Citation2016). Whereas there has been not any reports about the interaction between propofol and miRNA in EM, thereafter, we also researched articles to explore the miRNAs that are related with EM, and also can be regulated by propofol in EM.
From an array-based and global miRNA profiling, miR-9-5p was observed to be the most significantly decreased miRNA in early secretory endometrium (Liu et al. Citation2017). Thereafter, we found that propofol elevated miR-9-5p level, in addition, miR-9-5p was decreased in ectopic ESCs from patients with EM, indicating that propofol exhibited the inhibitory function in the progression of EM by regulating miR-9-5p. However, the mRNA that can be targeted by miR-9-5p and regulated by propofol in EM was left to be explored.
Using a cDNA microarray with 23,040 genes, TGFBI was elevated in EM tissues (Arimoto et al. Citation2003) and was targeted by miR-9-5p in Alzheimer’s disease (Schonrock et al. Citation2012). Herein, we found that propofol reduced TGFBI level, and TGFBI was increased in ectopic ESCs from patients with EM. However, the relation between miR-9-5p and TGFBI in EM, or the effects of propofol on miR-9-5p/TGFBI expression in EM has not been indicated.
At last, miR-9-5p inhibitor was found to attenuate the inhibitory effects of propofol on proliferation and invasion of ectopic ESCs, which was partially reversed by siRNA-TGFBI. In addition, miR-9-5p targeted 3’UTR of TGFBI in ectopic ESCs.
This study provided a novel potential therapeutic target for patients with EM. However, there exist a weakness in this study, i.e., we only collected a small portion of participants, we will collect much more participants in our future work.
Materials and methods
Tissue acquisition
From January 2016 to February 2018 at The First Affiliated Hospital of Nanchang University, the paired ectopic endometrial samples and eutopic endometrial samples were collected from 21 patients with EM, while control endometrium samples were collected from 21 patients with hysteromyoma. The basic clinical characteristics of 21 patients with hysteromyoma and 21 patients with EM were exhibited in Table . Each participant had regular schedule and received not any hormone therapies within 3 months before the surgery. Patients who suffered with polycystic ovary syndrome or inflammation were excluded from the study. Each participant signed and provided an informed consent prior to the conduction of the research. Ethics committee of The First Affiliated Hospital of Nanchang University approved the present study.
Table 1. Basic clinical characteristics of the participants.
Cell culture
Primary ESCs were isolated from control, eutopic and ectopic endometrial tissues as previously reported (Burney et al. Citation2009). In brief, samples were cut into pieces (1 mm) and digested by type II collagenase (0.1%) at 37°C for 1 h. Next, cells were collected by centrifugation (500 g for 5 min) followed with suspension in Ham’s F12/DMEM (1:1) and filter by nylon mesh (100-µm and 40-µm) to separate glandular epithelial cells. After incubation for 12 h at 37°C, the non-adherent glandular epithelial cells and blood cells were removed from the medium. Thereafter, the purified stromal cells were cultured in Ham’s F12/DMEM (1:1), supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin, and 0.25 µg/ml amphotericin B in an incubator with humidified atmosphere containing 5% CO2 at 37°C.
Transient transfection
miR-9-5p inhibitor, miR-negative control (NC) inhibitor, siRNA-TGFBI and siRNA-NC were obtained from the Applied Biosystems (China). Ectopic ESCs (3 × 105) were transfected with miR-9-5p inhibitor or miR-NC inhibitor, and siRNA-TGFBI or siRNA-NC transiently by Lipofectamine 2000 (Thermo), and the mixture was incubated in an incubator with humidified atmosphere containing 5% CO2 at 37°C for 48 h. Afterwards, ectopic ESCs were harvested for the conduction of the following experiments.
Cell treatment
Propofol was purchased from Corden Pharma Caponago S.P.A. (Milan, Italy) and dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich). Ectopic ESCs were seeded into 96-well plates and cultured in an incubator with humidified atmosphere containing 5% CO2 at 37°C overnight. On the next day, the ectopic ESCs were stimulated by propofol (1, 5 or 10 µg/ml), and incubated in an incubator with humidified atmosphere containing 5% CO2 at 37°C for 24, 48 and 72 h, respectively. While the ectopic ESCs in control group were treated by DMSO (0.2%). Thereafter, ectopic ESCs in each group were collected for the following studies.
RT-qPCR
Total RNA was extracted from ESCs by an RNA Isolation Kit (Takara). cDNA was synthesized with reverse transcription by PrimeScript RT reagent kit (Takara). The TaqMan miRNA assay (Qiagen) was conducted on an ABI 7500 Real-time PCR system (Bio-Rad Laboratories). U6 and GAPDH served internal references for the examination of miR-9-5p and TGFBI expression. The analysis was carried out with the method of 2-ΔΔCq.
Western blot
Total protein extraction kit (Kaiji Biological) was applied for the extraction of protein from ESCs. The BCA kit (Beyotime) was used to examine the concentration of protein in each sample. Thereafter, each protein sample was separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, followed by electro-transferring to the polyvinylidene difluoride membranes. Next, the polyvinylidene difluoride membranes were blocked by 5% non-fat milk for 2 h at room temperature, then treated by primary antibodies against TGFBIp overnight at 4°C and secondary antibodies for 2 h at room temperature. The optical density (OD) value in each protein band was examined by the enhanced chemiluminescent reagent. Each protein level was quantified by Image-Pro Plus software (Pierce Biotechnology). GAPDH served an internal reference for the examination of TGFBIp expression.
MTT assay
Briefly, cell proliferation of ectopic ESCs was examined by MTT assay (Sigma-Aldrich). Ectopic ESCs were seeded in the 96-well plates and cultured in an incubator with humidified atmosphere containing 5% CO2 at 37°C for 24 h. Subsequently, 20 µL of MTT solution was added into the above culture medium, and the mixture was placed in an incubator with humidified atmosphere containing 5% CO2 at 37°C for 4 h. Then 200 µL of DMSO was added into the above mixture to dissolve the formazan crystals, and the mixture was incubated for 15 min at 37°C. The OD value of each sample was examined at 490 nm.
Transwell assay
Ectopic ESCs (5 × 103 cells/well) were seeded into the upper Transwell chamber coated with Matrigel (8-µm pores; EMD Millipore) followed by addition of serum-free culture medium. Thereafter, the lower Transwell chamber was added with culture medium containing 20% FBS. Subsequently, ectopic ESCs were cultured in an incubator with humidified atmosphere containing 5% CO2 at 37°C for 24 h. The invaded cells were fixed by 4% paraformaldehyde for 10 min, and stained by 0.1% crystal violet for 15 min at room temperature. A microscope (Olympus) was applied for counting the migrated ectopic ESCs from 6 independent fields.
Dual luciferase report assay
The binding sites between TGFBI and miR-9-5p were predicted using bioinformatics analysis TargetScan 7.1. The 3’UTR of wild-type TGFBI (WT-TGFBI) and the mutant type TGFBI (MUT-TGFBI) were constructed into pGL3 (Promega Corporation, WI, USA), which were co-transfected with miR-9-5p inhibitor or miR-NC inhibitor into ectopic ESCs by Lipofectamine 2000 (Thermo). After incubation for 48 h, the luciferase activity was determined by dual luciferase reporter system (Promega Corporation), with Renilla (Promega Corporation) acting as the internal reference.
Statistical analysis
The GraphPad software (ver.6.01; GraphPad) was applied for analysis of data. Results were presented as means ± SD. Unpaired student’s test was applied for the determination of significance between 2 groups for results in ectopic ESCs. One-way analysis of variance followed by the Newman–Keuls test was applied for the determination of significance in multiple groups. P < 0.05 indicated statistical significance.
Author contributions
GL, QZ, JS, LX, and YZ carried out the experiments and analyzed the data. BH designed the experiments and wrote the manuscript.
Availability of data and materials
All data and materials were available freely. Due to the participants of this study did not agree for their data to be shared publicly, supporting data are not available.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Ahn JH, Choi YS, Choi JH. 2015. Leptin promotes human endometriotic cell migration and invasion by up-regulating MMP2 through the JAK2/STAT3 signaling pathway. Mol Hum Reprod. 21:792–802. doi: https://doi.org/10.1093/molehr/gav039
- Arimoto T, Katagiri T, Oda K, Tsunoda T, Yasugi T, et al. 2003. Genome-wide cDNA microarray analysis of gene-expression profiles involved in ovarian endometriosis. Int J Oncol. 22:551–560.
- Bartel DP. 2004. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 116:281–297. doi: https://doi.org/10.1016/S0092-8674(04)00045-5
- Burney RO, Hamilton AE, Aghajanova L, Vo KC, Nezhat CN, et al. 2009. MicroRNA expression profiling of eutopic secretory endometrium in women with versus without endometriosis. Mol Hum Reprod. 15:625–631. doi: https://doi.org/10.1093/molehr/gap068
- Chen J, Gu L, Ni J, Hu P, Hu K, et al. 2015. MiR-183 regulates ITGB1P expression and promotes invasion of endometrial stromal cells. BioMed Res Int. 2015:340218.
- Chen X, Lu P, Chen L, Yang SJ, Shen HY, et al. 2015. Perioperative propofol-paravertebral anesthesia decreases the metastasis and progression of breast cancer. Tumour Biol. 36:8259–8266. doi: https://doi.org/10.1007/s13277-015-4027-5
- Dai L, Gu L, Di W. 2012. MiR-199a attenuates endometrial stromal cell invasiveness through suppression of the IKKβ/NF-κB pathway and reduced interleukin-8 expression. Mol Hum Reprod. 18:136–145. doi: https://doi.org/10.1093/molehr/gar066
- Fabregat I, Fernando J, Mainez J, Sancho P. 2014. TGF-beta signaling in cancer treatment. Curr Pharm Des. 20:2934–2947. doi: https://doi.org/10.2174/13816128113199990591
- Feng S, Sun Y. 2018. Protective role of propofol in endometriosis and its mechanism. Exp Ther Med. 16:3646–3650.
- Freeman J, Crowley PD, Foley AG, Gallagher HC, Iwasaki M, et al. 2019. Effect of perioperative lidocaine, propofol and steroids on pulmonary metastasis in a murine model of breast cancer surgery. Cancers (Basel). 11:613. doi: https://doi.org/10.3390/cancers11050613
- Guan YT, Huang YQ, Wu JB, Deng ZQ, Wang Y, et al. 2016. Overexpression of chloride channel-3 is associated with the increased migration and invasion ability of ectopic endometrial cells from patients with endometriosis. Hum Reprod. 31:986–998. doi: https://doi.org/10.1093/humrep/dew034
- Guo SK, Shen MF, Yao HW, Liu YS. 2018. Enhanced expression of TGFBI promotes the proliferation and migration of glioma cells. Cell Physiol Biochem. 49:1097–1109. doi: https://doi.org/10.1159/000493293
- Hao W, Zhao ZH, Meng QT, Tie ME, Lei SQ, et al. 2017. Propofol protects against hepatic ischemia/reperfusion injury via miR-133a-5p regulating the expression of MAPK6. Cell Biol Int. 41:495–504. doi: https://doi.org/10.1002/cbin.10745
- Huang X, Teng Y, Yang H, Ma J. 2016. Propofol inhibits invasion and growth of ovarian cancer cells via regulating miR-9/NF-κB signal. Braz J Med Biol Res. 49:e5717. doi: https://doi.org/10.1590/1414-431x20165717
- Kim R. 2018. Effects of surgery and anesthetic choice on immunosuppression and cancer recurrence. J Transl Med. 16:8. doi: https://doi.org/10.1186/s12967-018-1389-7
- Lindsay SF, Luciano DE, Luciano AA. 2015. Emerging therapy for endometriosis. Expert Opin Emerg Drugs. 20:449–461. doi: https://doi.org/10.1517/14728214.2015.1051966
- Liu S, Gao S, Wang XY, Wang DB. 2012. Expression of miR-126 and Crk in endometriosis: miR-126 may affect the progression of endometriosis by regulating Crk expression. Arch Gynecol Obstet. 285:1065–1072. doi: https://doi.org/10.1007/s00404-011-2112-6
- Liu H, Zhang Z, Xiong W, Zhang L, Xiong Y, et al. 2017. Hypoxia-inducible factor-1alpha promotes endometrial stromal cells migration and invasion by upregulating autophagy in endometriosis. Reproduction. 153:809–820. doi: https://doi.org/10.1530/REP-16-0643
- Maheshwari A, Gurunath S, Fatima F, Bhattacharya S. 2012. Adenomyosis and subfertility: a systematic review of prevalence, diagnosis, treatment and fertility outcomes. Hum Reprod Update. 18:374–392. doi: https://doi.org/10.1093/humupd/dms006
- Nothnick WB. 2017. MicroRNAs and endometriosis: distinguishing drivers from passengers in disease pathogenesis. Semin Reprod Med. 35:173–180. doi: https://doi.org/10.1055/s-0037-1599089
- Nyholt DR, Low SK, Anderson CA, Painter JN, Uno S, et al. 2012. Genomewide association metaanalysis identifies new endometriosis risk loci. Nat Genet. 44:1355–1359. doi: https://doi.org/10.1038/ng.2445
- Painter JN, Anderson CA, Nyholt DR, Macgregor S, Lin J, et al. 2011. Genome-wide association study identifies a locus at 7p15.2 associated with endometriosis. Nat Genet. 43:51–54. doi: https://doi.org/10.1038/ng.731
- Schonrock N, Humphreys DT, Preiss T, Götz J. 2012. Target gene repression mediated by miRNAs miR-181c and miR-9 both of which are down-regulated by amyloid-β. J Mol Neurosci. 46:3243–3235. doi: https://doi.org/10.1007/s12031-011-9587-2
- Schrager S, Falleroni J, Edgoose J. 2013. Evaluation and treatment of endometriosis. Am Fam Physician. 87:107–113.
- Shang D, Liu Y, Yang P, Chen Y, Tian Y. 2012. TGFBI-promoted adhesion, migration and invasion of human renal cell carcinoma depends on inactivation of von Hippel-Lindau tumor suppressor. Urology. 79:966–e1-7. doi: https://doi.org/10.1016/j.urology.2011.12.011
- Sun H, Gao D. 2018. Propofol suppresses growth, migration and invasion of A549 cells by down-regulation of miR-372. BMC Cancer. 18:1252. doi: https://doi.org/10.1186/s12885-018-5175-y
- Vasileiou I, Xanthos T, Koudouna E, Perrea D, Klonaris C, et al. 2009. Propofol: a review of its non-anaesthetic effects. Eur J Pharmacol. 605:1–8. doi: https://doi.org/10.1016/j.ejphar.2009.01.007
- Vercellini P, Viganò P, Somigliana E, Fedele L. 2014. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 10:261–275. doi: https://doi.org/10.1038/nrendo.2013.255
- Wang BJ, Chi KP, Shen RL, Zheng SW, Guo Y, et al. 2019. TGFBI promotes tumor growth and is associated with poor prognosis in oral squamous cell carcinoma. J Cancer. 10:4902–4912. doi: https://doi.org/10.7150/jca.29958
- Xie F, Ling L, van Dam H, Zhou F, Zhang L. 2018. TGF-β signaling in cancer metastasis. Acta Biochim Biophys Sin (Shanghai). 50:121–132. doi: https://doi.org/10.1093/abbs/gmx123
- Xu K, Tao W, Su Z. 2018. Propofol prevents IL-13-induced epithelial-mesenchymal transition in human colorectal cancer cells. Cell Biol Int. 42:985–993. doi: https://doi.org/10.1002/cbin.10964
- Xu H, Yang JJ, Wang CH, Guo EY, Yang NH, et al. 2016. Effect of Wnt/β-catenin signal pathway on matrix metalloproteinase-7 and vascular endothelial growth factor gene expressions in endometriosis. Clin Exp Obstet Gynecol. 43:573–577.
- Yang N, Liang Y, Yang P, Ji F. 2017. Propofol suppresses LPS-induced nuclear accumulation of HIF-1α and tumor aggressiveness in non-small cell lung cancer. Oncol Rep. 37:2611–2619. doi: https://doi.org/10.3892/or.2017.5514
- Zheng X, Huang H, Liu J, Li M, Liu M, et al. 2018. Propofol attenuates inflammatory response in LPS-activated microglia by regulating the miR-155/SOCS1 pathway. Inflammation. 4:11–19. doi: https://doi.org/10.1007/s10753-017-0658-6
- Zheng Q, Xu Y, Lu J, Zhao J, Wei X, et al. 2016. Emodin inhibits migration and invasion of human endometrial stromal cells by facilitating the mesenchymal-epithelial transition through targeting ILK. Reprod Sci. 23:1526–1535. doi: https://doi.org/10.1177/1933719116645192