ABSTRACT
This study aimed to investigate the expression of miR-223-3p in a rat model of myocardial infarction and the effects of miR-223-3p on cardiomyocytes. Forty rats were randomly divided into mock surgical, model (acute myocardial infarction (AMI) group (AMIG)), AMI + miR-223-3p adenovirus (intervention group (IG)), and AMI + scramble-NC (negative control group (NCG)) groups. Cardiac function was assessed by small animal echocardiography. Reverse transcription polymerase chain reaction was used to quantify the expression of miR-223-3p. The expression levels of miR-233-3p and SOX6 mRNA in the blank control group (BCG) and mock surgical group (MSG) were lower than those in the AMIG, IG, and NCG (P < 0.05), while the cardiac function indices of the BCG and MSG were higher than those of the AMIG, IG, and NCG (P < 0.05). The expression levels of miR-233-3p and SOX6 mRNA in the AMIG and NCG were significantly higher than those in the IG, while their cardiac function indices were significantly lower than those in the IG. Our findings indicate that the silencing of miR-223-3p effectively improves the cardiac function of rats, and the overexpression of miR-223-3p promotes the apoptosis of cardiomyocytes. Thus, miR-223-3p may be a new therapeutic target for AMI.
Introduction
With the continuous improvement in human living standards, the incidence of cardiovascular disease is increasing. Cardiovascular diseases are a serious threat to human life and health, especially in the elderly population (Dreyer et al. Citation2017). As one of the most dangerous cardiovascular diseases, acute myocardial infarction (AMI) has a high mortality and disability rate (Townsend et al. Citation2016). In the U.S.A., approximately 790,000 cases of AMI are reported annually, and this number is increasing (Mihalko et al. Citation2018). With technological advancements in the medical field, the cure rate of patients with AMI is continuously increasing and the mortality of these patients is continuously decreasing. However, most patients still suffer from myocardial necrosis and other conditions after treatment. As a result, heart failure may occur, seriously affecting patient quality of life (Benjamin et al. Citation2017). Currently, clinical treatment for heart failure in patients with myocardial infarction (MI) primarily involves drug therapy or device implantation. However, the curative effects of these treatments are still very limited (Desta et al. Citation2015). Some studies have shown that the manifestation of cardiomyocyte necrosis in patients with AMI mainly occurs in cardiac muscle fiber. Therefore, the identification of factors involved in myocardial fibrosis could aid in the discovery of a new therapeutic target of AMI (Katarzyna Citation2017).
A microRNA (miRNA) is a single-stranded non-coding RNA that is 21–23 nucleotides long. Previous studies have shown that some miRNAs have close relationships with cardiovascular diseases. They exhibit regulatory effects on the growth and development of the heart (van Berlo and Molkentin Citation2016). miR-223 is an miRNA that is highly expressed in blood cells, such as granulocytes and bone marrow hematopoietic cells (Das and Halushka Citation2015). miR-223-3p is a derivative of miR-233. Previous studies have shown that miR-223-3p is strongly upregulated during the early stages of AMI, before the elevation of creatinine kinase isozyme (CK-MB) and troponin I (cTn I) (Rodriguez et al. Citation2012). miR-233 has also been reported to directly target the downstream cardiac troponin I-interacting kinase (TNNI3 K) and inhibit myocardial hypertrophy. Persistent myocardial hypertrophy can increase the risk of heart failure. Therefore, many studies have suggested that miR-233 could be a new therapeutic target for heart failure (Liu W et al. Citation2015).
However, the effect of miR-223 expression on cardiomyocyte apoptosis and cardiac function during MI has never, to the best of our knowledge, been reported. Thus, in this study, we investigated the expression of miR-223 in a rat MI model and examined its effects on cardiomyocyte apoptosis and cardiac function.
Materials and methods
Animals and materials
Sixty adult male Sprague-Dawley (SD) rats (6–8 weeks old, 200–240 g) were selected randomly and purchased from Slaccas Laboratory Animal Co., Ltd. All rats were raised under a 12-h light/dark cycle at 22°C, with free access to food and water. The medical small animal ventilator was purchased from Shanghai Yuyan Instruments Co., Ltd. The surgical instruments were purchased from RWD Life Science Co., Ltd. TRIzol reagent was purchased from Applied Invitrogen. The quantitative polymerase chain reaction (qPCR) kit and miScript reverse transcription kit were purchased from Takara. miR-223-3p mimics, miRNA NC, and internal reference U6 primer were synthesized and designed by GenePharma. The study was approved by the Committee on the Ethics of Animal Experiments of Fuwai Central China Cardiovascular Hospital. Sodium pentobarbital was purchased from Shanghai Yubai Biotechnology Co., Ltd. (57-33-0).
Establishment of the MI model
Fifty rats were randomly selected for surgery. The remaining 10 rats were enrolled in the blank control group (BCG) and maintained as previously described. Rats were anesthetized with intraperitoneal injections of sodium pentobarbital (3 mg/ml, 50 mg/kg). No signs of peritonitis, pain, or discomfort were observed. The rats were fixed on the operating table in the supine position. Then, endotracheal intubation was performed, and the ventilator was connected. After successful intubation, the ECG detector was connected to the limbs of the rats. ECG changes during surgery were recorded. The skin was cut along the ribs from the left third to fourth intercostal space. A dilator was used to fix the wound and the heart was exposed. The left coronary artery was ligated 2 mm below the left auricle. The following conditions indicated the successful establishment of an MI model: the anterior myocardium of the left ventricle appeared white, the heart began to weaken, and the ST-T segments observed in the ECG were elevated.
Grouping and adenovirus transfection
Ten rats were randomly selected for the mock surgical group (MSG). Chest opening was performed without ligation of the coronary artery. Thirty model rats were established, while ten rats died during anesthesia induction and surgery. The 30 rats were divided randomly into the model group (AMI group (AMIG)), AMI + miR-223-3p adenovirus group (intervention group (IG)), and AMI + miR-NC group (negative control group (NCG)) (n = 10 per group). After model establishment, the rats in the IG and NCG were administered an intramyocardial injection of 0.2 mL of either miR-223-3p adenovirus or miR-NC and Lipofectamine 2000 at a 1:1 ratio (Liu X et al. Citation2016) into the anterior wall of the left ventricle. After suturing, the rats were returned to their cage and fed. Cardiac function was examined using echocardiography, 48 h after transfection. First, the rats were connected to the echocardiogram detector. Then, the left ventricular ejection fraction (LVEF), fraction shortening (FS), interventricular septal thickness at end diastole (IVSD), left ventricular end diastolic diameter (LVEDD), left ventricular end systolic dimension (LVESD), and left ventricular posterior wall thickness at end diastole (LVPWD) scores were recorded and analyzed.
Expression of miR-223-3p and biological validation of the miRNA target Sox6
Using TargetScan, it was found that Sox6 is a target gene of miR-223. To assess the effects of miR-223-3p on Sox6 gene transcription, a luciferase reporter containing the 3′ untranslated region (UTR), both WT and mutated, of the predicted miRNA interaction sites was used. Levels of miR-223-3p were measured using individual TaqMan miRNA assays. After testing, the rats were anesthetized with the same dose of chloral hydrate and euthanized via cervical dislocation. Heart tissue was extracted and cut into sections, total RNA was extracted using TRIzol reagent, and the concentration and purity of RNA were determined by ultraviolet spectrophotometry. Next, 5 µl of total RNA was sampled from each group. Reverse transcription was performed using a kit in accordance with the manufacturer’s instructions. The reaction conditions used were as follows: incubation at 37°C for 50 min and then at 85°C for 10 s. Then, qPCR was performed under the following conditions: denaturation at 95°C for 30 s, followed by 45 cycles of incubation at 95°C for 10 s and annealing at 60°C for 30 s, and final extension at 72°C for 6 min. U6 was taken as the internal reference of miR-22. β-actin was used as the internal reference for SOX6 mRNA. The relative expression of miR-133 was statistically recorded and compared. The relevant primer sequences are shown in .
Table 1. Primer sequences.
Primary isolation and culture
The hearts of the rats were extracted and placed in a culture dish containing PBS. Then, the hearts were minced and placed in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% FBS. After oscillation at 37°C for 5 min, the supernatant was discarded. Then, 10 mL of collagenase was added to the culture. After oscillation for 1 min, the supernatant was removed and 6 mL of collagenase was added, followed by stirring for 15 min. This step was repeated twice more. Then, the resulting mixture was transferred to a centrifuge tube and centrifuged at 1000 rpm for 5 min. Next, 10 mL DMEM was added, and the cardiomyocytes were suspended and cultured in an incubator under 5% CO2 at 37°C. The cells were collected for subsequent experiments when they reached 80% confluence.
Transfection with miR-223-3p
The cells were grouped before transfection. The cells transfected with miR-223-3p mimics were included in the test group (TG), those transfected with miR-NC were included in the NCG, and untransfected cells were included in the BCG. Briefly, cells were inoculated in a six-well plate at a density of 3 × 105 cells/well. Lipofectamine 2000 and DNA were diluted and mixed according to the instructions of the manufacturer of the Lipofectamine 2000 transfection kit. After incubating at room temperature for 5 min, the above mixture was mixed with the cells and incubated at 37°C under 5% CO2. After 48 h of transfection, the expression of miR-223-3p was examined by PCR. The cells were collected for follow-up experiments.
SOX6 and cardiomyocyte apoptosis-related proteins
Cardiomyocytes were lysed with RIPA and total protein was collected. After separation with 10% SDS-PAGE, the protein was transferred to a PVDF membrane and 5% skim milk was added. The setup was sealed and incubated overnight at 4°C. After the addition of primary antibodies (SOX6, BAX, and Bcl-xL, dilution ratio 1:1000), the membranes were again incubated at 4°C overnight. After washing the membranes with PBS-T, secondary antibody, β-actin (l:500), was added. The membranes were then incubated at room temperature for 2 h. Finally, color development was conducted using ECL developing solution.
Detection of apoptosis rate of myocardial cells
The cells were digested with 0.25% trypsin, washed twice with PBS and suspended in 100 µL binding buffer. After that, they were dyed with 5 µL Annexin V-FITC in darkness at room temperature for 10 min, and then incubated with 5 µL PI solution at room temperature for 5 min. Apoptosis was detected by FACSVerse flow cytometer. FlowJo software (version 7.6.5; BD Biosciences) was used to analyse the data.
Dual luciferase reporter assay
Dual luciferase reporter assay was performed to determine whether SOX6 was a direct target gene of miR-223-3p. SOX6 3'UTR dual luciferase reporter plasmids (WT and MUT) were constructed by RiboBio. Lipofectamine 2000 was used to co-transfect them into cells with miR-223-3p inhibitor (anti-miR-223-3p) or a mimic control respectively. After incubation for 48 h, the luciferase activity was detected by a dual luciferase assay system.
Statistical analysis
Data were statistically analyzed using SPSS 219.0 (Beijing NTTimes Science and Technology Ltd.). The data are expressed as x ± s. One-way analysis of variance (ANOVA) was used for comparisons among groups followed by post hoc correction tests. The Bonferroni test was adopted for post hoc test. Spearman analysis was used for the correlation analysis of the miR-223-3p level and SOX6 mRNA and protein levels. The figures were plotted using GraphPad prism 6. P < 0.05 indicated statistically significant differences.
Ethics
This study was approved by the Committee on the Ethics of Animal Experiments of Fuwai Central China Cardiovascular Hospital.
Results
Expression of miR-233-3p and SOX6 mRNA
The miR-223-3p levels were 1.01 ± 0.09, 1.03 ± 0.11, 6.87 ± 0.74, 3.24 ± 0.56, and 6.50 ± 0.62 and the SOX6 mRNA levels were 1.18 ± 0.13, 1.19 ± 0.12, 7.65 ± 0.48, 3.17 ± 0.31, and 7.59 ± 0.52, respectively, in the BCG, MSG, AMIG, IG, and NCG. The miR-233-3p and SOX6 mRNA levels in the BCG and MSG were similar (P > 0.05) but lower than those in the AMIG, IG, and NCG (P < 0.05). However, these levels were significantly higher than those in the IG (P < 0.05) ( and ).
Figure 1. Expression of miR-233-3p and SOX6 mRNA in the myocardium of rats. The expression levels of miR-233-3p and SOX6 mRNA were not significantly different between the BCG and MSG (P > 0.05). The expression levels of miR-233-3p and SOX6 mRNA in the BCG and MSG were lower than those in the AMIG, IG, and NCG (P < 0.05). There was no significant difference in the expression levels of miR-233-3p and SOX6 mRNA between the AMIG and NCG (P > 0.05). However, miR-233-3p expression levels in the AMIG and NCG were higher than those in the IG (P > 0.05). * P < 0.05.
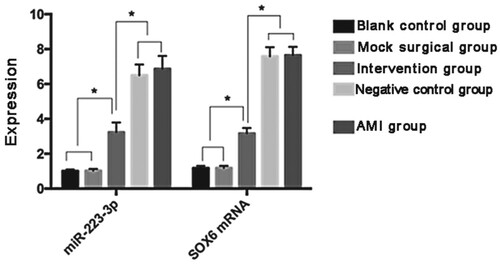
Table 2. Expression of miR-223-3p and SOX6 mRNA.
Comparison of cardiac function and description of myocardial tissue morphology
Forty-eight hours after transfection, the cardiac function of the rats was examined. The LVEF scores were 81.45% ± 3.29%, 79.37% ± 3.31%, 42.48% ± 0.79%, 69.86% ± 4.93%, and 43.11% ± 0.85% and the FS scores were 42.57% ± 4.03%, 41.33% ± 3.97%, 22.91% ± 1.04%, 36.16% ± 2.54%, and 23.74% ± 1.17%, respectively, for rats in the BCG, MSG, AMIG, IG, and NCG. The LVEF, FS, IVSD, LVEDD, LVESD and LVPWD scores were comparable between the BCG and MSG (P > 0.05). However, the LVEF and FS scores were higher than those in the AMIG, IG, and NCG (P < 0.05), and the IVSD, LVEDD, LVESD and LVPWD scores were lower than those in the AMIG, IG and NCG (P < 0.05). The LVEF, FS, IVSD, LVEDD, LVESD and LVPWD scores were comparable between the AMIG and NCG (P > 0.05). However, the LVEF and FS scores were lower than those in the IG, and the IVSD, LVEDD, LVESD and LVPWD scores were higher than those in the IG (P < 0.05). At the same time, scarring in myocardial tissue was observed in AMIG and NCG, with the hyperemia and edema in cardiac mesenchyme accompanied by a large amount of inflammatory cell infiltration. In the IG, hyperemia, edema and inflammatory cell infiltration in cardiac mesenchyme were all relieved. There was no obvious pathologic change in myocardial tissue in the BCG and MSG ( and ).
Figure 2. Comparison of cardiac function. LVEF and FS scores in the AMIG, IG, and NCG were lower than those in the BG and MSG (P < 0.05). The LVEF and FS scores in the IG were higher than those in the AMIG and NCG (P < 0.05). * P < 0.05.

Table 3. Cardiac function 48 h after transfection with adenovirus.
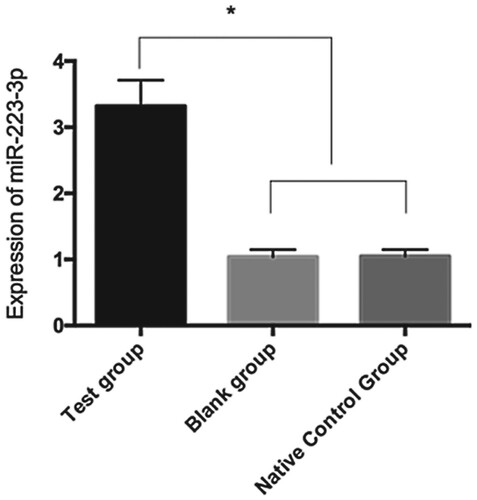
Expression of miR-223-3p after transfection
After transfection, the level of miR-223-3p in cardiomyocytes was 3.32 ± 0.39 in the TG, 1.04 ± 0.11 in the BG, and 1.05 ± 0.10 in the NCG. The miR-223-3p level in the TG was lower than those in the BG and NCG (P < 0.05), indicating successful transfection ().
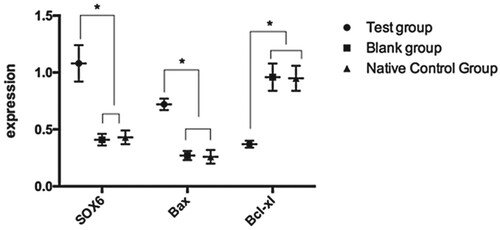
Expression of SOX6 and cardiomyocyte apoptosis-related proteins after transfection
The levels of SOX6, BAX, and Bcl-xL proteins in the TG, BG, and NCG were 1.08 ± 0.16, 0.41 ± 0.05, and 0.43 ± 0.06; 0.72 ± 0.05, 0.27 ± 0.04, and 0.26 ± 0.06; and 0.37 ± 0.03, 0.96 ± 0.12, and 0.95 ± 0.11, respectively. The levels of SOX6 and BAX in the TG were significantly higher than those in the BG and NCG. Bcl-xL expression was lower in the TG than in the BG and NCG (P < 0.05) ().
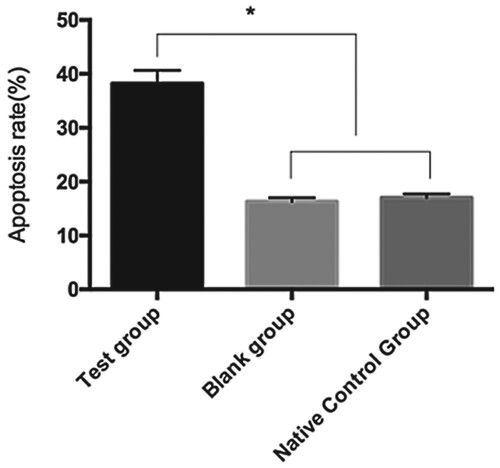
Cardiomyocyte apoptosis after transfection
The apoptosis rates of cardiomyocytes in the TG, BG, and NCG were 38.19% ± 2.47%, 16.33% ± 0.71%, and 17.02% ± 0.69%, respectively. The cardiomyocyte apoptosis rate in the TG was significantly higher than in the BG and NCG ().
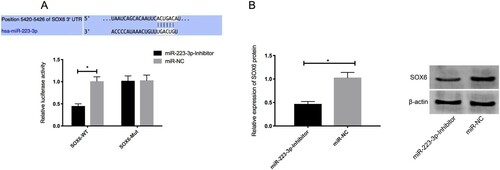
Dual luciferase reporter assay
Bioinformatics analysis was performed to predict the target genes of miR-223-3p. SOX6 was identified as the target gene of miR-223-3p. Luciferase reporter assay was performed to determine whether the 3'UTR of SOX6 could be directly targeted by miR-223-3p. The results showed that the overexpression of miR-223-3p further stimulated the luciferase activity of SOX6 3’UTR Wt (P < 0.05), but had no effect on the SOX6 3’UTR Mut. Moreover, Western blot showed that the expression of SOX6 protein in the cells transfected with miR-223-3p inhibitor was down-regulated ().
Figure 6. Overexpression of miR-223-3p on the luciferase activity of SOX6 3’UTR Wt and SOX6 3’UTR Mut (A) and expression of SOX6 protein in the cells transfected with miR-223-3p inhibitor (B). Overexpression of miR-223-3p further stimulated the luciferase activity of SOX6 3’UTR Wt (P < 0.05), but had no effect on the SOX6 3’UTR Mut. The expression of SOX6 protein in the cells transfected with miR-223-3p inhibitor was down-regulated. * P < 0.05.
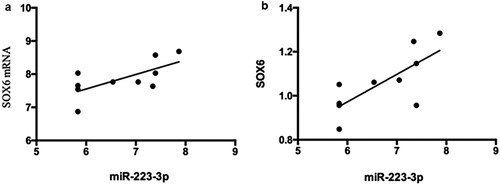
Correlation analysis of miR-223-3p level and SOX6 mRNA and protein levels
The miR-223-3p level and SOX6 mRNA and protein levels were positively correlated (r = 0.683, P < 0.05; r = 0.740, P < 0.05; respectively) ((a and b)).
Figure 7. Correlation between the expression of miR-223-3p and SOX6 mRNA and protein. 7a:The expression levels of miR-223-3p and SOX6 mRNA were positively correlated (r = 0.683, P < 0.05).7b: Correlation between the expression of miR-223-3p and SOX6 protein. The expression of miR-223-3p was positively correlated with that of SOX6 protein (r = 0.740, P < 0.05).
Discussion
AMI is a commonly occurring acute and critical disease that progresses as follows. First, lesions occur in the coronary artery. Then, the blood supply of the coronary artery significantly decreases or is interrupted. As a result, serious and persistent myocardial ischemia occurs, and myocardial necrosis is eventually caused (Lal et al. Citation2014). As an evolutionarily conserved endogenic non-coding small RNA, an miRNA can either inhibit the translation of a target mRNA or cause its degradation. Therefore, the regulatory effects of miRNAs on gene expression have been realized. miRNAs are involved in various physiological and pathological processes due to this function (Lesizza et al. Citation2017). There are some cardiac-specific miRNAs that are highly expressed in the cardiovascular system. They play an important role in cardiac angiogenesis, myocardial hypertrophy, heart failure, and other pathophysiological processes (Mehta et al. Citation2016). miR-223-3p is a cardiac-specific miRNA, and a previous study showed that it is moderately expressed in cardiomyocytes (Schirle et al. Citation2014). However, few studies have been conducted regarding the effect of miR-223-3p on cardiomyocyte apoptosis in patients with AMI. To identify a new therapeutic target for patients with AMI, the level of miR-223-3p in rats with AMI and the effects of miR-223-3p on cardiomyocytes were investigated in this study.
The AMI rat model was first established by ligating the coronary artery. miR-223-3p was silenced via the adenovirus transfection method. The expression of miR-223-3p and its target gene, Sox6, in myocardial tissue was examined 48 h after transfection. miR-223-3p and SOX6 mRNA and protein were found to be highly expressed in the myocardial tissues of AMIG rats. However, their expression levels decreased in the IG rats, indicating successful transfection. Further analysis demonstrated that the miR-223-3p level and SOX6 mRNA and protein levels were positively correlated. SOX6 plays an important role in cell death and proliferation. As a pro-apoptotic factor, it can regulate the apoptosis of cardiomyocytes (Bartel Citation2004). A study (Qian et al. Citation2011) found that the miR-223-3p expression level in ischemic cardiac microvascular endothelial cells (CMECs) is significantly higher than that in normal CMECs. Another study (Wang et al. Citation2015) indicated that the expression of miR-223-3p in the ischemic myocardium is higher than that in the normal myocardium. Another study (Jia et al. Citation2016) reported that the increased levels of serum miR-223-3p in the ischemic myocardium are one of the diagnostic biomarkers for AMI. All the above-mentioned findings are consistent with the findings of the present study. Subsequently, the cardiac function in each group of rats was tested. There was no significant difference in LVEF and FS scores between the BG and MSG (P > 0.05). However, the LVEF and FS scores were higher in the BG and MSG than those in the AMIG, IG, and NCG (P < 0.05). The LVEF and FS scores were not significantly different between the AMIG and NCG (P > 0.05). However, these scores were lower in the AMIG and NCG than those in the IG (P < 0.05). The results indicated that the adenovirus-mediated silencing of the expression of miR-223-3p in the myocardium of AMI rats can effectively improve cardiac function. Dai et al. demonstrated that (Dai et al. Citation2014) demonstrated that the overexpression of miR-223 promotes the occurrence of myocardial hypertrophy. Knockout of miR-223 effectively reduces pathological myocardial hypertrophy and heart failure. Therefore, inhibition of the function of miR-223 effectively inhibits myocardial hypertrophy and heart failure and improves cardiac function. The results of this study corroborated our previous findings. The cardiomyocytes of rats in the BG were used to create a primary culture and transfected with miR-223-3p. Overexpression of miR-223-3p was observed in the cardiomyocytes. The apoptosis rate and expression of apoptosis-related proteins in various cardiomyocytes were examined. The apoptosis rate was found to be higher than those in the BG and NCG (P < 0.05). Both BAX and Bcl-xL are members of the apoptosis-related Bcl protein family. BAX is a pro-apoptosis protein. Bcl-xL is an anti-apoptosis protein (van Rooij et al. Citation2008). The expression levels of both SOX6 and Bax proteins in the TG were significantly higher than those in the BG and NCG. The expression levels of the Bcl-cl protein in the TG were lower than those in the BG and NCG (P < 0.05). There was no significant difference in SOX6, BAX, and Bcl-xL protein levels between the BG and NCG (P > 0.05). This result indicated that the overexpression of miR-223-3p inhibits Bcl-xL and promotes BAX protein levels, thereby promoting the apoptosis of cardiomyocytes. The effect of miR-223-3p on apoptosis-related proteins has not yet been reported. A previous study (Li et al. Citation2013) showed that the overexpression of miR-223 in cultured umbilical vein endothelial cells can increase their apoptosis rate; endothelial cell proliferation and tubule formation induced by the vascular endothelial growth factor were inhibited. The results of this study confirmed our conclusions.
In summary, miR-223-3p was highly expressed in the AMI rat model. The silencing of miR-223-3p can effectively improve the cardiac function of rats. Moreover, the overexpression of miR-223-3p promotes the apoptosis of cardiomyocytes by regulating SOX6. Due to the sample size being too small and current experimental materials, techniques, and methods, there may have been some errors. However, our results provide some evidence indicating that miR-223-3p may be a new therapeutic target for AMI.
However, there are still some limitations of this study. For example, the relationships between SOX6, BAX, and Bcl-xL proteins were not studied. As SOX6 is the pro-apoptotic factor of cardiomyocytes, BAX and Bcl-xL are more likely to be downstream factors of SOX6. If this inference is confirmed, the mechanism through which miR-223-3p promotes cardiomyocyte apoptosis can be explained. Therefore, the relationships between SOX6, BAX, and Bcl-xL proteins should be studied in the future to further elucidate the mechanism of action of miR-223-3p in AMI and the specific route through which miR-223-3p induces cardiomyocyte apoptosis.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are openly available in figshare at http://doi.org/10.6084/m9.figshare.12301061.
References
- Bartel DP. 2004. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 116(2):281–297. doi: https://doi.org/10.1016/S0092-8674(04)00045-5
- Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, et al. 2017. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 135(10):e146–e603. doi: https://doi.org/10.1161/CIR.0000000000000485
- Dai GH, Ma PZ, Song XB, Liu N, Zhang T, Wu B, Ahrens I. 2014. MicroRNA-223-3p inhibits the angiogenesis of ischemic cardiac microvascular endothelial cells via affecting RPS6KB1/hif-1a signal pathway. PLoS One. 9(10):e108468. doi: https://doi.org/10.1371/journal.pone.0108468
- Das S, Halushka MK. 2015. Extracellular vesicle microRNA transfer in cardiovascular disease. Cardiovasc Pathol. 24(4):199–206. doi: https://doi.org/10.1016/j.carpath.2015.04.007
- Desta L, Jernberg T, Lofman I, Hofman-Bang C, Hagerman I, Spaak J, Persson H. 2015. Incidence, temporal trends, and prognostic impact of heart failure complicating acute myocardial infarction. The SWEDEHEART Registry (Swedish web-system for enhancement and development of evidence-based care in heart disease evaluated according to recommended therapies): a study of 199,851 patients admitted with index acute myocardial infarctions, 1996 to 2008. JACC Heart Fail. 3(3):234–242. doi: https://doi.org/10.1016/j.jchf.2014.10.007
- Dreyer RP, Sciria C, Spatz ES, Safdar B, D’Onofrio G, Krumholz HM. 2017. Young women with acute myocardial infarction: current perspectives. Circ Cardiovasc Qual Outcomes. 10(2):e003480. https://pubmed.ncbi.nlm.nih.gov/28228455/. doi: https://doi.org/10.1161/CIRCOUTCOMES.116.003480
- Jia Z, Wang J, Shi Q, Liu S, Wang W, Tian Y, Lu Q, Chen P, Ma K, Zhou C. 2016. SOX6 and PDCD4 enhance cardiomyocyte apoptosis through LPS-induced miR-499 inhibition. Apoptosis. 21(2):174–183. doi: https://doi.org/10.1007/s10495-015-1201-6
- Katarzyna R. 2017. Adult stem cell therapy for cardiac repair in patients after acute myocardial infarction leading to ischemic heart failure: an overview of evidence from the recent clinical trials. Curr Cardiol Rev. 13(3):223–231. doi: https://doi.org/10.2174/1573403X13666170502103833
- Lal H, Ahmad F, Parikh S, Force T. 2014. Troponin I-interacting protein kinase: a novel cardiac-specific kinase, emerging as a molecular target for the treatment of cardiac disease. Circ J. 78(7):1514–1519. doi: https://doi.org/10.1253/circj.CJ-14-0543
- Lesizza P, Prosdocimo G, Martinelli V, Sinagra G, Zacchigna S, Giacca M. 2017. Single-dose intracardiac injection of pro-regenerative microRNAs improves cardiac function after myocardial infarction. Circ Res. 120(8):1298–1304. doi: https://doi.org/10.1161/CIRCRESAHA.116.309589
- Li C, Fang Z, Jiang T, Zhang Q, Liu C, Zhang C, Xiang Y. 2013. Serum microRNAs profile from genome-wide serves as a fingerprint for diagnosis of acute myocardial infarction and angina pectoris. BMC Med Genomics. 6:16. doi: https://doi.org/10.1186/1755-8794-6-16
- Liu W, Ling S, Sun W, Liu T, Li Y, Zhong G, Zhao D, Zhang P, Song J, Jin X, et al. 2015. Circulating microRNAs correlated with the level of coronary artery calcification in symptomatic patients. Sci Rep. 5:16099. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4633594/. doi: https://doi.org/10.1038/srep16099
- Liu X, Zhang Y, Du W, Liang H, He H, Zhang L, Pan Z, Li X, Xu C, Zhou Y, et al. 2016. MiR-223-3p as a novel microRNA regulator of expression of voltage-gated K+ channel Kv4.2 in acute myocardial infarction. Cell Physiol Biochem. 39(1):102–114. doi: https://doi.org/10.1159/000445609
- Mehta LS, Beckie TM, DeVon HA, Grines CL, Krumholz HM, Johnson MN, Lindley KJ, Vaccarino V, Wang TY, Watson KE, et al. 2016. Acute myocardial infarction in women: a scientific statement from the American Heart Association. Circulation. 133(9):916–947. doi: https://doi.org/10.1161/CIR.0000000000000351
- Mihalko E, Huang K, Sproul E, Cheng K, Brown AC. 2018. Targeted treatment of ischemic and fibrotic complications of myocardial infarction using a dual-delivery microgel therapeutic. ACS Nano. 12(8):7826–7837. doi: https://doi.org/10.1021/acsnano.8b01977
- Qian L, Van Laake LW, Huang Y, Liu S, Wendland MF, Srivastava D. 2011. miR-24 inhibits apoptosis and represses Bim in mouse cardiomyocytes. J Exp Med. 208(3):549–560. doi: https://doi.org/10.1084/jem.20101547
- Rodriguez AE, Hernandez JA, Benito R, Gutierrez NC, Garcia JL, Hernandez-Sanchez M, Risueno A, Sarasquete ME, Ferminan E, Fisac R, et al. 2012. Molecular characterization of chronic lymphocytic leukemia patients with a high number of losses in 13q14. PLoS One. 7(11):e48485. doi: https://doi.org/10.1371/journal.pone.0048485
- Schirle NT, Sheu-Gruttadauria J, MacRae IJ. 2014. Structural basis for microRNA targeting. Science. 346(6209):608–613. doi: https://doi.org/10.1126/science.1258040
- Townsend N, Wilson L, Bhatnagar P, Wickramasinghe K, Rayner M, Nichols M. 2016. Cardiovascular disease in Europe: epidemiological update 2016. Eur Heart J. 37(42):3232–3245. doi: https://doi.org/10.1093/eurheartj/ehw334
- van Berlo JH, Molkentin JD. 2016. Most of the dust has settled: cKit+ progenitor cells are an irrelevant source of cardiac myocytes in vivo. Circ Res. 118(1):17–19. doi: https://doi.org/10.1161/CIRCRESAHA.115.307934
- van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN. 2008. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A. 105(35):13027–13032. doi: https://doi.org/10.1073/pnas.0805038105
- Wang YS, Zhou J, Hong K, Cheng XS, Li YG. 2015. MicroRNA-223 displays a protective role against cardiomyocyte hypertrophy by targeting cardiac troponin I-interacting kinase. Cell Physiol Biochem. 35(4):1546–1556. doi: https://doi.org/10.1159/000373970