ABSTRACT
Refractory wounds can be a serious threat to the quality of life and survival of patients. Various surgical methods have been attempted; however, they do not cure these refractory wounds; therefore, new and promising treatment methods to improve wound healing and angiogenesis are urgently warranted. Adipose-derived stromal vascular fraction (SVF) cells have been clinically used to promote healing. Hence, we aimed to evaluate the clinical efficacy of SVF cells for the treatment of refractory wounds. Forty patients with refractory wounds were randomly divided into two groups: patients in the experimental group were treated with SVFs (n = 20) and those in the control group were given conventional treatment (n = 20). The general data of age, sex ratio, wound size, frequency of every caused wound, and etiology were compared between the two groups. No significant difference was found between the two groups (P = 0.089). Clinical efficacy was also evaluated between the two groups. The experimental group had a shorter wound healing time and a significantly higher healing rate than the control group (P = 0.023 and P = 0.035, respectively). Therefore, SVFs may be a new and effective treatment for refractory wounds (P = 0.043).
Introduction
Refractory wounds cannot heal via the normal and orderly and timely repair process. These wounds often have delayed healing or do not heal at all commonly due to diabetes, ischemia, and stress. Clinically, wounds are considered refractory when they do not heal after more than 1 month of treatment, with no healing tendency. The 1-month time frame is not completely absolute and depends on several factors, such as wound size and cause and the general health status of the individual. Generally, when the wound cannot shrink by 10%–15% per week or by 50% for more than 1 month, it is considered as a chronic wound. In developed countries, the main reasons for refractory wounds are diabetic foot, pressure ulcer, and venous leg ulcer. In developing countries, such as China, patients with chronic wound account for 1.5%–3.0% of surgery inpatients, and the main causes are traumatic infection (67.5%), pressure ulcer (9.2%), venous ulcer (6.5%), diabetic ulcer (4.9%), and others (11.9%).
Refractory wounds are common and result in a long course of treatment with many possible complications. They can also influence the patient’s appearance. Refractory wounds are a growing worldwide health problem (Yan et al. Citation2018). They are deficient in blood supply; therefore, neovascularization is important for treating refractory wounds (Boccafoschi et al. Citation2005). Inflammatory factors surrounding the tissues consistently stimulate the wound, resulting in fibrous tissue proliferation and scar tissue formation (Wu et al. Citation2007; Yang et al. Citation2010). However, conventional treatments for refractory wounds are not ideally effective in a clinical setting (Han et al. Citation2010). Conventional treatment involves removing the secretions and necrotic tissue on the surface of the wound, covering the wound with a vaseline gauze, and wrapping the sterile gauze.
Adipose-derived stromal vascular fraction (SVF) is a heterogeneous population of cells, including adipose-derived mesenchymal stem cells (ADSCs) (Ferrara and Alitalo Citation1999). ADSCs can be harvested from many tissues and easily preserved in a specific medium. As a result, they are widely used to heal skin wounds via paracrine signaling (Onodera et al. Citation2004). ADSC-CMs dose dependently decrease cell viability and the expression of fibrosis molecules and tissue inhibitor of metalloproteinases-1 and significantly increase the expression of matrix metalloproteinase-1. Collagen production and the ratio of collagen type I and type III (Col1/Col3) are also suppressed by ADSC-CMs in a dose-dependent manner (Ma et al. Citation2020). During wound healing, ADSCs have the ability to rapidly migrate cells to the wounded sites and differentiate into dermal fibroblasts (DF), endothelial cells, and keratinocytes. Additionally, ADSCs and DFs are the major sources of the extracellular matrix (ECM) proteins involved in maintaining skin structure and function. They interact with skin cells to regulate skin homeostasis and healing. Evidence suggests that their secretomes ensure (i) changes in the inflammatory phenotype of macrophages implicated in the inflammatory phase, (ii) the formation of new blood vessels, thereby promoting angiogenesis by increasing endothelial cell differentiation and cell migration, and (iii) the formation of granulation tissues, skin cells, and ECM production, resulting in proliferation and remodeling. These characteristics have enabled the establishment of therapeutic strategies for wound healing and skin aging and have resulted in more insights in many clinical investigations (Mazini et al. Citation2020).
Studies have found that ADSCs contain many cell components, such as mesenchymal stem cells, endothelial precursor cells, macrophages, and smooth muscle cells (Yan et al. Citation2010). Angiogenesis is very important for the treatment of refractory wounds, and the cells within SVFs can improve angiogenesis by differentiating into vascular endothelial cells (Wu et al. Citation2007) and secreting angiogenesis-promoting and antiapoptotic growth factors (Yang et al. Citation2010); (Han et al. Citation2010). In this study, we aimed to investigate the clinical efficacy of using ADSCs to heal refractory wounds as well as to provide valuable evidence for the effect of ADSC therapy on refractory wounds.
Materials and methods
Patients
This study enrolled 40 patients with refractory wounds, which lasted for more than 4 weeks and could not be repaired in an orderly and timely manner after regular medical and surgical treatment to restore their anatomical and functional integrity. Thus, these wounds were considered as difficult wounds. These patients were hospitalized in our hospital from June 2014 to June 2018. The causes of the wounds were venous insufficiency, post-traumatic lower extremity ischemia, lymphedema, burns, wound infection, and scar ulcers. The patients were divided into an experimental group (n = 20, 9 males, 11 females, 42.9 ± 19.5 years old) and a control group (n = 20, 12 males, 8 females, 48.2 ± 14.9 years old) according to the different treatments.
This study was approved by the Ethics Committee of Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medical School. All patients provided signed informed consent.
Treatment
Patient wounds were thoroughly debrided before treatment to control infection and to ensure that there were no new wounds. No obvious necrotic tissue was observed on the surfaces of the wounds. Then, the patients in the experimental group were treated with ADSCs, whereas those in the control group were given a routine dressing change. The wounds of the patients in both groups were covered with silver ion dressing. The dressing was regularly changed until the wound healed.
The specific procedures of ADSCs treatment were as follows:
Abdominal liposuction: Swollen anesthetic fluid was subcutaneously injected into the abdomen of the patients, and a 3-mm diameter blunt suction needle connected with a 20-mL syringe was used for liposuction. Manual suction was gently performed to fully protect the adipocyte activity.
Extraction of ADSCs from adipose tissues (Conde-Green et al. Citation2016; Kilroy et al. Citation2018): The extracted adipose tissues were washed with the same amount of phosphate-buffered saline (PBS) to remove blood, broken tissues, and anesthetics and then transferred into a 50-mL centrifugal tube. Collagenase type I was prepared and added to the adipose tissue at a ratio of 1:1 and incubated in an air shaker at 190 rpm for 60 min at 37°C. After digestion, the mature fat and supernatant of the upper layer were removed by centrifugation at 25°C for 5 min, and the sediment was resuspended in PBS to form a suspension. The suspension was filtered with a 200 mesh screen to remove cell debris and other impurities and then transferred into a 10-mL centrifugal tube for centrifugation at 600 × g for 5 min at 25°C. The centrifugal sediment was suspended in normal saline to prepare the ADSC cell suspension. A drop was taken from the cell suspension and added into the same amount of erythrocyte lysate. The total cell number is 1 × 109. After incubation at room temperature for 5 min, 10 µL of the liquid was transferred onto a slide. Cell counting was used to count the total cell number, and 5 × 106/mL ADSCs were prepared from the cell suspension.
Wound injection: The area of the wound was calculated. The ADSC suspension was transferred into a 10-mL syringe and evenly injected at the edge and base of the wound (0.5 mL/cm2). The wound dressings of the two groups were regularly changed according to the exudation. The wounds were photographed and the data were recorded. The patients were followed-up for 8 weeks.
Evaluation of wound healing
Wound healing time: The time for complete epithelialization of the wound
Wound healing rate: Wound healing rate (%) = (original area of the wound − residual area of the wound)/original area of the wound × 100%
The proportion of effective cases: Markedly effective, wound healing rate ≥ 80%; effective, wound healing rate 50%–80%; ineffective, wound healing rate <50%. The proportion of effective cases = (markedly effective cases + effective cases)/total cases
We followed these patients via hospital records because tall patients were hospitalized.
Statistical analysis
The SPSS16.0 software was used for statistical analysis. Continuous data were expressed as mean ± standard deviation. The data were analyzed via the t-test. The Fisher’s exact probability method was used to compare the counting data. Some other advanced statistical analysis methods, such as adjusted regression, were used to control the possible confounding effects of these differences. Significance was set at P < 0.05.
Results
Baseline characteristics
The average wound size of the experimental group was 53.9 ± 92.7 cm2 and 40.2 ± 77.8 cm2 in the control group. The general data of age, sex ratio, wound size, the frequency of every wound cause, and etiology were comparable between the 2 groups. They were no significant difference between the two groups (p = 0.089)
Wound healing rate comparison is solely based on some clinical outcomes
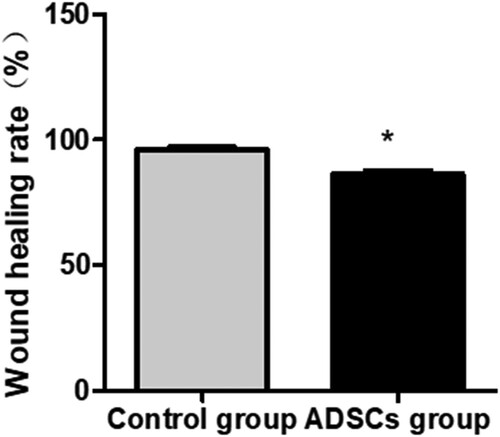
Compared with the control group, the wound healing time of the experimental group was shorter (38.60 ± 8.03 days vs. 55.25 ± 10.96 days; P = 0.023). The wound healing rate of the experimental group was higher than that of the control group (73.05% ± 11.86% and 96.05% ± 0.05% and 56.80% ± 9.56% and 86.35% ± 10.61% at 4 and 8 weeks after the treatment, respectively; P = 0.035). Four weeks after the treatment, 18 cases in the experimental group were markedly effective, 1 case was effective, and 1 case was ineffective; on the other hand, 12 cases in the control group were markedly effective, 4 cases were effective, and 4 cases were ineffective. The proportion of effective cases in the experimental group was higher than that in the control group (P = 0.043). Eight weeks after the operation, 20 cases in the experimental group were markedly effective, while 15 cases in the control group were markedly effective and 5 cases were effective (Figures –).
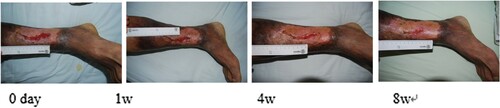
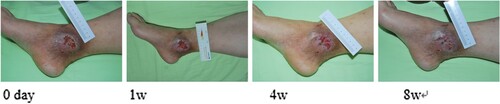
Figure 1. Liu Hua, female, 71 years old, suffered from skin ulceration of the right ankle with infection. The infection was recurrent and uncured for more than 1 month and recovered after 8 weeks of ADSC treatment.
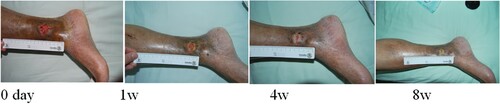
Figure 2. Ding Jiasheng, male, 55 years old, suffered from skin ulceration of the right heel with infection. The infection was recurrent and uncured for more than 3 months. The healing rate was 99% after 8 weeks of ADSC treatment.
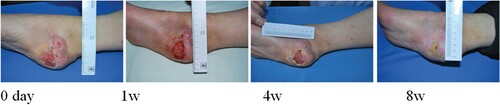
Figure 3. Zhang Wei, male, 55 years old, suffered from skin ulceration and infection after right axillary surgery. The infection was recurrent and uncured for more than 1 month. The healing rate was 95% after 8 weeks of ADSC treatment.
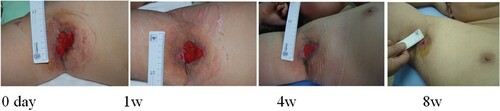
Figure 4. Wang Guiqin, female, 53 years old, suffered from residual burn wounds on her left lower extremity. Her wound was recurrent and uncured for more than 2 months. The healing rate was 87% after 8 weeks of ADSC treatment.
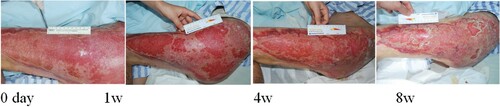
Figure 5. Wan Pengfei, male, 57 years old, suffered from skin ulceration and infection after undergoing left axillary surgery. The infection was recurrent and uncured for more than 1 month. The wound healing rate was 79% after 8 weeks of routine dressing change treatment.
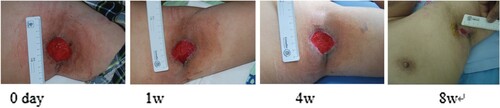
Figure 6. Zhao Xiujuan, female, 59 years old, suffered from skin ulceration of the right ankle with infection. The infection was recurrent and uncured for more than 1.5 months. The wound healing rate was 89% after 8 weeks of routine dressing change treatment.
Discussion
Studies have shown that ADSCs and their contained cells can secrete a variety of bioactive molecules by differentiating into vascular endothelial cells, fibroblasts, and epidermal cells to promote angiogenesis, limit inflammation and apoptosis, and participate in wound repair. This results in wound epithelialization and granulation tissue formation and accelerates wound healing (Zhang et al. Citation2012; Bonafede et al. Citation2016; Foubert et al. Citation2016).
Vascular endothelial cells differentiated from ADSCs can increase blood perfusion and vascular tissue density to improve vascular tissue construction (Vorauer-Uhl et al. Citation2002). Meanwhile, vascular smooth muscle cells differentiated from ADSCs can express vascular smooth muscle and tuning proteins (Deniz et al. Citation2013; Ozturk and Karagoz Citation2015). Furthermore, CD34+ endothelial precursor cells in ADSCs can promote angiogenesis by directly or indirectly secreting growth factors (Nguyen et al. Citation1993).
Adipose tissues are endocrine organs with strong secretory capacity. Adipose stem cells in ADSCs can secrete vascular endothelial growth factor, hepatocyte growth factor, basic fibroblast growth factor, granulocyte–macrophage-colony stimulating factor, and transforming growth factor to promote angiogenesis (Zhang et al. Citation2012; Yan et al. Citation2018). They can also promote the proliferation and migration of cells in adjacent tissues via cell contact or paracrine signaling and can effectively improve the healing speed of wounds (Uysal et al. Citation2009).
The application of ADSCs has several advantages (Kwon et al. Citation2008; Uysal et al. Citation2009). First, adipose tissue is abundant and can be collected via liposuction, which causes slight trauma or pain to the patient (Nguyen et al. Citation1993). ADSCs extracted from the adipose tissue do not require in vitro culturing; hence, complex procedures and possible contamination caused by cell culturing are prevented (Burd et al. Citation2007). Moreover, excessive cell passages can result in cell differentiation into a tumorigenic cell lineage. Therefore, ADSCs are safer and more practical than other cells (Burd et al. Citation2007). Second, ADSCs can self-proliferate and have long-term survival and multiple differentiation potential, with more mesenchymal stem cells and stronger cell activity than bone marrow (Atalay et al. Citation2014). Finally, compared with mesenchymal stem cells derived from other tissues, the cell viability of ADSCs is less affected by acquisition technology and patient’s age and body mass index (Cardoso et al. Citation2016).
In this study, ADSCs were applied to refractory wounds with different origins, and the healing results were comprehensively evaluated. This further confirmed the superiority of ADSCs for the treatment of refractory wounds. Through the continuous in-depth study of ADSCs for the treatment of refractory wounds, more valuable data for the clinical application of ADSCs will become available. This will give rise to solutions to the problems of treating refractory wounds.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 81272108 and 81671922) and from the Fundamental Research Funds for the Central Universities.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Considering the nature of this research, the study participants did not agree for their data to be shared publicly; thus, supporting data are not available.
Additional information
Funding
References
- Atalay S, Coruh A, Deniz K. 2014. Stromal vascular fraction improves deep partial thickness burn wound healing. Burns. 40(7):1375–1383. doi: https://doi.org/10.1016/j.burns.2014.01.023
- Boccafoschi F, Habermehl J, Vesentini S, Mantovani D. 2005. Biological performances of collagen-based scaffolds for vascular tissue engineering. Biomaterials. 26(35):7410–7417. doi: https://doi.org/10.1016/j.biomaterials.2005.05.052
- Bonafede R, Scambi I, Peroni D, Potrich V, Boschi F, Benati D, Bonetti B, Mariotti R. 2016. Exosome derived from murine adipose-derived stromal cells: neuroprotective effect on in vitro model of amyotrophic lateral sclerosis. Exp Cell Res. 340(1):150–158. doi: https://doi.org/10.1016/j.yexcr.2015.12.009
- Burd A, Ahmed K, Lam S, Ayyappan T, Huang L. 2007. Stem cell strategies in burns care. Burns. 33(3):282–291. doi: https://doi.org/10.1016/j.burns.2006.08.031
- Cardoso AL, Bachion MM, Morais JDM, Fantinati MS, Almeida VLLD, Júnior L, Souza R. 2016. Adipose tissue stromal vascular fraction in the treatment of full thickness burns in rats. Acta Cir Bras. 31(9):578–585. doi: https://doi.org/10.1590/S0102-865020160090000002
- Conde-Green A, Kotamarti VS, Sherman LS, Keith JD, Lee ES, Granick MS, Rameshwar P. 2016. Shift toward mechanical isolation of adipose-derived stromal vascular fraction: review of upcoming techniques. Plast Reconstr Surg Glob Open. 4(9):e1017. doi: https://doi.org/10.1097/GOX.0000000000001017
- Deniz M, Borman H, Seyhan T, Haberal M. 2013. An effective antioxidant drug on prevention of the necrosis of zone of stasis: N-acetylcysteine. Burns. 39(2):320–325. doi: https://doi.org/10.1016/j.burns.2012.06.015
- Ferrara N, Alitalo K. 1999. Clinical applications of angiogenic growth factors and their inhibitors. Nat Med. 5(12):1359–1364. doi: https://doi.org/10.1038/70928
- Foubert P, Gonzalez AD, Teodosescu S, Berard F, Doyle-Eisele M, Yekkala K, Tenenhaus M, Fraser JK. 2016. Adipose-derived regenerative cell therapy for burn wound healing: a comparison of two delivery methods. Adv Wound Care. 5(7):288–298. doi: https://doi.org/10.1089/wound.2015.0672
- Han SK, Kim HR, Kim WK. 2010. The treatment of diabetic foot ulcers with uncultured, processed lipoaspirate cells: a pilot study. Wound Repair Regen. 18(4):342–348. doi: https://doi.org/10.1111/j.1524-475X.2010.00593.x
- Kilroy G, Dietrich M, Wu X, Gimble JM, Floyd ZE. 2018. Isolation of murine adipose-derived stromal/stem cells for adipogenic differentiation or flow cytometry-based analysis. Methods Mol Biol. 1773:137–146. doi: https://doi.org/10.1007/978-1-4939-7799-4_11
- Kwon DS, Gao X, Liu YB, Dulchavsky DS, Danyluk AL, Bansal M, Chopp M, McIntosh K, Arbab AS, Dulchavsky SA. 2008. Treatment with bone marrow-derived stromal cells accelerates wound healing in diabetic rats. Int Wound J. 5(3):453–463. doi: https://doi.org/10.1111/j.1742-481X.2007.00408.x
- Ma J, Yan X, Lin Y, Tan Q. 2020. Hepatocyte growth factor secreted from human adipose-derived stem cells inhibits fibrosis in hypertrophic scar fibroblasts. Curr Mol Med. 20(7):558–571. doi: https://doi.org/10.2174/1566524020666200106095745
- Mazini L, Rochette L, Admou B, Amal S, Malka G. 2020. Hopes and limits of adipose-derived stem cells (ADSCs) and mesenchymal stem cells (MSCs) in wound healing. Int J Mol Sci. 21(4):1306. doi: https://doi.org/10.3390/ijms21041306
- Nguyen TT, Cox CS, Traber DL, Gasser H, Redl H, Schlag G, Herndon DN. 1993. Free radical activity and loss of plasma antioxidants, vitamin E, and sulfhydryl groups in patients with burns: the 1993 Moyer Award. J Burn Care Rehabil. 14(6):602–609. doi: https://doi.org/10.1097/00004630-199311000-00004
- Onodera H, Ikeuchi D, Nagayama S, Imamura M. 2004. Weakness of anastomotic site in diabetic rats is caused by changes in the integrity of newly formed collagen. Dig Surg. 21(2):146–151. doi: https://doi.org/10.1159/000078381
- Ozturk S, Karagoz H. 2015. Experimental stem cell therapies on burn wound: do source, dose, timing and method matter? Burns. 41(6):1133–1139. doi: https://doi.org/10.1016/j.burns.2015.01.005
- Uysal AC, Mizuno H, Tobita M, Ogawa R, Hyakusoku H. 2009. The effect of adipose-derived stem cells on ischemia-reperfusion injury: immunohistochemical and ultrastructural evaluation. Plast Reconstr Surg. 124(3):804–815. doi: https://doi.org/10.1097/PRS.0b013e3181b17bb4
- Vorauer-Uhl K, Fürnschlief E, Wagner A, Ferko B, Katinger H. 2002. Reepithelialization of experimental scalds effected by topically applied superoxide dismutase: controlled animal studies. Wound Repair Regen. 10(6):366–371. doi: https://doi.org/10.1046/j.1524-475X.2002.t01-1-10605.x
- Wu Y, Chen L, Scott PG, Tredget EE. 2007. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 25(10):2648–2659. doi: https://doi.org/10.1634/stemcells.2007-0226
- Yan X, Chen B, Lin Y, Li Y, Xiao Z, Hou X, Tan Q, Dai J. 2010. Acceleration of diabetic wound healing by collagen-binding vascular endothelial growth factor in diabetic rat model. Diabetes Res Clin Pract. 90(1):66–72. doi: https://doi.org/10.1016/j.diabres.2010.07.001
- Yan X, Lin Y, Jiang Y, Xu Y, Tan Q. 2018. Adipose-derived SVFs with hyaluronic acid accelerate diabetic wound healing in diabetic porcine model. Int J Clin Exp Med. 11(2):735–740.
- Yang M, Sheng L, Li H, Weng R, Li QF. 2010. Improvement of the skin flap survival with the bone marrow-derived mononuclear cells transplantation in a rat model. Microsurgery. 30(4):275–281. doi: https://doi.org/10.1002/micr.20779
- Zhang H-C, Liu X-B, Huang S, Bi X-Y, Wang H-X, Xie L-X, Wang Y-Q, Cao X-F, Lv J, Xiao F-J. 2012. Microvesicles derived from human umbilical cord mesenchymal stem cells stimulated by hypoxia promote angiogenesis both in vitro and in vivo. Stem Cells Dev. 21(18):3289–3297. doi: https://doi.org/10.1089/scd.2012.0095