?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A commercial herbal oil with different bottle materials on the Indonesian markets was studied. The effects of the different specimens viz. PET-new, PET-open, and AL-new on the main antibacterial components of herbal oil were investigated. Step-1, analysis of antibacterial activity (against S. aureus and E. coli) and testing of non-toxic (in-vivo). Step-2, detection of the main antibacterial compounds (6 types) during the storage times. The market and environmental aspects were also considered. The results, all specimens had antibacterial activity and they showed non-toxic. At the 0-month, six types of main antibacterial compounds were detected in all specimens. The longer the storage time, the more main antibacterial compounds were lost. The most loss (4 types) was observed in AL-new (12-months) and PET-open. PET-new showed the least loss (1 type) at 6-months and 12-months, namely eugenol. Interestingly, PET-new was able to retain curcumene, farnesol, and p-cymene for up to 12 months of storage. PET-new was also more economical (8.7% cheaper) and environmental-friendly than AL-new. The sprayer cap types are strongly recommended for PET herbal oil packaging.
Introduction
Indonesia has a huge biodiversity of herbal plants. Since a long time ago, Indonesian people always use ingredients from several herbal plants for traditional medicine. So far, the potency of herbal plants as a medical ingredient in the herbal oils or essential oils has been long recognized. Herbal oils are widely traded in Indonesia. They are often produced from a mixture of herbal plants such as the Ashitaba, Neem, Pulai, Agarwood, Javanese Ginger, and Star anise groups. Ashitaba leaf (Angelica keiskei) contains cytotoxic, antidiabetic, antiobesity, and antioxidant compounds (Caesar and Cech Citation2016). Neem leaf (Azadirachta indica) is called a magic tree because it can treat malaria and fever (Al-Hashemi and Hossain Citation2016). The bioactivity of Pulai tree (Alstonia scholaris) is promising as antidiabetic, antianxiety, and anticancer (Pankti et al. Citation2012). The sedative-hypnotic effect of Agarwood (Aquilaria beccarain) is beneficial for treating insomnia (Wang et al. Citation2017). The medical activity of Javanese ginger (Curcuma zanthorrhiza) to gastrointestinal disorders, pain, wounds, cancer, anti-inflammatory, and anti-aging (Dosoky and Setzer Citation2018). The medical activity of Star anise (Illicium verum) includes digestion stimulating, antiparasitic, antibacterial, antioxidant, antifungal, and antipyretic (Ding et al. Citation2017).
One of the commercial herbal oil products in Indonesia is selected in this study for its antibacterial compounds. The herbal oil is produced from several herbal plants with the right dose, so it is expected to bring up the antibacterial activity of herbal oil. Herbal oil is packaged in bottles with different materials. The packaging materials of the bottle are certainly responsible for the maintenance of bioactive components. The effects of oxidation, degradation or decomposition, and chemical migration due to oils-packaging interactions can be prevented by the selection of exact packaging material (Hu et al. Citation2020; Huyan et al., Citation2019). Aluminum bottle and polyethylene terephthalate (PET) bottle have been applied as herbal oil packaging, then the effect of the different materials on antibacterial components in herbal oil is deeply investigated. The difference in bottle materials affects the selling price of herbal oil that needs to be considered.
Materials and methods
Herbal oil
The herbal oil was the original product from Indonesia that attracts many consumers due to its medicinal efficacy. This herbal oil in one type, which has the same compositions and brand, and purchased from one distributor. However, it has two different bottle materials namely aluminum bottle and PET bottle. The aluminum bottle is equipped with a sprayer cap and the PET bottle is not equipped (Figure and Table ).
Figure 1. Different materials of the bottle for herbal oil packaging. PET-new: a PET plastic bottle. PET-open: a bottle material is the same as PET-new, but it has been opened for daily use for one month. During daily use, the bottle cap is fully opened. AL-new: an aluminum bottle has a sprayer cap type in which only its 2nd-cap is opened when daily use. The brand, volume, production code, production permit, and expired date in all different specimens are the same.

Table 1. The herbal oil compositions in all specimens.
In this experiment, three specimens i.e. PET-new and PET-open (bottle material is PET), and AL-new (bottle material is aluminum) were selected (Figure ). Table , PET-new and AL-new were noted as new products, they are being directly analyzed after purchasing. PET-open was a used product, it is being directly analyzed after daily use for one month. The types of PET and aluminum bottle caps were different (Figure ).
Table 2. Storage times of the herbal oils.
The steps in this research were as follows:
Step-1: All different specimens at 0-months (Table ) were tested for their antibacterial activity and toxicity. The antibacterial activity and non-toxic were proven in all the different specimens and the research leads to Step-2.
Step-2: The study emphasized the effect of different bottle materials on the antibacterial compound content in herbal oils. The alterations of antibacterial compound content were noted as loss-detected or not-detected on the main types of antibacterial compounds. The specimens were stored at various times to lets the packaging and oils have sufficient interaction (Table ).
Toxicity assay
The toxicity of specimens was tested using Brine Shrimp Lethality Test (BSLT) by a slight modification of the Weli et al. (Citation2019) method. Hatching of Artemia salina eggs: 2 g of cysts (Artemia salina eggs) was placed into a plastic container containing artificial seawater (NaCl 40 g/L freshwater, 15–35 ppt salinity) with constant aeration at room temperature (±27°C) for 48 h. The mature nauplii were separated from their eggshells for the experiment.
Experiment: several different concentrations (1, 10, 100, and 1000 mg/ml) of specimens were prepared in dimethyl sulfoxide (DMSO). In each experimental well (6-well plate cell culture) which had been contained 5 ml seawater, each specimen concentration (100 µl) was placed, then 40 nauplii were added by a dropper. The negative control, 100 µl of DMSO was prepared as a substitute for the specimens. After 24 h was maintained under the light, the experimental well plate was observed under a stereomicroscope with 400× magnification. If the nauplii did not exhibit any internal or external movement during the time of observation, so they were considered dead. Mortality was calculated by using the formula:
The final data on the toxicity test was expressed as a lethal concentration of 50 subjects (LC50), the smaller the LC50 value the more toxic.
Antibacterial activity assay
The disc diffusion test was employed to the antibacterial activity of specimens, with two types of pathogenic bacteria namely Staphylococcus aureus (S. aureus) (Gram-positive bacteria) and Escherichia coli (E. coli) (Gram-negative bacteria) (Jaswir et al. Citation2014). Initially, the nutrient agar medium (NA) prepared in a sterile condition (consists of 5 g/L peptone, 3 g/L beef extract, 5 g/L NaCl, and 20 g agar in distilled water). The NA plate in each Petri-dish was inoculated (spread plate technique) with 100 µL of the pathogenic bacterial strains (107 CFU/mL), and it was allowed to dry for 15 min. Sterile paper discs (Whatman, the diameter of 6 mm) were soaked with 10 µl of each herbal oil (195 mg/ml), then placed on the surface of the inoculated plates. The specimens were substituted by DMSO as a negative control. The inoculated plates were stored at 4°C (2 h) to allow the diffusion of herbal oil to the medium, then incubated at 37°C (24 h) in an inverted position. Finally, the diameter (mm) of the clear zones around the paper discs was measured.
Detection of antibacterial compounds by liquid chromatography–high Resolution Mass spectrometry (LC-HRMS)
To ensure the presence of antibacterial compounds in herbal oil, a qualitative analysis was performed using LC-HRMS. The LC-HRMS has the Q-exactive type that produced by Thermo Fisher Scientific using hypersil gold aQ 50 × 1 mm × 1.9 µ particle size of the column.
Before injected on LC-HRMS, the specimens were first dissolved into absolute methanol (LCMS grade). After vortexed (±3 min) and spin down (±3 min) then the samples were filtered using the minisart filter PTFE (pore size 0.22 µm). About 1 ml of samples ready to be injected into LC-HRMS with mobile phase A (0.1% formic acid in H2O) and mobile phase B (0.1% formic acid in acetonitrile). The running time of each specimen was 30 min with positive polarity, flow rate of 40 µL/min, oven column temperature 30°C with elution gradient as follows; 0–2 min 5% B, 15–22 min 60% to 95% B, and kept in 95% B for 3 min then let it down to 5% B at 30 min.
The detected chromatogram was analyzed using the compound discoverer software 3 based on the online library that is the mzCloud. To find out the types of antibacterial compounds that had been detected, each compound was searched for its bioactivity via the PubChem page and supported by scientific evidence from previous studies.
Statistical analysis
Quantitative values were presented as means ± standard deviation (SD) from the 3 replications. Statistical analysis was done using the Minitab 18 software. ANOVA (Analysis of variance) and Tukey test were done at significance p < 0.05. Meanwhile, the result of detected antibacterial compounds were qualitative data.
Results and discussion
Toxicity
Toxicity test was done for proving that herbal oil is non-toxic as an external medicine. In most cases, Artemia salina larvae were applied as an experimental animal in toxicity test, due to it can be used for initial screening as a substitute for cell lines (in-vitro) with more easily, quickly, cheaply, and ethically (Hisem et al. Citation2011; Primahana et al. Citation2015; Arumugam et al. Citation2019). The BSLT method well correlated with in-vitro tests on cell lines, where extracts of dichloromethane:methanol (2:1) from microalgae and podophylloytoxin drugs were proven to be toxic on Artemia salina and cell lines (human nasopharyngal carcinoma and human colon adenocarcinoma) (Mian et al. Citation2003; Jaki et al. Citation1999; Hisem et al. Citation2011). At the very least, the BSLT method was precisely chosen for initial screening in the toxicity test of herbal oil, although further tests on human cells are still needed.
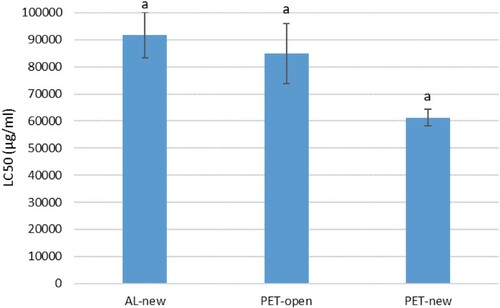
Figure , LC50 of PET-new (61291.90 ± 3099.52 µg/ml) is the strongest followed by PET-open (84904.61 ± 11131.53 µg/ml) and AL-new (91729.35 ± 8480.04 µg/ml), respectively. However, the different bottle materials do not significantly affect the LC50. Thus, all different bottle materials were proven safe or did not cause toxic to the herbal oil, this is because LC50 > 1000.00 µg/mL has been agreed to be inactive or non-toxic (Tanamatayarat, Citation2016; Supraja et al. Citation2018). Aluminum packaging materials were proven to be non-toxic (Geueke et al. Citation2018), while PET packaging materials were considered non-toxic packaging if the styrene monomer does not migrate into the product (Pilevar et al. Citation2019).
Antibacterial activities
The composition of all different specimens in this study was the same which consists of the mixture of the various herbal plants, so it is very rich in phytochemicals such as antibacterial compounds. Therefore, the specimens must be proved for their antibacterial activities on both Gram-positive and Gram-negative bacteria. S. aureus and E. coli were selected to represent the strains of Gram-positive and Gram-negative bacteria. Those bacteria strains have cell wall structures in different that lead to different susceptibility against the actions of antibiotics or antibacterial compounds (Papuc et al. Citation2017). Moreover, S. aureus and E. coli were selected because they belong to the Multi-Drug Resistant (MDR) or Multiple Antibiotic Resistance (MAR) which are easy to infect (Shafay et al. Citation2015).
Table , all the different specimens can inhibit the growth of E. coli and S. aureus bacteria, which are marked by the presence of inhibition zones. The highest inhibition zone on E. coli is recorded by PET-open and on S. aureus is recorded by PET-new, however only slightly higher than the other specimens. All the different specimens were proven to have antibacterial activity. It due to the composition (Table ) of the specimens consist of the herbs which are rich in antibacterial components. Zingiber officinale, Olea europaea, Citronella, Aquilaria, Nigella sativa, Piper betle, and Cocos nucifera herbs had been reported to be rich in antibacterial compounds as bactericidal against both S. aureus and E. coli (Sivasothy et al. Citation2011; Pereira et al. Citation2007; Timung et al. Citation2016; Hendra et al. Citation2016; Kokoska et al. Citation2008; Taukoorah et al. Citation2016; Nitbani et al. Citation2016). Hence, the effect of the different specimens on the content of main antibacterial compounds had to be studied in more depth.
Table 3. Inhibition zone on E. coli and S. aureus by the herbal oils.
Detection of main antibacterial compounds
LC-HRMS for the detection of antibacterial compounds in natural products was recognized, it has high mass resolution and high calculation of mass accuracy (Klen et al. Citation2015). Table , herbal oil contains the main antibacterial compounds namely (R)-3-hydroxy myristic acid, curcumene, farnesol, eucalyptol, eugenol, and p-cymene. The compounds contained in PET-new and AL-new at 0-month are justified as the main antibacterial compounds because they have been detected with the dominant area. The main antibacterial compounds in PET-open at 0-month were similar to the main antibacterial compounds of PET-new. Furthermore, the effect of different bottle materials on the alterations of the main antibacterial compounds is focused, whether the main antibacterial compounds are specifically detected or not detected.
Table 4. Detection of main antibacterial compounds in the herbal oils during storage times.
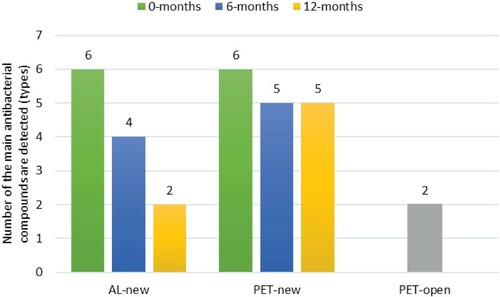
In Table , all the main antibacterial compounds were detected at 0-months, wherein that all the different specimens are fresh or new at 0-months. However, the longer storage time (at 6-months and 12-months) of specimens, some main antibacterial compounds in PET-new and Al-new were not detected,likewise some antibacterial compounds in PET-open. Both (R)-3-hydroxy myristic acid and eucalyptol are not affected by storage time, but eugenol is affected or damaged during the storage time in all the different specimens. Interestingly, PET-new can retain curcumene, farnesol, and p-cymene during the storage time, nevertheless, these compounds are lost in other specimens.
Figure , four main antibacterial compounds in AL-new (12-months) and PET-open were lost. The longer the storage time, the more of the main antibacterial compounds are lost. In contrast, PET-new more retains the main antibacterial compounds, even though it has been stored for 12 months, wherein only one compound is not detected namely eugenol. The PET bottle was proven to be safer than the aluminum bottle for packing herbal oils. The contact between PET plastics and phenolic compounds was safe, as PET plastics did not react with phenyl ring groups (Wang et al. Citation2020). Also, PET plastic packaging was able to inhibit olive oil oxidation for 30 days of storage time at 40°C. This is due to the migration phenomenon of PET plastic material into olive oil was very low, so chemical interactions were suppressed (Kassouf et al., Citation2014). Thus, PET plastic bottles equipped with spray cap types were strongly recommended, in that the loss of the main antibacterial compounds in PET-open was noted high. As with olive oil without packaging, more hydroperoxides were produced due to the oxidation process by oxygen from the outside (Kassouf et al. Citation2014).
Market and environment aspect
The herbal oil was launched with two types of bottle packaging namely aluminum bottle (Al-new) and PET bottle (PET-new). The price of AL-new with the same volume and composition was 8.7% more expensive than PET-new. On the other side, the price of PET bottles was cheaper and plastic materials were easy to be recycled. In terms of environmental conservation, PET packaging was also more environmentally than aluminum packaging. Taniguchi et al. (Citation2019) had been identified PET hydrolytic enzymes (PHEs) and they reported the degradation of PET by a consortium of microbial and resident bacteria, Ideonella sakaiensis.
Conclusion
The PET bottle was proven more capable of maintains the content of the main antibacterial compounds than that of the aluminum bottle. This bottle was also more economical and environmental-friendly compared to the aluminum bottle. Direct contact of herbal oil with outside air could reduce the number of main antibacterial compounds. Therefore, the PET bottles equipped with the sprayer caps are strongly recommended as herbal oil packaging.
Acknowledgments
The authors thank the Department of Agricultural Engineering and Central Laboratory of Life Science, Universitas Brawijaya, Malang-Indonesia
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Al-Hashemi ZSS, Hossain MA. 2016. Biological activities of different neem leaf crude extract used locally in Ayurvedic medicine. Pacific Sci Rev A: Nat Sci Eng. 18:128–131.
- Arumugam DG, Sivaji S, Dhandapani KV, Nookala S, Ranganathan B. 2019. Panchagavya mediated copper nanoparticles synthesis, characterization and evaluating cytotoxicity in brine shrimp. Biocatal Agri Biotechnol. 19:101132. doi: https://doi.org/10.1016/j.bcab.2019.101132
- Caesar LK, Cech NB. 2016. A review of the medical uses and pharmacology of ashitaba. Planta Med. 82:1236–1245. doi: https://doi.org/10.1055/s-0042-110496
- Chávez-Andrade GM, Tanomaru-Filho M, Rodrigues EM, Gomes-Cornélio AL, Faria G, Bernardi MIB, Guerreiro-Tanomaru JM. 2017. Cytotoxicity, genotoxicity and antibacterial activity of poly(vinyl alcohol)-coated silver nanoparticles and farnesol as irrigating solutions. Arch Oral Biol. 84:89–93. doi: https://doi.org/10.1016/j.archoralbio.2017.09.028
- Chen J, Tang C, Zhang R, Ye S, Zhao Z, Huang Y, Xu X, Lan W, Yang D. 2020. Metabolomics analysis to evaluate the antibacterial activity of the essential oil from the leaves of Cinnamomum camphora (Linn.) Presl. J Ethnopharmacol. 253:112652. doi: https://doi.org/10.1016/j.jep.2020.112652
- Constantino JA, Delgado-Rastrollo M, Pacha-Olivenza MA, Pérez-Giraldo C, Quiles M, González-Martín ML, Gallardo-Moreno AM. 2016. In vivo bactericidal efficacy of farnesol on Ti6Al4V implants. Rev Esp Cir Ortop Traumatol. 60(4):260–266.
- Cutillas AB, Carrasco A, Martinez-Gutierrez R, Tomas V, Tudela J. 2018. Thyme essential oils from Spain: aromatic profile ascertained by GC-MS, and their antioxidant, antilipoxygenase and antimicrobial activities. J Food Drug Anal. 26:529–544. doi: https://doi.org/10.1016/j.jfda.2017.05.004
- da Silva GNS, Pozzatti P, Rigatti F, Hörner R, Alves SH, Mallmann CA, Heinzmann BM. 2015. Antimicrobial evaluation of sesquiterpene α-curcumene and its synergism with imipenem. J Microbiol Biotech Food Sci. 4(5):434–436. doi: https://doi.org/10.15414/jmbfs.2015.4.5.434-436
- Delgado B, Fernandez PS, Palop A, Periago PM. 2004. Effect of thymol and cymene on Bacillus cereus vegetative cells evaluated through the use of frequency distributions. Food Microbiol. 21:327–334. doi: https://doi.org/10.1016/S0740-0020(03)00075-3
- Delmondes GA, Bezerra DS, Dias DQ, Borges AS, Araújo IM, da Cunha GL, Bandeira PFR, Barbosa R, Coutinho HDM, Felipe CFB, et al. 2019. Toxicological and pharmacologic effects of farnesol (C15H26O): a descriptive systematic review. Food Chem Toxicol. 129:169–200. doi: https://doi.org/10.1016/j.fct.2019.04.037
- Ding X, Yang CW, Yang ZB. 2017. Effect of star anise (Illicium verum Hook.f.), essential oil, and leavings on growth performance, serum, and liver antioxidant status of broiler chickens. J Appl Poult Res (JAPR). 26(4):459–466. doi: https://doi.org/10.3382/japr/pfx014
- Dosoky NS, Setzer WN. 2018. Chemical composition and biological activities of essential oil of Curcuma species. Nutriens. 10(9):1196. doi: https://doi.org/10.3390/nu10091196
- Geueke B, Groh K, Muncke J. 2018. Food packaging in the circular economy: overview of chemical safety aspects for commonly used materials. J Cleaner Prod. 193:491–505. doi: https://doi.org/10.1016/j.jclepro.2018.05.005
- Gomes F, Leite B, Teixeir P, Cerca N, Azeredo J, Oliveira R. 2011. Farnesol as antibiotics adjuvant in Staphylococcus epidermidis control in vitro. Am J Med Sci. 341(3):191–195. doi: https://doi.org/10.1097/MAJ.0b013e3181fcf138
- Hendra H, Moeljopawiro S, Nuringtyas TR. 2016. Antioxidant and antibacterial activities of agarwood (Aquilaria malaccensis Lamk.) leaves. Adv Sci Technol Soc. 1755:140004-1–140004-9.
- Hisem D, Hrouzek P, Tomek P, Tomsickova J, Zapomelova E, Skacelova K, Lukesova A, Kopecky J. 2011. Cyanobacterial cytotoxicity versus toxicity to brine shrimp Artemia salina. Toxicon. 57:76–83. doi: https://doi.org/10.1016/j.toxicon.2010.10.002
- Hu K, Huyan Z, Ding S, Dong Y, Yu X. 2020. Investigation on food packaging polymers: effects on vegetable oil oxidation. Food Chem. 315:126299. doi: https://doi.org/10.1016/j.foodchem.2020.126299
- Huang CB, George B, Ebersole JL. 2010. Antimicrobial activity of n-6, n-7 and n-9 fatty acids and their esters for oral microorganisms. Arch Oral Biol. 55:555–560. doi: https://doi.org/10.1016/j.archoralbio.2010.05.009
- Huyan Z, Ding S, Mao X, Wu C, Yu X. 2019. Effects of packaging materials on oxidative product formation in vegetable oils: hydroperoxides and volatiles. Food Packag Shelf Life. 21:100328. doi: https://doi.org/10.1016/j.fpsl.2019.100328
- Jaki B, Orjala J, Burgi HR, Sticher O. 1999. Biological screening of cyanobacteria for antimicrobial and molluscicidal activity, brine shrimp lethality, and cytotoxicity. Pharm Biol. 37(2):138–143. doi: https://doi.org/10.1076/phbi.37.2.138.6092
- Jaswir I, Tope AHT, Raus RA, Monsur HA, Ramli N. 2014. Study on anti-bacterial potentials of some Malaysian brown seaweeds. Food Hydrocolloids. 42:276. doi: https://doi.org/10.1016/j.foodhyd.2014.03.008
- Kassouf A, El Rakwe M, Chebib H, Ducruet V, Rutledge DN, Maalouly J. 2014. Independent components analysis coupled with 3D-front-face fluorescence spectroscopy to study the interaction between plastic food packaging and olive oil. Anal Chim Acta. 839:14–25. doi: https://doi.org/10.1016/j.aca.2014.06.035
- Klen TJ, Wondra AG, Vrhovsek U, Vodopivec BM. 2015. Phenolic profiling of olives and olive oil process-derived matrices using UPLC-DAD-ESI-QTOF-HRMS analysis. J Agric Food Chem. 63:3859–3872. doi: https://doi.org/10.1021/jf506345q
- Kokoska L, Havlik J, Valterova I, Sovova H, Sajfrtova M, Jankovska I. 2008. Comparison of chemical composition and antibacterial activity of Nigella sativa seed essential oils obtained by different extraction methods. J Food Prot. 71(12):2475–2480. doi: https://doi.org/10.4315/0362-028X-71.12.2475
- Kossakowska-Zwierucho M, Szewczyk G, Sarna T, Nakonieczna J. 2020. Farnesol potentiates photodynamic inactivation of Staphylococcus aureus with the use of red light-activated porphyrin TMPyP. J Photochem Photobiol B: Biol. 206:111863. doi: https://doi.org/10.1016/j.jphotobiol.2020.111863
- Liu CH, Huang HY. 2012. Antimicrobial activity of curcumin-loaded myristic acid microemulsions against Staphylococcus epidermidis. Chem Pharm Bull. 60(9):1118–1124. doi: https://doi.org/10.1248/cpb.c12-00220
- Mahboubi M, Heidarytabar R, Mahdizadeh E, Hosseini H. 2017. Antimicrobial activity and chemical composition of Thymus species and Zataria multiflora essential oils. Agri Nat Res. 51:395–401.
- Mian P, Heilmann J, Burgi HR, Sticher O. 2003. Biological screening of terrestrial and freshwater cyanobacteria for antimicrobial activity, brine shrimp lethality, and cytotoxicity. Pharm Biol. 41(4):243–247. doi: https://doi.org/10.1076/phbi.41.4.243.15672
- Miladi H, Zmantar T, Kouidhi B, Al Qurashi YMA, Bakhrouf A, Chaabouni Y, Mahdouani K, Chaieb K. 2020. Synergistic effect of eugenol, carvacrol, thymol, p-cymene and Ɣ-terpinene on inhibition of drug resistance and biofilm formation of oral bacteria. Microb Pathog. 112:156–163. doi: https://doi.org/10.1016/j.micpath.2017.09.057
- Muktar B, Bello IA, Sallau MS. 2018. Isolation, characterization and antimicrobial study of lupeol acetate from the root bark of fig-mulberry sycamore (Ficus sycomorus LINN). J Appl Sci Environ Manage. 22(7):1129–1133.
- Mulyaningsih S, Sprorer F, Reichling J, Wink M. 2011. Antibacterial activity of essential oils from Eucalyptus and selected components against multidrug-resistant bacteria patoghens. Pharm Biol. 49(9):893–899. doi: https://doi.org/10.3109/13880209.2011.553625
- Nitbani FO, Jumina, Siswanta D, Solikhah EN. 2016. Isolation and antibacterial activity test of lauric acid from crude coconut oil (Cocos nucifera L.). Procedia Chem. 18:132–140. doi: https://doi.org/10.1016/j.proche.2016.01.021
- Okusa PN, Stevigny C, Nevraumont M, Gelbeke M, Antwerpen PV, Braekman JC, Duez P. 2014. Ferulaldehyde and lupeol as direct and indirect antimicrobial compounds from Cordia gilletii (Boraginaceae) root barks. Nat Prod Commun. 9(5):619–622.
- Oloyede HOB, Ajiboye HO, Salawu MO, Ajiboye TO. 2017. Influence of oxidative stress on the antibacterial activity of botulin, betulinic acid and ursolic acid. Microb Pathog. 111:338–344. doi: https://doi.org/10.1016/j.micpath.2017.08.012
- Pankti K, Payal G, Manodeep C, Jagadish K. 2012. A phtyopharmacological review of Alstonia Scholaris: a paranomic herbal medicine. Int J Res Ayur Phar (IJRAP). 3(3):367–371.
- Papuc C, Goran GV, Predescu CN, Nicorescu V, Stefan G. 2017. Plant polyphenols as antioxidant and antibacterial agents for shelf-life extension of meat and meat products: classification, structures, sources, and action mechanisms. Compr Rev Food Sci Food Saf. 16:1243–1268. doi: https://doi.org/10.1111/1541-4337.12298
- Pereira AP, Ferreira ICFR, Marcelino F, Valentão P, Andrade PB, Seabra R, Estevinho L, Bento A, Pereira JA. 2007. Phenolic compounds and antimicrobial activity of olive (Olea europaea L. Cv. Cobrançosa) leaves. Molecules. 12:1153–1162. doi: https://doi.org/10.3390/12051153
- Pilevar Z, Bahrami A, Beikzadeh S, Hosseini H, Jafari SM. 2019. Migration of styrene monomer from polystyrene packaging materials into foods: Characterization and safety evaluation. Trends Food Sci Technol. 91:248–261. doi: https://doi.org/10.1016/j.tifs.2019.07.020
- Primahana G, Ernawati T, Puspa Dewi NL, Dwiyatmi ID, Darmawan A, Hanafi M. 2015. Synthesis of 2-allylphenyl cinnamate and brine shrimp lethality test activity evaluation. Procedia Chem. 16:694–699. doi: https://doi.org/10.1016/j.proche.2015.12.014
- Qian W, Sun Z, Wang T, Yang M, Liu M, Zhang J, Li Y. 2020. Antimicrobial activity of eugenol against carbapenem-resistant Klebsiella pneumoniae and its effect on biofilms. Microb Pathog. 139:103924. doi: https://doi.org/10.1016/j.micpath.2019.103924
- Rattanachaikunsopon P, Phumkhachorn P. 2010. Assessment of factors influencing antimicrobial activity of carvacrol and cymene against Vibrio cholerae in food. J Biosci Bioeng. 110(5):614–619. doi: https://doi.org/10.1016/j.jbiosc.2010.06.010
- Rialita T, Nurhadi B, Puteri RD. 2018. Characteristics of microcapsule of red ginger (Zingiber officinale var. Rubrum) essential oil produced from different Arabic gum ratios on antimicrobial activity toward Escherichia coli and Staphylococcus aureus. Int J Food Prop. 21(1):2500–2508. doi: https://doi.org/10.1080/10942912.2018.1528455
- Ribes S, Ruiz-Rico M, Pérez-Esteve E, Fuentes A, Barat JM. 2019. Enhancing the antimicrobial activity of eugenol, carvacrol and vanillin immobilised on silica supports against Escherichia coli or Zygosaccharomyces rouxii in fruit juices by their binary combinations. LWT-Food Sci Technol. 113:108326. doi: https://doi.org/10.1016/j.lwt.2019.108326
- Schubert K, Sieger B, Meyer F, Giacomelli G, Bohm K, Rieblinger A, Lindenthal L, Sachs N, Wanner G, Bramkamp M. 2017. The antituberculosis drug ethambutol selectively blocks apical growth in CMN group bacteria. Am Soc Microbiol. 8(1):13–16.
- Shafay SME, Ali SS, Sheekh MME. 2015. Antimicrobial activity of some seaweeds species from Red Sea, againts mulitdrug resistant bacteria. Egyptian J Aqua Res. 42:65–74. doi: https://doi.org/10.1016/j.ejar.2015.11.006
- Sivasothy Y, Chong WK, Hamid A, Eldeen IM, Sulaiman SF, Awang K. 2011. Essential oils of Zingiber officinale var. rubrum Theilade and their antibacterial activities. Food Chem. 124:514–517. doi: https://doi.org/10.1016/j.foodchem.2010.06.062
- Supraja N, Prasad TNVKV, Gandhi AD, Anbumani D, Kavitha P, Babujanarthanam R. 2018. Synthesis, characterization, and evaluation of antimicrobial efficacy and brine shrimp lethality assay of Alstonia scholaris stem bark extract mediated ZnONPs. Biochem Biophys Rep. 14:69–77.
- Tanamatayarat P. 2016. Antityrosinase, antioxidative activities, and brine shrimp lethality of ethanolic extracts from Protium serratum (Wall. ex Colebr.). Engl. Asian Pacific J Trop Biomed. 6(12):1050–1055. doi: https://doi.org/10.1016/j.apjtb.2016.10.001
- Taniguchi I, Yoshida S, Hiraga K, Miyamoto K, Kimura Y, Oda K. 2019. Biodegradation of PET: current status and application aspects. Am Chem Soc (ACS). 9:4089–4105.
- Taukoorah U, Lall N, Mahomoodally F. 2016. Piper betle L. (betel quid) shows bacteriostatic, additive, and synergistic antimicrobial action when combined with conventional antibiotics. S Afr J Bot. 105:133–140. doi: https://doi.org/10.1016/j.sajb.2016.01.006
- Timung R, Barik CR, Purohit S, Goud VV. 2016. Composition and anti-bacterial activity analysis of citronella oilobtained by hydrodistillation: process optimization study. Ind Crops Prod. 94:178–188. doi: https://doi.org/10.1016/j.indcrop.2016.08.021
- Wal A, Srivastava RS, Wal P, Rai A, Sharma S. 2015. Lupeol as a magical drug. Phar Biolog Eval. 2(5):142–151.
- Wang S, Wang C, Peng D, Liu X, Wu C, Guo P, Wei J. 2017. Agarwood essential oil displays sedative-hypnotic effect through the GABAergic system. Molecules. 22(12):2190. doi: https://doi.org/10.3390/molecules22122190
- Wang K, Zhang Y, Zhong Y, Luo M, Du Y, Wang L, Wang H. 2020. Flotation separation of polyethylene terephthalate from waste packaging plastics through ethylene glycol pretreatment assisted by sonication. Waste Manage. 105:309–316. doi: https://doi.org/10.1016/j.wasman.2020.02.021
- Weli AM, Al-Harrasi A, Al Baiti NH, Philip A, Hossain A, Gilani SA, Banioraba N. 2019. Biological and toxicological evaluation of aerial parts extracts of locally grown Cleome austroarabica. J King Saud Univ Sci. 32(1):755.