 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
We aimed to evaluate the efficacy of microwave ablation and entecavir as a combination treatment for primary liver cancer and their effects on hepatitis B virus (HBV) and liver function. A total of 258 patients were randomly allocated into the study group (n = 129) for treatment with microwave ablation and entecavir or the control group (n = 129) for treatment with microwave ablation alone. The levels of liver function indicators and serum HBV-DNA were determined in all patients before and after treatment. While no difference in total effective rate was observed, the levels of TBIL, AST, and ALT were decreased and the level of ALP was increased after treatment in both groups, and the change was more significant in the study group. Compared with that in the control group, the HBV-DNA levels were significantly decreased in the study group. The study group also had higher KPS scores, higher 2-year survival rates, lower recurrence rates, and a lower incidence of adverse reactions. Compared with microwave ablation alone, microwave ablation and entecavir as a combination treatment can improve liver function, serum HBV-DNA levels, and QOL in patients with primary liver cancer.
Introduction
Primary liver cancer is characterized by a high mortality rate and a short course, and it is one of the major contributors to cancer mortality worldwide. Approximately 782,000 new cases and 745,000 deaths are reported annually (Global Burden of Disease Liver Cancer et al. Citation2017; Meloni et al. Citation2017). Clinically, transplantation or surgical excision is the most common course of treatment (Xiao et al. Citation2018). Unfortunately, most patients are already in the later stages of disease at the time of diagnosis, rendering surgical excision or transplantation impossible due to poor liver function, the excessively high position of the tumor, and a high risk of severe complications (Bruix and Sherman Citation2011). Microwave ablation is a newly developed approach for the treatment of liver cancer that targets multiple foci and greatly increases blood coagulation volume in a short period of time (Poulou et al. Citation2015). Some studies have reported better clinical outcomes for microwave ablation in the treatment of malignant liver tumors with diameters less than 3 cm (Leung et al. Citation2015), whereas other studies have reported that oral administration of antiviral drugs (e.g. nucleotide analogs) can slow the progression of hepatocellular carcinoma (Lee et al. Citation2016).
Entecavir is a cyclopentyl guanine nucleotide analog developed for antiviral treatment. It is an effective inhibitor of hepatitis B virus (HBV) replication and has minimal impact on liver function. However, it is also capable of transforming into an active tri-phosphate with the help of a phosphokinase (Sun et al. Citation2016; Luo et al. Citation2018). However, to date, few studies have examined the efficacy of microwave ablation and entecavir as a combination treatment for primary liver cancer.
As HBV infection is the largest contributor to primary liver cancers (Saitta et al. Citation2015), serum HBV-DNA levels have been adopted as an indicator of the presence and replication of HBV in the body (Li et al. Citation2015). A study has also revealed that the extent of liver damage in some patients with liver cancer can be determined via the measurement of serum levels of HBV-DNA (Huang et al. Citation2018). Therefore, quantitative assessment of HBV-DNA is very useful in antiviral treatment. Other liver function indicators, valued for their extensive, convenient, and affordable applications, have long been used in patients with liver cancer (Li-Qing and Juan Citation2017). Specifically, the levels of ALP, AST, TBIL, and ALT are common indicators of liver function and damage (Zhang, Chan-Yuan, et al. Citation2015).
In this study, the efficacy of microwave ablation and entecavir as a combination treatment for primary liver cancer was compared with the efficacy of microwave ablation alone. We also discussed the effects of these different treatments on HBV and liver function and described potential applications for clinical treatment.
Materials and methods
General characteristics
A total of 258 patients with primary liver cancer who were admitted to our hospital were included as study participants and were randomly distributed into the study group (n = 129; 89 males and 40 females) for combination treatment with microwave ablation and entecavir or the control group (n = 129; 84 males and 45 females) for treatment with microwave ablation alone. The study group included 43 patients with foci on the left lobe, 73 with foci on the right lobe, and 13 with foci at the juncture. In this group, 82 patients had tumors at a single point and 47 had tumors at multiple points. The control group included 42 patients with foci on the left lobe, 76 with foci on the right lobe, and 11 with foci at the juncture. In this group, 86 patients had tumors at a single point and 43 had tumors at multiple points.
The inclusion criteria were as follows: patients diagnosed with primary liver cancer but no reported history of mental illness and no chemotherapy treatment in the previous month. Included patients were also able to provide complete medical records and were more likely to comply with study guidance and follow-up interventions.
The exclusion criteria were as follows: patients who were pregnant or lactating, allergic to the study drugs, or who reported a combination of severe injuries of the visceral organs, autoimmune diseases, and coagulation disorders that cannot be corrected.
The study was approved by the Ethics Committee of the First Affiliated Hospital of Bengbu Medical College, and all participants provided written informed consent.
Treatment methods
Patients fasted (water and food) for 6 h prior to treatment. Patients in the control group received microwave ablation with an ECO-100 multifunctional microwave instrument (Nanjing ECO) from a dorsal position with local anesthesia for 6–10 min at a power frequency between 40 and 70 W. Treatment variables depended on the size of the foci as well as other factors that arose during treatment, such as the patient’s tolerance. During treatment, percutaneous transhepatic puncture of the liver tumor was performed under CT guidance, and in situ treatment was administered to patients with a tumor diameter of ≤3 cm. For tumors >3 cm, needles were arranged at multiple points, from deep to shallow and from inside to out. After treatment, local puncture points were pressed for minutes, and measures were taken to protect the liver, stop bleeding, and prevent infection. Patients were required to return monthly for follow-ups and undergo further microwave ablation until complete disappearance of any residual foci was achieved. In the study group, microwave ablation was conducted as described above, and 0.5 mg entecavir tablets (Shangdong Lukang Pharmaceutical Co., Ltd. GYZ Zi H20130061) were administered to patients ODAT. Treatment continued for 20 weeks to observe the efficacy.
Indicators of liver function
Before and 14 d after treatment, 5 mL of blood was taken from the veins of fasting patients and centrifuged to separate serum. Supernatants were collected and stored at −80°C for analyses of ALT, AST, ALP, and TBIL levels using a PUZS-300 automatic biochemical analyzer (Perlong Medical) and HBV-DNA levels using an ABI7000 Quantitative PCR Instrument (ABI, the United States). The HBV-DNA Quantitative PCR Test Kit (Shanghai Beinuo Life Science Co., Ltd., Product No.: 1061123) was used for quantitative PCR. Specifically, 50 µL of concentrate was added to 50 µL each of negative control, positive control, and experimental serum samples. The preparations were then mixed, incubated at 100°C for 10 min, and centrifuged for 10 min at 12,000 rpm; 4 µL of supernatant was removed for PCR amplification under the following conditions: pre-denaturation at 50°C for 2 min and 94°C for 2 min, 40 cycles of 94°C for 10 s and 60°C for 30 s, and a final extension step at 35°C for 10 min. All procedures were performed in strict accordance with the manufacturer’s instructions.
Observation indicators
(1) Efficacy of treatment (Liao et al. Citation2017): a total tumor diameter increase of >25% was considered PD; a total tumor diameter decrease below 50% was considered SD; a total tumor diameter decrease of ≥50% for at least 1 month was considered PR; and complete disappearance of the tumor for at least 1 month was considered CR. Effective rate / % = (CR + PR) / total number of cases × 100%. (2) KPS scores were used to assess the function and status of patients before and after treatment; with higher scores indicating healthier status. (3) The levels of liver function indicators and HBV-DNA were determined in all patients before and after treatment. (4) Patients were required to return for a 2-year follow-up to document survival and recurrence rates. (5) Patients were observed for adverse reactions after treatment.
Statistical analyses
SPSS version 22.0 (IBM Corp, Armonk, NY, USA) was used for statistical analyses. Nominal data were expressed as [n (%)] and were subject to chi-squared tests between groups. Measurement data were expressed as ± SD and were subject to independent-samples t-tests between groups or paired t-tests in the same group. Survival was analyzed using the Kaplan–Meier method and was subject to Log-rank tests. P < 0.05 was considered a statistically significant difference.
Results
Comparison of general clinical characteristics
There were no significant differences between the two groups in gender, age, tumor size and location, number of foci, vascular invasion, presence of cirrhosis, and AFP levels before surgery. There are 35% patients are positive for hepatitis B and/or HBV viruses. The two groups also had no difference. (P > 0.05, Table ).
Table 1. Comparison between the two groups in general clinical characteristics n [%].
Comparison of clinical efficacy
The two groups were observed and compared for clinical efficacy. After treatment, the study group showed a total effective rate of 79.84%, which was higher than the total effective rate of 68.22% for the control group (X2 = 3.74, P > 0.05, Table ).
Table 2. Comparison of clinical efficacy between the groups n [%].
Changes in liver function indicators before and after treatment
Changes in liver function were evaluated in both groups before and after treatment. While no difference was observed before treatment (P > 0.05), the TBIL, AST, and ALT levels decreased and the ALP level increased in both groups after treatment (P < 0.05), and these changes were more significant in the study group (P < 0.05, Table ).
Table 3. Changes in liver function indicators before and after treatment (x ± SD).
Changes in HBV-DNA levels before and after treatment
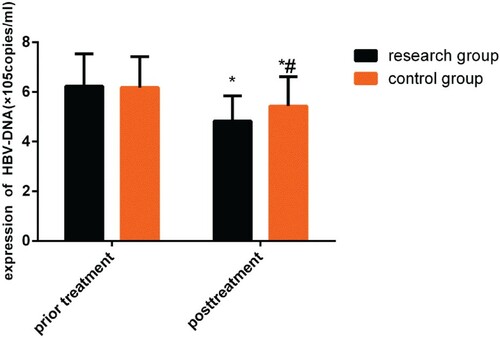
The HBV-DNA levels in the two groups were compared before and after treatment. While no difference was observed (P > 0.05) before treatment, the HBV-DNA levels decreased in both groups after treatment (P < 0.05), and this change was more significant in the study group (P < 0.05, Figure ).
Figure 1. Changes in HBV-DNA levels before and after treatment. While no differences were observed before treatment (P > 0.05), both groups demonstrated a decrease in HBV-DNA level after treatment (P < 0.05), and this change was more significant in the study group (P < 0.05).
Note: *P < 0.05 compared with conditions before treatment; #P < 0.05 compared with the study group after treatment.
Comparison of KPS scores before and after treatment
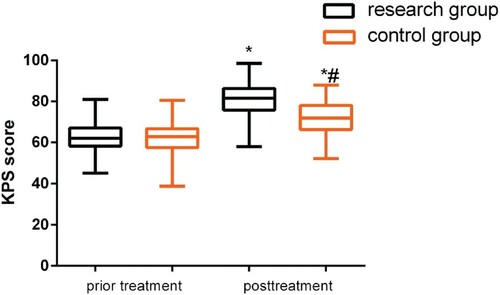
KPS scores were compared between the groups before and after treatment. While no difference was observed (P > 0.05) before treatment, both groups demonstrated an increase in KPS scores after treatment (P < 0.05), and this was more significant in the study group (P < 0.05, Figure ).
Figure 2. Comparison of KPS scores before and after treatment. While no differences were observed before treatment (P > 0.05), both groups demonstrated an increase in KPS scores after treatment (P < 0.05), and this change was more significant in the study group (P < 0.05).
Note: *P < 0.05 compared with conditions before treatment; #P < 0.05 compared with the study group after treatment.
Comparison of survival rates and recurrence rates
The survival and recurrence rates were recorded in both groups at the 2-year follow-up (Figure ). The 1-year survival rate was 87.60% in the study group and 81.40% in the control group (P > 0.05). The 2-year survival rate was 75.19% in the study group and 60.47% in the control group (P < 0.05). The recurrence rate was 43.41% in the study group and 61.24% in the control group (P < 0.05, Table ).
Figure 3. Comparison of survival rates. The study group had a 1-year survival rate of 87.60%, and the control group had a 1-year survival rate of 81.40% (P > 0.05). The study group had a 2-year survival rate of 75.19%, and the control group had a 2-year survival rate of 60.47% (P < 0.05).
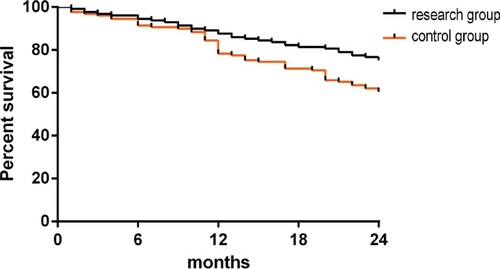
Table 4. Comparison of recurrence rate and LOS n [%].
Comparison of adverse reactions
The incidences of adverse reactions were compared between the groups after treatment (Table ), and no significant differences were found in nausea, vomiting, and fever (P > 0.05). However, a lower incidence of hepatalgia, renal function injury, elevated transaminase level, and reduced blood platelet level were noted in the study group (P < 0.05).
Table 5. Comparison of the incidence of adverse reactions n [%].
Discussion
HBV, HCV, obesity, excessive alcohol consumption, and type 2 diabetes are risk factors that contribute to primary liver cancer (Marrero et al. Citation2003; Liu and Wu Citation2010; Razavi et al. Citation2014; Marengo et al. Citation2015; Ozakyol Citation2017). Among these, obesity is most correlated with the increasing incidences of liver cancer as obesity becomes more prevalent (Marengo et al. Citation2015), and the contributions of behavioral changes should never be neglected in preventive medicine. A trend in the younger population has been observed (Xie et al. Citation2017). Microwave ablation uses electromagnetic waves emitted from microwave electrodes to quickly generate a local temperature of 100°C. The resultant oscillation of molecules produces frictional heating, ultimately generating thermocoagulation and tissue necrosis within the tumors (Lubner et al. Citation2013). However, this technique is limited as the high temperature may injure surrounding tissues and the coagulation zone may not extend to an entire large tumor, resulting in incomplete ablation (Liang et al. Citation2013). Entecavir targets the activity of HBV reverse transcriptase through three links of HBV-DNA positive strand synthesis, base guidance, and reverse transcription from the precursor mRNA to synthesize negative-strand DNA (Xie et al. Citation2017) in order to improve the functions of T and B lymphocytes and the patient’s immunity (Xie et al. Citation2017).
HBV is a Hepadnaviridae infectious factor and a double-stranded circular DNA virus (Sanada et al. Citation2017). HBV-DNA functions as a reliable indicator of viral replication and is a core element of the virus (Li et al. Citation2015). During HBV replication, the immunopathological reaction of the host body injures liver cells and leads to liver tissue repair and fibrillation (Chen et al. Citation2017). A positive HBV-DNA test result indicates that HBV is infectious and replicating, and the infection may be more serious and active if serum HBV-DNA levels are higher (Yu-Feng et al. Citation2018.). A reduction in ALP levels from liver synthesis may indicate that synthesis functions of liver cells has been damaged (Liu et al. Citation2017). AST is a widely used indicator of liver function that is released through mitochondria, and elevated AST levels indicate pathological changes or thanatosis of liver cells (Mu-Fa et al. Citation2015). Serum TBIL levels are used to ascertain the existence of hematolysis in cholochrome metabolism. Elevated TBIL levels correspond to damage to the biliary tract and liver, and serum TBIL levels are used in the diagnosis of primary liver cancers (Zhang et al. Citation2015). ALP levels are mainly used for the detection of diseases such as primary liver cancer and cholangitis jaundice, and elevated ALP levels serve as a key indicator of liver cancer (Zhang et al. Citation2012).
Studies have demonstrated the increased clinical efficacy of percutaneous microwave ablation under CT guidance in the treatment of malignant lung tumors (Guo et al. Citation2013) and efficacy of entecavir against short-term virology of HBV-associated hepatocellular carcinoma (Kim et al. Citation2015). In the present study, the study group had a higher effective rate than the control group, although this effect was not statistically significant. This indicates that microwave ablation and entecavir as a combination treatment has limited short-term efficacy on patients with primary liver cancers, although both groups yielded favorable efficacy, and further studies are required to determine the long-term efficacy. A previous study (Peng et al. Citation2010) found that lamivudine and microwave ablation combination therapy inhibits the replication of HBV, alleviates liver inflammation, and protects liver function in patients with primary liver cancer. Another study (Jingzhi et al. Citation2017) examined the efficacy of TACE with entecavir as a combination treatment for HBV-associated primary liver cancers and observed a significant decrease in ALT levels and greater efficacy as compared with TACE treatment alone. In the present study, changes in liver function indicators were recorded before and after treatment. While no differences were observed before treatment (P > 0.05), the two groups demonstrated a decrease in TBIL, AST, and ALT levels and an increase in ALP level after treatment (P < 0.05), which was more significant in the study group. This indicates that microwave ablation and entecavir combination treatment can improve the levels of liver function indicators compared with microwave ablation alone. Other studies have supported the capacity of entecavir to reduce the HBV-DNA carrying capacity and non-response rates in patients with compensated HBV cirrhosis (Yuan et al. Citation2018). However, to date, few studies have evaluated the impact of microwave ablation and entecavir combination treatment on the levels of serum HBV-DNA in patients with liver cancer. In the present study, compared with the control group, the study group yielded a more significant decrease (P < 0.05) in the HBV-DNA level, although the groups showed little difference before treatment (P > 0.05). This indicates that microwave ablation and entecavir combination treatment can reduce HBV-DNA levels, inhibit replication of HBV, and improve treatment efficacy in patients with primary liver cancer. Other reports have also demonstrated the capacity of entecavir therapy to improve KPS and QOL scores in patients with HBV-associated advanced liver cancer (Yan-Li and Gastroenterology Citation2017). In the present study, compared with the control group, the study group yielded a more significant increase (P < 0.05) in KPS scores, although the groups showed little difference before treatment (P > 0.05), which indicated that microwave ablation and entecavir combination treatment can improve QOL of patients with liver cancer with greater efficacy. Zhang (Zhang, Zhou, et al. Citation2015) et al. discussed the effect of antiviral therapy on tumor recurrence, death, and survival of patients with HBV-associated primary liver cancers and showed that entecavir can improve patients’ 3-year disease-free survival and recurrence rates. In a previous study, tenofovir was shown to be better than entecavir for preventing HCC. In the present study, similar observations were recorded during the 2-year follow-up, and there were no significant differences in the 1-year survival rate. However, the study group had a higher 2-year survival rate and lower recurrence rate, indicating that microwave ablation and entecavir combination treatment can lower the recurrence rate of primary liver cancers and prolong patient survival. However, further studies are warranted to verify the role of microwave ablation and entecavir as a combination treatment in improving long-term survival as no significant difference was observed between the two groups in the improvement of the 1-year survival rate.
Despite good clinical efficacy, microwave therapy for liver cancer may result in complications or adverse reactions during or after treatment, which may pose a threat to patients in serious cases (Zhang, Jing, et al. Citation2015). In the present study, the incidences of adverse reactions after treatment were compared between the two groups, and the study group demonstrated a lower incidence of hepatalgia, renal function injury, elevated transaminase level, and reduced blood platelets compared with the control group (P < 0.05). Previous reports have indicated that entecavir can prevent the re-activation of HBV in patients with primary liver cancer and reduce the degree of adverse reaction if administered continuously (Yan et al. Citation2015). As demonstrated in the present study, however, microwave ablation treatment in combination with entecavir can reduce adverse reactions in patients with liver cancer while improving safety.
A comprehensive discussion on the efficacy of microwave ablation and entecavir combination treatment on primary liver cancer and their effects on HBV and liver function was performed in this study. However, the long-term efficacy and survival of patients with liver cancer undergoing this regimen are yet to be analyzed. Additional systematic studies are necessary to clarify the treatment mechanisms and clinical value. Because entecavir has been used more in patients with primary liver cancer and we had a limited number of treatment options and patients included in this study, we did not administer entecavir alone to treat primary liver cancer as a comparison. As an increasing number of studies focus on the abovementioned aspects, microwave ablation and entecavir combination treatment will play a more important role in the treatment of patients with liver cancer.
In conclusion, microwave ablation and entecavir combination treatment can improve the liver function, serum HBV-DNA level, and QOL of patients with primary liver cancer and reduce the incidence of adverse reactions and recurrence rates.
Data availability statement
Due to the nature of this research, the participants of this study did not agree for their data to be shared publicly, so supporting data is not available.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Global Burden of Disease Liver Cancer C, Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, Al-Raddadi R, Alvis-Guzman N, Amoako Y, et al. 2017. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the global burden of disease study 2015. JAMA Oncol. 3(12):1683–1691.
- Bruix J, Sherman M, American Association for the Study of Liver D. 2011. Management of hepatocellular carcinoma: an update. Hepatology. 53(3):1020–1022.
- Chen Q, Chen R, Zeng YX, Wang Y, Zhou FY. 2017. Correlation analysis between HBV-DNA load and HBV serum and liver fibrosis markers in patients with chronic hepatitis B virus infection.
- Guo Y, Sun Y, Song P, Yuji AN, Weina HE. 2013. Clinical study of CT-guided percutaneous microwave ablation therapy(PMAT) for lung cancer.
- Huang G, Li PP, Lau WY, Pan ZY, Zhao LH, Wang ZG, Wang MC, Zhou WP. 2018. Antiviral therapy reduces hepatocellular carcinoma recurrence in patients with low HBV-DNA levels. Ann Surg. 268(6):943–954.
- Jingzhi YU, Jiancheng W, Wei Z. 2017. Clinical efficacy of TACE combined with entecavir for hepatitis B related primary liver cancer.
- Kim YW, Kwon JH, Chung E, Lee SW, Lee JY, Jang JW, Chung KW, Nam SW. 2015. Short term virologic efficacies of telbivudine versus entecavir against hepatitis B-related hepatocellular carcinoma. Gastroenterol Res Pract. 2015:181065.
- Lee J, Yoo SH, Sohn W, Kim HW, Choi YS, Won JH, Heo JY, Park SJ, Park YM. 2016. Obesity and hepatocellular carcinoma in patients receiving entecavir for chronic hepatitis B. Clin Mol Hepatol. 22(3):339–349.
- Leung U, Kuk D, D’Angelica MI, Kingham TP, Allen PJ, DeMatteo RP, Jarnagin WR, Fong Y. 2015. Long-term outcomes following microwave ablation for liver malignancies. Br J Surg. 102(1):85–91.
- Li-Qing B, Juan S. 2017. Diagnostic value of common biochemical indicators of liver function for primary hepatocellular cancer.
- Li C, Wu B, Chen X, Duan Z, Tian P. 2015. Study on correlation between HBV Pre-S1 antigen with HBV DNA,HBV M and liver function in patients with hepatitis B.
- Liang P, Yu J, Lu MD, Dong BW, Yu XL, Zhou XD, Hu B, Xie MX, Cheng W, He W. 2013. Practice guidelines for ultrasound-guided percutaneous microwave ablation for hepatic malignancy. World J Gastroenterol. 19(33):5430–5438.
- Liao JS, Liu Q, Huan-Jun LI, Jia Y. 2017. Effects and safety of Apatinib mesylate in the treatment of advanced primary liver cancer.
- Liu J, Meng QX, Cui QC, Yang R, Chen X. 2017. Simple hepatic cysts: comparative study on ultrasound-guided percutaneous puncture lauromacrogol injection and anhydrous ethanol sclerotherapy.
- Liu Y, Wu F. 2010. Global burden of aflatoxin-induced hepatocellular carcinoma: a risk assessment. Environ Health Perspect. 118(6):818–824.
- Lubner MG, Brace CL, Ziemlewicz TJ, Hinshaw J, Lee FT. 2013. Microwave ablation of hepatic malignancy. Semin Intervent Radiol. 30(1):056–066.
- Luo A, Jiang X, Ren H. 2018. Entecavir-based combination therapies for chronic hepatitis B: a meta-analysis. Medicine (Baltimore). 97(51):e13596.
- Marengo A, Rosso C, Bugianesi E. 2015. Liver cancer: connections with obesity, fatty liver, and cirrhosis. Annu Rev Med. 67(67):103.
- Marrero JA, Su GL, Wei W, Emick D, Conjeevaram HS, Fontana RJ, Lok AS. 2003. Des-gamma carboxyprothrombin can differentiate hepatocellular carcinoma from nonmalignant chronic liver disease in American patients. Hepatology. 37(5):1114–1121.
- Meloni MF, Chiang J, Laeseke PF, Dietrich CF, Sannino A, Solbiati M, Nocerino E, Brace CL, Lee Jr. FT. 2017. Microwave ablation in primary and secondary liver tumours: technical and clinical approaches. Int J Hyperthermia. 33(1):15–24.
- Mu-Fa C, Feng G, Xiao-Fang FU. 2015. The study on the relationship between AFU and ALT, AST, GGT of serum in liver disease.
- Ozakyol A. 2017. Global epidemiology of hepatocellular carcinoma (HCC epidemiology). J Gastrointest Cancer. 48(3):238–240.
- Peng Q, Xiao B, Cheng T, Tan Y, Zhuoliang LI, Zhouyao YU. 2010. Lamivudine therapy after percutaneous microwave coagultion therapy for patients with primary hepatocellular carcinoma complicated with hepatitis B virus:an analysis of 40 cases.
- Poulou LS, Botsa E, Thanou I, Ziakas PD, Thanos L. 2015. Percutaneous microwave ablation vs radiofrequency ablation in the treatment of hepatocellular carcinoma. World J Hepatol. 7(8):1054–1063.
- Razavi H, Waked I, Sarrazin C, Myers RP, Idilman R, Calinas F, Vogel W, Mendes Correa MC, Hezode C, Lazaro P, et al. 2014. The present and future disease burden of hepatitis C virus (HCV) infection with today’s treatment paradigm. J Viral Hepat. 21(Suppl. 1):34–59.
- Saitta C, Tripodi G, Barbera A, Bertuccio A, Smedile A, Ciancio A, Raffa G, Sangiovanni A, Navarra G, Raimondo G, et al. 2015. Hepatitis B virus (HBV) DNA integration in patients with occult HBV infection and hepatocellular carcinoma. Liver Int. 35(10):2311–2317.
- Sanada T, Hirata Y, Naito Y, Yamamoto N, Kikkawa Y, Ishida Y, Yamasaki C, Tateno C, Ochiya T, Kohara M, et al. 2017. Transmission of HBV DNA Mediated by Ceramide-Triggered Extracellular Vesicles. Cell Mol Gastroenterol Hepatol. 3(2):272–283.
- Sun HY, Jang JW, Kwon JH, Jung SM, Jang B, Choi JY. 2016. Preemptive antiviral therapy with entecavir can reduce acute deterioration of hepatic function following transarterial chemoembolization. Clin Mol Hepatol. 22(4):458–465.
- Xiao R, Tian H, Xiaodan HU. 2018. Influencing factors of microwave ablation on tumor-free survival rate in treatment of primary liver cancer.
- Xie R, Gui L, Huang Q, Xiongfeng BI, Yang J. 2017. The study progress of lamivudine and entecavir for the treatment of patients with HBV reactivation of primary hepatocellular carcinoma(HCC).
- Yan-Li MA, Gastroenterology DO. 2017. Clinical study of antiviral treatment in hepatitis B related advanced liver cancer.
- Yan D, Yao XS, Gao QZ, Liu DZ, Zeng HY, Li CR, Shi ZH, Guo YJ, Li H. 2015. Application of nucleos (t) ide analogues in transarterial interventional therapy for hepatitis B virus related hepatocellular carcinoma. Chinese Journal of Interventional Imaging and Therapy. 12(5):293–297.
- Yu-Feng X, Da-Qiao Z, Jing-Song H, Chun-Shan W, Wei-Chao Z, Zhi-Yi H, De-Ti P, Mu-Min S, Tung-Ting S, Kam-Wah MD. 2018. Clinical and histopathological features of chronic hepatitis B virus infected patients with high HBV-DNA viral load and normal alanine aminotransferase level: A multicentre-based study in China. PLoS One. 13(9):e0203220.
- Yuan G, Airong HU, Yaoren HU, Zeng C, Zhu D, Shi X. 2018. Clinical efficacy and long-term prognosis of entecavir and adefovir dipivoxil in the treatment of compensatory hepatitis B cirrhosis.
- Zhang XJ, Chan-Yuan XU, Ying-Xian LI, Tian GJ, Jian JM, Chi XL. 2015. Combined detection of serum indexes of liver function in the differential diagnosis of primary hepatocellular carcinoma and intrahepatic colangiocarcinoma.
- Zhang CM, Jing HE, Zhang YX, Zhang XL, Wang Y, Wei GH, Xing XY. 2015. Nursing care for patients with primary liver cancer receiving CT-guided percutaneous microwave ablation treatment.
- Zhang MY, Wen ZL, Yan-Hua XU. 2012. Clinical significance of alkaline phosphatase, γ-glutamyl transpeptidase and cholinesterase detection in chronic hepatitis B, liver cirrhosis, hepatocellular carcinoma and biliary tract disease.
- Zhang H, Zhou Y, Yuan G, Zhou G, Yang D, Zhou Y. 2015. Antiviral therapy improves the survival rate and decreases recurrences and fatalities in liver cancer patients following curative resection: A meta-analysis. Mol Clin Oncol. 3(6):1239–1247.
