 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective: This study aimed to compare the clinical efficacy and safety of a combination of methotrexate (MTX) plus calcium folinate (CF) and actinomycin D (ACT-D) treatment in patients with low-risk trophoblastic tumors. Methods: We included 95 and 101 patients who received chemotherapy with MTX plus CF (MTX + CF group) and ACT-D (ACT-D group). The cure rate, drug resistance rate, number of treatment courses, and adverse reactions were compared between both groups. Results : Age, International Federation of Gynecology and Obstetrics (FIGO) stage and score, prechemotherapy β-human chorionic gonadotropin (HCG) levels, and the nature of the last pregnancy were associated with the development of resistance. Furthermore, age, FIGO stage III, and a hydatidiform mole in the last pregnancy were independent risk factors for gestational trophoblastic neoplasia (GTN) after MTX + CF or ACT-D chemotherapy (P < 0.05). Both MTX + CF and ACT-D had a high cure rate and a low rate of resistance in gestational trophoblastic tumors. The adverse reaction incidence was lower in the MTX + CF group than in the ACT-D group. Conclusions: Advanced age, FIGO stage III, and a hydatidiform mole in the last pregnancy may be associated with the development of resistance to MTX + CF or ACT-D chemotherapy in patients with gestational trophoblastic tumors.
Introduction
Gestational trophoblastic neoplasia (GTN) encompasses tumors formed by the abnormal development of placental trophoblasts (Hsieh et al. Citation2015; Mei et al. Citation2015). GTN is extremely sensitive to chemotherapeutic drugs; however, before the discovery of effective chemotherapeutic agents, the 2-year survival rate of patients was 15–40% (Li et al. Citation2018). According to the International Federation of Gynecology and Obstetrics (FIGO) 2000 scoring criteria, patients with GTN can be divided into two groups: a low-risk group with prognosis scores <7 and a high-risk group with prognosis scores ≥7.
More than 90% of patients are in the low-risk group (Uberti et al. Citation2015), which has a cure rate approaching 100% because of the development of novel drugs for GTN (a solid tumor with the highest cure rate). Cure rates as high as 90% can be achieved in patients with extensive metastases (Osborne et al. Citation2015). Therefore, chemotherapy plays an important role in the treatment of GTN.
Methotrexate (MTX) is an antifolate antitumor drug that inhibits the proliferation of tumor cells. MTX arrests cells in the S phase of the cell cycle, mainly by binding to dihydrofolate reductase, inhibiting its activity, and thereby inhibiting the formation of tetrahydrofolate. These effects further interfere with DNA synthesis, which inhibits the growth and reproduction of tumor cells. Thus, MTX is a cell-cycle specific drug (Fujieda et al. Citation2016; Claxton et al. Citation2018). Trophoblasts are very sensitive to MTX, which effectively inhibits trophoblast proliferation in patients, preventing embryonic development (Elias et al. Citation2014).
Calcium folinate (CF), a formyl derivative of tetrahydrofolate, has been used as an antidote to folic acid (FA) antagonists, and it effectively prevents the side effects of MTX chemotherapy (Sun et al. Citation2016). Cotreatment with high-dose MTX and CF produces successful clinical outcomes in patients with ectopic pregnancy (Wang and Gynaecology Citation2016). Actinomycin D (ACT-D) is an anticancer drug (Yang et al. Citation2016) that specifically binds to deoxyguanosine residues in DNA and is embedded in the minor groove of the DNA double helix. Consequently, it acts by impairing the function of RNA polymerase and inhibiting the synthesis of RNA, particularly mRNA (Cortes et al. Citation2016).
GTN is typically treated using single-agent chemotherapy with agents such as 5-fluorouracil (5-Fu), MTX, or ACT-D. To date, however, an effective first-line single-drug chemotherapy regimen that can be administered at home and abroad has not been established. Currently, the choice of chemotherapeutic regimen depends on geographical location and the experience or preference of the clinician rather than on the previously reported efficacy and side effects of various chemotherapeutic regimens (Lu et al. Citation2003; Matsui et al. Citation2005).
Methods and Materials
Subjects
In this study, 196 patients with GTN who were admitted to our hospital between January 2017 and July 2017 were enrolled. This number included 95 patients who were treated with MTX plus CF chemotherapy (MTX + CF group) while the remaining 101 were treated with ACT-D chemotherapy (ACT-D group). This study included patients diagnosed with GTN according to the FIGO 2002 diagnostic criteria (Eysbouts et al. Citation2017); patients with hydatidiform moles who showed a <10% decrease in serum β-human chorionic gonadotropin (HCG) levels within 3 weeks or a plateau or rise for 2 weeks; those with a history of abortion, full-term birth, or who carried an ectopic pregnancy for 1 month and above; and those who received initial treatment for GTN who were aged ≥ 18 years and provided informed consent.
This study excluded patients who were allergic to a variety of drugs; patients with mental disorders; those who would be unable to conform to the treatment plan for individual reasons; those who had received other treatments such as chemotherapy and had shown significant improvement; patients with other tumors; patients with severe dysfunction of other basic organs such as the heart, liver, and kidney; patients with chemotherapy contraindications; and patients with incomplete clinical data. This study was approved by the ethics committee of Fujian Maternal and Child Health Hospital.
FIGO 2000 GTN clinical staging and prognosis scoring criteria
This study evaluated the clinical stage of GTN and the prognosis score of patients in each group using the FIGO 2000 criteria (Ngan Citation2010). A prognosis score < 7 indicated low risk and a score ≥ 7 indicated high risk. Lung metastases > 3 cm and metastatic tumors were each assigned a score of 1 (Tables and ).
Table 1. International Federation of Gynecology and Obstetrics (FIGO Citation2010) gestational trophoblastic neoplasia (GTN) staging criteria.
Table 2. International Federation of Gynecology and Obstetrics (FIGO 2000) gestational trophoblastic neoplasia (GTN) prognostic criteria.
Chemotherapy
Patients in the MTX + CF group received intramuscular injections of MTX at 1.0 mg kg−1·day−1 (EbeThis Study Pharma Ges.m.b.H.Nfg.KG, Guoyaozhunzi: H20030446) on day 1, 3, 5, and 7. After 24 h, the patients received intramuscular injections of CF at 0.1 mg kg−1·day−1 (Jiangsu Hengrui Pharmaceutical Co., Ltd., Guoyaozhunzi H20000584) on day 2, 4, 6, and 8. The duration of treatment was 2 weeks.
Efficacy criteria
During the treatment period, the serum β-HCG levels of patients were examined weekly. The criteria for a diagnosis of drug resistance were as follows: no logarithmic decrease or a less than 50% decrease (from baseline), or an increase in serum β-HCG levels after two consecutive courses of chemotherapy or no shrinkage or increase in size of the tumor or development of new lesions on imaging examination. The criterion for determining cure rate was a serological complete response (SCR), defined as serum β-HCG values within the normal range for four consecutive weeks.
The FIGO withdrawal criteria were as follows: patients with low-risk GTN with detection of serum β-HCG who received at least one course of chemotherapy after the first negative conversion, and GTN patients with a slow decrease in HCG levels or diffuse lesions who were required to undergo an additional two to three courses of chemotherapy. Patients who received chemotherapy with a single-agent and developed resistance had to continue chemotherapy for an additional two to three courses even after they showed negative HCG results.
Observation index
In this study, we quantitated the average number of courses of chemotherapy and the occurrence of adverse reactions; examined the blood of each patient every 3 days for red blood cell, white blood cell, and platelet counts; and evaluated bone marrow suppression according to the WHO chemotherapeutic adverse reaction grading standard (Table ). Granulocyte colony-stimulating factor (G-CSF) agents were expected to have been used for supportive care in patients with ≥ grade III bone marrow suppression. Liver and kidney functions were assessed weekly to monitor related toxicity, and the occurrences of other adverse events such as gastrointestinal reactions, oral mucosal redness, and ulcers were recorded.
Table 3. World Health Organization (WHO) grading of myelosuppression.
Analysis of drug resistance-related factor
Patients in each group who developed resistance to their treatment regimen were combined into a drug resistance group for the analysis, while those in each group who achieved SCR were combined into a single group. Univariate analysis was performed to compare age, chemotherapy, and other factors between the two groups, whereas the multivariate logistic regression analysis was used to identify factors associated with the development of drug resistance.
Data analysis
Data were analyzed using the statistical package for the social sciences (SPSS) version 19.0 software (IBM, SPSS, Chicago, IL, USA). Values were expressed as means ± standard error () and were analyzed using the Student’s t-test. Numerical data were expressed as percentages and were analyzed using the chi-square (X2) test. Pressure-related factors were analyzed using single factor analysis with the X2 test, and statistically significant factors in the single factor analysis were further assessed using multivariate analysis. Logistic regression was used and a P < 0.05 indicated statistical significance.
Results
Comparison of baseline data between both groups
There were no significant differences between the MTX + CF and ACT-D groups in age, FIGO stage and prognosis score, β-HCG level, and last pregnancy (P > 0.05). MTX + CF and ACT-D were both considered secondary chemotherapeutic regimens because some patients had been previously treated with 5-day single-agent regimens of 5-Fu or MTX. Among the 95 patients in the MTX + CF group, 85 were newly diagnosed with GTN and 10 who initially received a 5-day single-drug regimen of 5-Fu or MTX were switched to the MTX + CF regimen. Of the 101 patients in the ACT-D group, 95 were newly diagnosed patients, 5 had been treated with 5-Fu or MTX for 5 days, and 1 had previously received ACT-D. No significant difference was observed in secondary chemotherapy between the two groups (P > 0.05, Table ).
Table 4. Two sets of baseline data.
Comparison of efficacy in both groups
SCR was achieved in 63 patients in the MTX + CF group, consisting of 53 patients who were initially treated and 10 patients who were cured with the MTX + CF regimen. The cure rate was 66.32% while the remaining 32 patients developed drug resistance after chemotherapy at a rate of 33.68%. SCR was achieved in 72 patients in the ACT-D group, consisting of 66 patients who were initially treated and 6 patients who were cured with the ACT-D regimen at a rate of 71.29%. The remaining 29 patients developed drug resistance after chemotherapy at a rate of 28.71%. No significant difference in efficacy was observed between the MTX + CF and ACT-D groups (P > 0.05, Table ).
Table 5. Curative effect in the two groups.
Comparison of number of chemotherapy courses of patients in each group
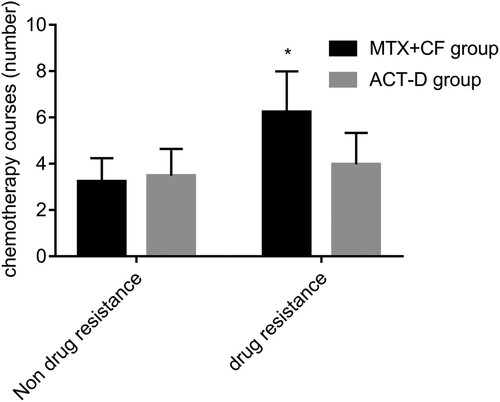
The number of chemotherapy courses required to achieve SCR in patients who did not develop drug resistance was determined to be 3.23 ± 1.01 and 3.48 ± 1.16 in the MTX + CF and ACT-D groups, respectively. No significant difference was observed in the number of chemotherapy courses required to achieve SCR in patients who did not develop resistance between both groups (P > 0.05). The average number of treatment courses was higher in the MTX + CF group than in the ACT-D group (P < 0.05, Figure ).
Figure 1. No significant difference was observed in the number of courses of treatment required to achieve serological complete response (SCR) in patients who did not develop resistance between the two groups (P > 0.05). The average number of courses of treatment in methotrexate plus calcium folinate (MTX + CF) group were higher than those in actinomycin D (ACT-D) group (P < 0.05). P < 0.05, compared with ACT-D group.
Comparison of myelosuppression between groups
Myelosuppression occurred in 17 (17.89%) patients in the MTX + CF group, including 15 (15.79%) and 2 (2.11%) patients with grade I-II and grade III-IV myelosuppression, respectively. Myelosuppression was observed in 80 (79.21%) patients in the ACT-D group, including 66 (65.35%) and 14 (13.86%) patients with grade I-II and grade III-IV myelosuppression, respectively. Bone marrow suppression improved after treatment with G-CSF and none of the patients discontinued chemotherapy because of myelosuppression. The degree of myelosuppression in the MTX + CF group was significantly lower than that in the ACT-D group (P < 0.05). Patients in both groups mostly showed grade I-II myelosuppression (Table ).
Table 6. Myelosuppression in two groups.
Comparison of other adverse reactions between groups
No significant differences were observed in the occurrence of adverse reactions such as liver and kidney toxicity or alopecia between the groups (P > 0.05). The number of gastrointestinal reactions and oral ulcers in the MTX + CF group was significantly lower than that in the ACT-D group (P < 0.05, Table ).
Table 7. Incidence of other adverse reactions in the two groups.
Single factor analysis of drug resistance
This study combined 32 and 29 patients who developed drug resistance in the MTX + CF and ACT-D groups, respectively, into a drug-resistant group (n = 61) for the analysis. Furthermore, 63 and 72 patients in the MTX + CF and ACT-D groups, respectively, who achieved SCR were combined into an SCR group (n = 135). The univariate analysis revealed no significant differences in age, chemotherapy regimen, FIGO stage, last pregnancy, or secondary chemotherapy between the groups (P > 0.05). Significant differences in age, FIGO stage, FIGO score, prechemotherapy β-HCG levels, and the nature of the last pregnancy were observed between the groups. The age of patients, FIGO score, and β-HCG levels before chemotherapy were significantly higher in the drug-resistant group than in the SCR group (P < 0.05, Table ).
Table 8. Single factor analysis of drug resistance.
Multivariate logistic regression analysis
The univariate analysis results showed significant differences in age, FIGO score, prechemotherapy β-HCG levels, FIGO stage, and last pregnancy between the groups. The multivariate logistic regression analysis showed that advanced age, FIGO stage III, and a hydatidiform mole in the last pregnancy may be independent risk factors for the development of resistance after MTX + C or AACT-D chemotherapy in patients with GTN (P < 0.05, Table ).
Table 9. Multivariate logistic regression analysis.
Discussion
GTN is a group of malignant tumors that originate from embryonic trophoblasts. Before the availability of chemotherapeutic drugs, the mortality rate of patients with GTN was 90% and all patients with metastasis died within a short time (Savage et al. Citation2015). The recent discovery and use of sensitive chemotherapeutics and high-sensitivity and -specificity monitoring of tumor β-HCG, has increased the total cure rate of patients with GTN to 85–90%, making it the only curable tumor (Yarandi et al. Citation2016; Kang et al. Citation2017). The current first-line chemotherapeutic regimen used worldwide consists of ACT-D or MTX administered as single agents to decrease the incidence of adverse reactions to chemotherapy. In addition, CF (Uberti et al. Citation2015) is commonly used with ACT-D. To date, the clinical efficacy and side effects of GTN chemotherapy have not been investigated. This study compared the clinical efficacy of two treatment regimens (MTX combined with CF and ACT-D) and determined factors associated with the development of resistance to each regimen in the treatment of trophoblastic tumors. The data obtained provide insights into establishing a safe and effective chemotherapeutic regimen for patients with GTN and improving patient prognosis.
The baseline data showed that both groups of patients used single-agent chemotherapy, whereas the high-risk patients were preferentially coadministered multiple drugs with chemotherapy. The potential for patients to develop drug resistance should be considered when designing a chemotherapeutic regimen including ACT-D. MTX has a lower cure rate than ACT-D for low-risk GTN (Shahbazian et al. Citation2014), probably because ACT-D is a cell cycle targeting drug that is relatively non-phase-specific and has a strong inhibitory effect on RNA synthesis (Wang et al. Citation2016). ACT-D affects the cell cycle in multiple phases but is especially potent in G1 and, thus, it broadly inhibits or kills cancer cells (Wang et al. Citation2016). MTX is a cell cycle-specific drug whose effect is relatively limited to the S phase and, therefore, it may not be as damaging to cancer cells as ACT-D is (Shiozawa et al. Citation2016). Incidence of gastrointestinal response to MTX in the treatment of low-risk GTN is higher than that of ACT-D (Kang et al. Citation2017). However, in this study, the incidence of bone marrow suppression, gastrointestinal reactions, and oral ulcers were significantly lower in low-risk GTN patients in the MTX + CF group than in those in the ACT-D group (P < 0.05). In contrast to previously reported results, this study showed no significant difference in the occurrence of adverse reactions such as liver and kidney toxicity and alopecia (P > 0.0.5). These findings may be attributed to the use of CF, which prevents the toxicity of MTX, limiting its damage to normal cells and reversing its effects on bone marrow and gastrointestinal mucosa, which markedly reduces its myelosuppression and other adverse reactions.
No significant differences in cure and drug resistance rates were observed between the MTX + CF and ACT-D groups, which is inconsistent with the findings of previous studies (Osborne et al. Citation2015; Kanno et al. Citation2018). This may be attributed to the reduction in the adverse reactions of CF, which minimizes autoimmune responses and improves patient prognosis. Compared to treatment with MTX alone, cotherapy with MTX and CF significantly decreased resistance rates and adverse reactions (Maestá et al. Citation2017). Randomized controlled trials have shown that the efficacy and adverse effects of MTX-FA were significantly lower than those of ACT-D in treating patients with low-risk GTN (Shobeiri et al. Citation2014), which is consistent with the results of this study. This is probably because CF has similar effects to FA and is an antidote to FA antagonists (Chen et al. Citation2017). Therefore, the effects of MTX-FA are very similar to those of MTX-CF.
Drug resistance and relapse are the two main causes of chemotherapy failure in GTN (Xiao hua and Shi Citation2003; Williams et al. Citation2014). Univariate analysis showed that age, FIGO stage and score, prechemotherapy β-HCG level, and the nature of the last pregnancy were closely related to drug resistance. Multivariate logistic regression analysis showed that advanced age, FIGO stage III, and a hydatidiform mole in the last pregnancy may be independent risk factors for the development of resistance after MTX + CF or ACT-D chemotherapy in patients with GTN. GTN is a type of disease that occurs during the reproductive age, and the development of follicular defects and abnormal chromosome number is more frequent in older women. Therefore, the rate of occurrence of GTN increases in older women, which is consistent with previously reported findings that a FIGO prognosis score > 13 may be a related factor responsible for the development of resistance in patients with GTN (Zhu Citation2015; Jiang et al. Citation2018). This study did not explain the relationship between a total FIGO score of 13 points and the development of drug resistance. This study indicates that the probability of developing drug resistance increases with an increase in the total FIGO score. Moreover, the FIGO score is a risk factor for pressure tolerance. The β-HCG level reflects the number of trophoblasts that are active in patients with GTN. High serum HCG levels indicate an increase in the number of trophoblasts that are active in the body and worsening of prognosis. In addition, high serum HCG levels indicate a high burden of GTN, the presence of drug-resistant strains, and poor response to chemotherapy (Guzel et al. Citation2014; Fan et al. Citation2015).
GTN patients receiving a single drug are prone to drug resistance. The rate of resistance in this population is between 10% and 50% (Alazzam et al. Citation2012; Li et al. Citation2018). A patient receiving monotherapy requires more courses of chemotherapy or combination chemotherapy with no cross-resistance to continue chemotherapy. Thus, the number of chemotherapeutics is increased, adverse reactions are exacerbated, and the length of hospitalization is prolonged.
This is associated with a considerable economic burden on patients and their families (Mathew et al. Citation2018). The addition of CF decreases the side effects of ACT-D combined with MTX chemotherapy and is beneficial to patient prognosis. A large multicenter randomized controlled trial should be performed to determine the most appropriate chemotherapy regimen for patients with GTN.
Both MTX + CF and ACT-D regimens have high cure rates and low rates of development of resistance in GTN patients. The incidence of adverse reactions was lower in the MTX + CF group than in the ACT-D group. Patients receiving the MTX + CF regimen were more prone to develop. Advanced age, FIGO stage III, and a hydatidiform mole in the last pregnancy may be independent risk factors for the development of resistance after MTX + CF/ACT-D chemotherapy in patients with GTN.
Acknowledgements
None.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data is not available.
Reference
- Alazzam M, Tidy J, Osborne R, Coleman R, Hancock BW, Lawrie TA. 2012. Chemotherapy for resistant or recurrent gestational trophoblastic neoplasia. Cochrane Database Syst Rev. 12(12):CD008891.
- Chen N, Hematology DO, Hospital XC. 2017. Different administration routes of calcium folinate on the prevention of oral mucositis in children with acute lymphoblastic leukemia. J Clin Nurs Pract. 38:10–11.
- Claxton L, Taylor M, Soonasra A, Bourret JA, Gerber RA. 2018. An economic evaluation of tofacitinib treatment in rheumatoid arthritis after methotrexate or after 1 or 2 TNF inhibitors from a U.S. payer perspective. Eur J Med Chem. 138:170–181.
- Cortes CL, Veiga SR, Almacellas E, Hernándezlosa J, Ferreres JC, Kozma SC, Ambrosio S, Thomas G, Tauler A. 2016. Effect of low doses of actinomycin D on neuroblastoma cell lines. Mol Cancer. 15(1):1–13.
- Elias KM, Harvey RA, Hasselblatt KT, Seckl MJ, Berkowitz RS. 2014. Type I interferons modulate methotrexate resistance in gestational trophoblastic neoplasia. Gynecol Oncol. 133(6):190–191.
- Eysbouts YK, Ottevanger PB, Lfag M, Inthout J, Short D, Harvey R, Kaur B, Sebire NJ, Sarwar N, Fcgj S. 2017. Can the FIGO 2000 scoring system for gestational trophoblastic neoplasia be simplified? A new retrospective analysis from a nationwide dataset. Ann Oncol. 28(8):1856–1861.
- Fan S, Pan M, Qin H. 2015. The relationship between the expression of β-HCG mRNA in peripheral blood and the hematogenous metastasis of gestational trophoblastic neoplasia. Pract J Cancer. 6:25–28.
- Fujieda M, Tsuruga K, Sato T, Kikuchi H, Tamaki W, Ishihara M, Yamamoto M, Oishi T, Tanaka H, Daibata M. 2016. Monitoring of Epstein-Barr virus load and killer T cells in patients with juvenile idiopathic arthritis treated with methotrexate or tocilizumab. Mod Rheumatol. 27(1):66–71.
- Guzel AI, Kokanali MK, Erkilinc S, Topcu HO, Oz M, Ozgu E, Erkaya S, Gungor T. 2014. Predictive role of the neutrophil lymphocyte ratio for invasion with gestational trophoblastic disease. Asian Pac J Cancer Prev. 15(10):4203–4206.
- Hsieh FJ, Wu CC, Lee CN, Chen TM, Chen CA, Chen FC, Chen CL, Hsieh CY. 2015. Vascular patterns of gestational trophoblastic tumors by color Doppler ultrasound. Cancer. 74(8):2361–2365.
- Jiang F, Wan X-R, Xu T, Feng F-Z, Ren T, Yang J-J, Zhao J, Yang T, Yjgo X. 2018. Evaluation and suggestions for improving the FIGO 2000 staging criteria for gestational trophoblastic neoplasia: A ten-year review of 1420 patients. Gynecol Oncol. 149(3):539–544.
- Kang H, Zhao Q, Yang S, Duan W, Oncology DO. 2017. A prospective study of actinomycin D combined with methotrexate and methotrexate only in the treatment of low-risk gestational trophoblastic tumor. Beijing Med J. 11(3):199–201.
- Kanno T, Matsui H, Akizawa Y, Usui H, Shozu M. 2018. Treatment results of the second-line chemotherapy regimen for patients with low-risk gestational trophoblastic neoplasia treated with 5-day methotrexate and 5-day etoposide. J Gynecol Oncol. 29(6):e89.
- Li J, Li S, Yu H, Wang J, Xu C, Lu X. 2018. The efficacy and safety of first-line single-agent chemotherapy regimens in low-risk gestational trophoblastic neoplasia: A network meta-analysis. Gynecol Oncol. 148(2):247–253.
- Lu WG, Ding ZM, Xie X, Ye DF, Chen HZ, Feng SW. 2003. [Single methotrexate chemotherapy for low-risk gestational trophoblastic tumor]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 25(4):414–417.
- Maestá I, Nitecki R, Horowitz NS, Goldstein DP, de Freitas Segalla Moreira M, Elias KM, Berkowitz RS. 2017. Effectiveness and toxicity of first-line methotrexate chemotherapy in low-risk postmolar gestational trophoblastic neoplasia: the New England Trophoblastic Disease Center experience. Gynecol Oncol. 148(1):161–167.
- Maria L, Tae KY. 2010. Revised International Federation of Gynecology and Obstetrics (FIGO) staging systems in gynecologic malignancies. Korean Journal of Obstetrics & Gynecology. 53(8):669.
- Mathew RA, Johnson S, Jose S, Chakraborty S, Kenneth N, Gowda V. 2018. A comparative study of monotherapy versus combination therapy in patients with stage-1 hypertension in terms of efficacy and cost effectiveness and to assess the medication adherence. Int J Res Pharm Sci. 9(1):147–153.
- Matsui H, Suzuka K, Yamazawa K, Tanaka N, Mitsuhashi A, Seki K, Sekiya S. 2005. Relapse rate of patients with low-risk gestational trophoblastic tumor initially treated with single-agent chemotherapy. Gynecol Oncol. 96(3):616–620.
- Mei P, Ding Y, Ling Y, Deng Y, Lai W, Yun H, Zhang H, Wu X, Hong F, Hui D. 2015. Tegafur substitution for 5-Fu in combination with actinomycin D to treat gestational trophoblastic neoplasm. Plos One. 10(11):e0143531.
- Ngan HY. 2010. The practicability of FIGO 2000 staging for gestational trophoblastic neoplasia. Int J Gynecol Cancer. 14(2):202–205.
- Osborne RJ, Filiaci V, Schink JC, Mannel RS, Alvarez SA, Kelley JL, Provencher D, Scott MD, Covens AL, Lage JM. 2015. Phase III trial of weekly methotrexate or pulsed dactinomycin for low-risk gestational trophoblastic neoplasia: a gynecologic oncology group study. J Clin Oncol. 29(7):825–831.
- Savage P, Cooke R, Onions J, Krell J, Kwan A, Camarata M, Dancy G, Short D, Seckl MJ, Swerdlow AJ. 2015. Effects of single-agent and combination chemotherapy for gestational trophoblastic tumors on risks of second malignancy and early menopause. J Clin Oncol. 33(5):472–478.
- Shahbazian N, Razi T, Razi S, Yazdanpanah L. 2014. Comparison of the efficacy of methotrexate and actinomycin D in the treatment of patients with stage I low risk gestational trophoblastic neoplasia (GTN). Med J Islam Repub Iran. 28(28):78–78.
- Shiozawa K, Yamane T, Murata M, Yoshihara R, Tsumiyama K, Imura S, Shiozawa S. 2016. MMP-3 as a predictor for structural remission in RA patients treated with MTX monotherapy. Arthritis Res Ther. 18(1):1–9.
- Shobeiri MJ, Vejdani R, Melli MS, Madarek EOS, Garebaghi PM, Khoei SA, Ghojazadeh M, Asgharzadeh A. 2014. Comparison of methotrexate-folinic acid versus pulsed actinomycin-d in treatment of stage i, low risk gestational trophoblastic neoplasia: a randomized clinical trial. Iran J Obstet Gynecol Infertil. 17(91):1–11.
- Sun N, Sun Q, Liu Q, Zhang T, Zhu Q, Wang W, Cao M, Zang QI. 2016. α-fetoprotein-producing gastric carcinoma: a case report of a rare subtype and literature review. Oncol Lett. 11(5):3101–3104.
- Uberti EM, Fajardo MC, Da CA, Frota SS, Braga A, Ayub AC. 2015. Treatment of low-risk gestational trophoblastic neoplasia comparing biweekly eight-day methotrexate with folinic acid versus bolus-dose actinomycin-D, among Brazilian women. Rev Bras Ginecol Obstet. 37(6):258–265.
- Wang N, Gynaecology DO. 2016. The effect of intracavity ultrasound guided puncture ectopic pregnancy sac injection of methotrexate in treatment of ectopic pregnancy. China Cont Med Educ. 7(3):112–120.
- Wang Y, Miao JW, Wang T, Wang Y, Wu YM, Kong WM, Su L, Duan W. 2016. Comparison of MACT and 5Fu+ACT-D chemotherapy regimens in the treatment of low-risk gestational trophoblastic neoplasia. J Chemother. 28(2):135–139.
- Williams J, Short D, Dayal L, Strickland S, Harvey R, Tin T, Savage PM, Seckl MJ. 2014. Effect of early pregnancy following chemotherapy on disease relapse and fetal outcome in women treated for gestational trophoblastic neoplasia. J Reprod Med. 59(5–6):248–254.
- Xiao hua FU, Shi YF. 2003. Progress in study on the mechanism of multiple drug resistance in the treatment of gestational trophoblastic tumor. Foreign Med Sci. 5(3):113–123.
- Yang J, Xiang Y, Wan X, Feng F, Ren T. 2016. Primary treatment of stage IV gestational trophoblastic neoplasia with floxuridine, dactinomycin, etoposide and vincristine (FAEV): A report based on our 10-year clinical experiences. Gynecol Oncol. 143(1):68–72.
- Yarandi F, Mousavi A, Abbaslu F, Aminimoghaddam S, Nekuie S, Adabi K, Hanjani P. 2016. Five-day intravascular methotrexate versus biweekly actinomycin-D in the treatment of low-risk gestational trophoblastic neoplasia: a clinical randomized trial. Int J Gynecol Cancer. 26(5):971–976.
- Zhu J. 2015. 2 regimens for the treatment of high-risk gestational trophoblastic tumor and drug resistance related factors. China Pharm. 8(13):313–319.
