Abstract
Date palm is the most important crop in Oman, occupying approximately 50% of the area devoted to agriculture. Little information is available on leaf spot diseases of date palm in Oman and in the world. This study was conducted to identify the most common fungal pathogens associated with leaf spot diseases of date palm. A total of 198 symptomatic leaf samples were collected from seven districts in Oman. Isolations revealed that most isolates belonged to the genus Alternaria. Molecular-based identification using multigene sequences revealed that the isolates belonged to Alternaria alternata, A. burnsii, A. tomato, Curvularia subpapendorfii, Didymella microchlamydospora, Fusarium brachygibbosum, F. incarnatum-equiseti and Nigrospora lacticolonia. In pathogenicity tests, A. alternata was found to be the most aggressive. F. incarnatum-equiseti was the least aggressive, only inducing symptoms in leaves injured prior to inoculation. The other species caused moderate leaf spot symptoms on the leaves. This study shows that several fungal species belonging to different genera can be found associated with leaf spot symptoms of date palms, and they vary in their aggressiveness on date palm leaves. It is the first study to report C. verruculosa, D. microchlamydospora, and N. lacticolonia as leaf spot pathogens of date palm in the world.
Introduction
Date palm (Phoenix dactylifera L.) is the most important crop in the Arabian Peninsula, where it has been cultivated for thousands of years. It has been the main source of calories for people living in this part of the world (Al-Yahyai and Khan Citation2015; Khierallah et al. Citation2015). Date palm is cultivated for its fruits, which are eaten at the Rutab (ripen) or Tamar (semi-dry) stages. In addition, date palm leaves and trunk had been used in the construction of shelter. Date palm is a strategic crop for many countries in Asia and Africa with a total worldwide production of more than 8 million tonnes every year (FAO Citation2019). Date palm production in Oman exceeds 360,000 tons annually, placing Oman as the 9th producer of dates in the world (FAO Citation2019).
Date palms are susceptible to several diseases and pests that limit dates production. Diseases cause considerable losses in date palms groves. Bayoud is the most serious disease affecting date palm, especially in countries like Algeria and Morocco. This disease is caused by Fusarium oxysporum f.sp. albedinis (El-Modafar Citation2010). The number of date palm trees that have been killed due to the disease is more than 12 million in Morocco and three million in Algeria. Besides F. oxysporum f.sp. albedinis, several studies reported the association of different Fusarium species with date palm diseases. These include F. fujikuroi, the cause of pollen rot of date palm in Iraq (Abedalred et al. Citation2019), F. solani and F. proliferatum, associated with chlorosis and other date palm diseases in Saudi Arabia (Abdalla et al. Citation2000; Saleh et al. Citation2017), F. solani associated with sudden decline of date palms in Pakistan (Ali Maitlo et al. Citation2013) and F. proliferatum associated with date bunch fading in Iran (Mansoori Citation2012). Thielaviopsis paradoxa and T. punctulata have been recorded as causal agents of black scorch or neck bending on date palm (Khairi et al. Citation2010; Al-Sadi et al. Citation2012; Al-Sadi Citation2013; Mirzaee et al. Citation2014; Saeed et al. Citation2016).
Leaf spot diseases in date palms received less attention from researchers. There are several fungi capable of causing leaf spot diseases in date palm, including Nigrospora sphaerica, (Abass et al. Citation2013), and Graphiola phoenicis (Fröhlich et al. Citation1997; Abdul Sattar et al. Citation2012; Khairi Citation2015). A preliminary study in Oman showed that Alternaria is a common leaf spot pathogen in date palm (Al-Nadabi et al. Citation2018).
The main objective of our study was to investigate the main fungal pathogens associated with date palm leaf spot in Oman. Specific objectives include (1) Isolation of fungal species associated with leaf spot disease; (2) molecular characterization of the isolated fungal species; and (3) testing pathogenicity of the isolated fungal species. Findings from this study will improve our knowledge about the most common foliar pathogens of date palms, which will lay the ground for future management strategies.
Materials and methods
Collection of samples
A total of 198 leaf samples were obtained from 198 date palm trees developing leaf spot symptoms during February to March 2018. The survey was conducted in Al-Batinah governorates (Sohar, Musanaa and Suwaiq districts), Al-Sharqiya governorates (Sur and Dama we Taeen) and Al-Dakiliya governorate (Samail and Izki) in Oman. The survey was in 5–10 farms in each district and five samples were collected from each farm. The samples were collected in sterile plastic bags and kept at 4°C. Samples were processed within 48 hr of collection.
Isolation
Leaf samples were washed and cut into 0.5 × 0.5 cm2 pieces, followed by surface sterilization with 1% sodium hypochlorite for one minute. The leaf pieces were transferred after washing and drying into 2.5% potato dextrose agar (PDA). The plates were incubated at 25°C for 10 days. Four replicates were used for each leaf sample. Growing fungal colonies were subcultured onto new plates.
Identification of fungi
Preliminary identification was based on morphological characteristics. Then the isolates were identified using sequences of the internal transcribed spacer region of the ribosomal RNA (ITS rRNA). Further identification of the isolated fungi was based on the use of additional genes specific to different fungal genera as described in relevant literature: glyceraldehyde-3-phosphate dehydrogenase (GAPDH) for Curvularia and Alternaria (Woudenberg et al. Citation2015; Al-Jaradi et al. Citation2018); beta-tubulin (TUB) for Nigrospora (Kee et al. Citation2019); translation elongation factor (TEF) for Curvularia and Nigrospora (Al-Jaradi et al. Citation2018; Kee et al. Citation2019); (ALT) for Alternaria (Woudenberg et al. Citation2015); and partial RPB2 for Alternaria, Fusarium and Didymella (Woudenberg et al. Citation2015; Akram Ahmadpour et al. Citation2017; Moslemi et al. Citation2017; Osorio et al. Citation2017; Senanayake et al. Citation2017). DNA extraction was as described by Al-Sadi (Citation2016) (Table ). Polymerase Chain Reaction (PCR) for each gene was performed using PuRe Taq Ready-to-go PCR beads (GE Healthcare, Buckinghamshire, UK), 1 µl of each primer, 1 µl DNA, and sterile distilled water up to 22 µl (Al-Sadi et al. Citation2012). PCR conditions for the ITS region were as described by Al-Sadi et al. (Citation2011). For the amplification of the other genes, primers EF-728F and EF-986R were used for the TEF region (O’Donnell and Cigelnik Citation1997); BT2A and BT2B were used for the TUB region (Glass and Donaldson Citation1995); gpd1 and gpd2 were used for the GPDH region (Berbee et al. Citation1999); rpb1 and rpb2 were used for the RPB2 region (Woudenberg et al. Citation2013); and alt1 and alt2 were used for the ALT region (Woudenberg et al. Citation2013). The PCR conditions were as described previously (Woudenberg et al. Citation2013; Samson et al. Citation2014; Sarr et al. Citation2014; Raspé et al. Citation2016).
Table 1. Fungal species and their accession numbers.
PCR purification and sequencing were conducted at Macrogen Inc. (Korea). Sequences were compared with reference sequences obtained from the GenBank database. Multiple sequence alignments were generated with MEGA v.7.0.26. Maximum likelihood (ML) analyses were performed using RAxMLGUI v. 1.3 (Silvestro and Michalak Citation2012). The optimal ML tree search was conducted with 1000 separate runs, and the resulting trees were printed with MEGA v.7.0.26.
Pathogenicity test
Pathogenicity test was conducted for eight identified fungal species namely Alternaria burnsii (2356), A. alternata (2029), A. tomato (2372), Curvularia subpapendorfii (2315), Didymella microchlamydospora (2332), Fusarium brachygibbosum (2016), F. incarnatum-equiseti (2397) and Nigrospora lacticolonia (2269). Two sets of date palm seedlings were used: one with injury on the leaves and one without injury. The leaves were injured using sterile scalpel, about 3 mm. Spores obtained from two-week-old cultures of these fungi were inoculated on the injured/ non-injured leaves of three-month-old date palm seedlings using 5 μl of spore suspension (42 spores μl−1) per inoculation site. The spore suspension was prepared using sterile distilled water. Control seedlings were inoculated with sterile distilled water. Five replicate seedlings were used per fungal isolate/treatment. The inoculated seedlings were covered with transparent polyethylene bags for two days post inoculation. This was followed by incubation of the seedlings separately at 22–24°C for 10 days. Symptom development on the inoculated leaves was recorded by determining the % leaf area covered with leaf spot within 10 mm of the inoculation site and after 10 days following inoculation. Re-isolations were conducted from the leaves that developed leaf spot symptoms to confirm infection by the same isolate used for inoculation. The experiment was conducted twice.
Statistical analysis
Data of the area covered with leaf spot was compared among isolates using Tukey’s Studentized range test (SAS, v.8) at P < 0.05.
Results
Survey
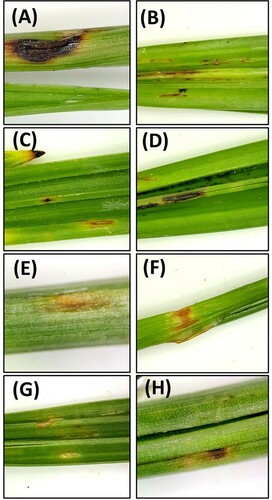
The survey showed that all date palm cultivars suffer from leaf spot symptoms (Figure ). However, the severity of the disease did not exceed 25% of the leaf area in any of the surveyed farms, with most leaf spots covering <5% of the leaf area.
Identification of fungi
Seventy fungal isolates were isolated and initially identified using morphological traits. Sequence analyses showed that our samples belong to five different genera namely Alternaria, Curvularia, Didymella, Fusarium and Nigrospora. The most dominant genus was Alternaria, which was detected in all the surveyed districts (Table ).
Table 2. Frequency of isolation of fungal genera from the seven districts.
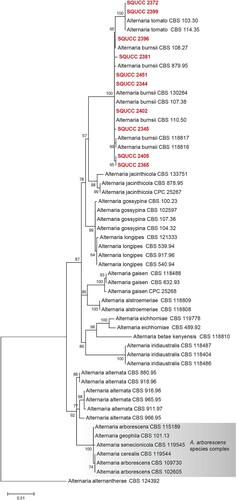
Alternaria species were identified based on the combined sequences of the ITS, RPB2 and Alt a 1 regions (Figure ) using A. alternantherae (CBS 124392) as an outgroup. The alignment comprised 54 strains and the manually adjusted dataset comprised 1751 characters including gaps. Based on the phylogenetic analysis, two isolates (SQUCC 2372, SQUCC 2399) were identified as A. tomato and eight isolates were identified as A. burnsii (SQUCC 2396, SQUCC 2381, SQUCC 2451, SQUCC 2344, SQUCC 2402, SQUCC 2345, SQUCC 2405, SQUCC 2365).
Figure 2. ML tree obtained from the combined ITS, RPB2 and Alt a 1 sequence alignment analysis of the species in section Alternaria. Bootstrap support values above 50% are shown at the nodes and newly generated sequences are in red bold. Alternaria alternantherae (CBS 124392) is used as the outgroup taxon.
Curvularia species were identified based on the combined ITS, TEF and GPDH genes (Figure ), using Bipolaris maydis (CBS 136.29) as an outgroup in the phylogenetic tree. The alignment comprised 98 strains and the manually adjusted dataset comprised 1910 characters including gaps. Based on the phylogenetic analysis two isolates were identified as C. subpapendorfii (SQUCC 2305, SQUCC 2315).
Figure 3. ML tree obtained from the combined ITS, TEF and GPDH sequence alignment analysis of the species of Curvularia. Bootstrap support values above 50% are shown at the nodes and newly generated sequences are in red bold. Bipolaris maydis (CBS 136.29) is used as the outgroup taxon.

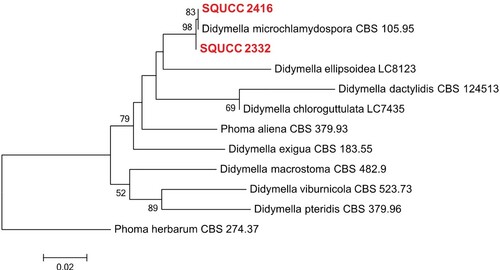
Didymella phylogenetic tree was constructed using the combined ITS and RPB2 sequence data. After the exclusion of ambiguously aligned regions, the final dataset included 12 isolates with an alignment length of 1119 characters (including gaps). Two Didymella isolates from the present study (SQUCC 2416, SQUCC 2332) grouped with D. microchlamydospora (Figure ).
Figure 4. ML tree obtained from the combined ITS and RPB2 sequence alignment analysis of the selected species of Didymella. Bootstrap support values above 50% are shown at the nodes and newly generated sequences are in red bold. Phoma herbarum (CBS 274.37) is used as the outgroup taxon.
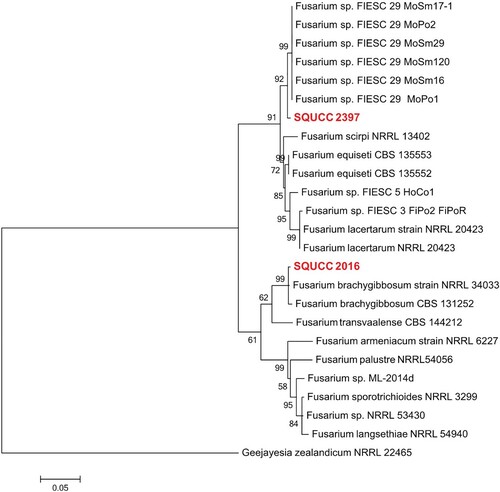
Fusarium isolates were identified based on RPB2 sequence data (Figure ). The aligned dataset consisted of 25 isolates and 873 characters including gaps. In the phylogenetic analysis, one Fusarium isolate (SQUCC 2397) from the present study clustered with species in the Fusarium section incarnatum-equiseti. The Fusarium isolate SQUCC 2016 group with F. brachygibbosum isolates with a high bootstrap support (ML: 99%).
Figure 5. Phylogram generated from ML analysis based on RPB2 sequence data of species in Fusarium sections incarnatum-equiseti and sambucinum species complexes. Isolates derived from this study are in red bold. RAxML bootstrap support values above 50 are given at the nodes. The tree is rooted with Geejayesia zealandicum (NRRL 22465).
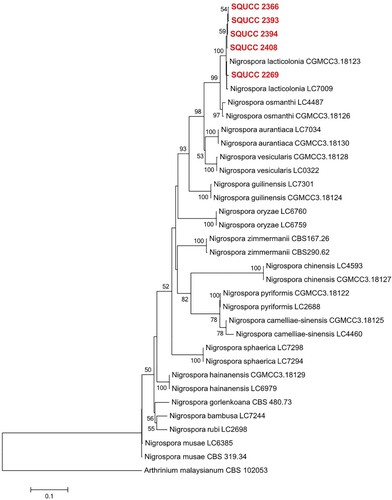
Sequences of 34 isolates of Nigrospora were included in the combined ITS, TEF and TUB phylogram (Figure ). The aligned data matrix consisted of 1461 characters (including gaps). In the phylogenetic tree, two GenBank isolates of Nigrospora lacticolonia (CGMCC 3.18123, LC7009), including the five newly generated sequences (SQUCC 2366, SQUCC 2393, SQUCC 2394, SQUCC 2408, SQUCC 2269) clustered in a clade with a strong bootstrap support (ML: 100%).
Pathogenicity test
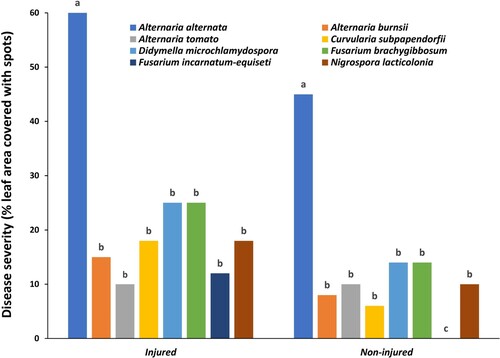
Inoculation of date palm leaves with the eight fungal isolates (Alternaria burnsii, A. alternata, A. tomato, Curvularia subpapendorfii, Didymella microchlamydospora, Fusarium brachygibbosum, F. incarnatum-equiseti and Nigrospora lacticolonia) resulted in the production of leaf spot symptoms in all leaves injured before inoculation (Figures and ). On the other hand, except for F. incarnatum-equiseti, the other seven fungal isolates induced leaf spots in the non-injured leaves (Figure ). Severity of the leaf spot symptoms varied from one isolate to the other, with A. alternata being significantly (P < 0.05) the most aggressive. The spots produced by A. alternata covered 60% and 45% of the leaf area within 10 days of inoculation in the injured and non-injured leaves, respectively. The spots started small and light brown, which enlarged with time and became dark brown to black in color (Figure ). No clear differences were observed in the color of the symptoms caused by the different fungal species. F. incarnatum-equiseti was the least aggressive, which caused no infections in the non-injured leaves (Figure ).
Discussion
Date palm is affected by different fungal pathogens. Most studies in the past focused on wilt and root-associated diseases, with less attention been given to foliar pathogens (El-Modafar Citation2010; Al-Sadi et al. Citation2012; Khierallah et al. Citation2015). Isolations from date palm leaves showed that Alternaria is the most dominant genus. A. alternata, A. burnsii and A. tomato proved to be pathogenic on date palm leaves. This agrees with previous studies that indicated the prevalence of this genus and its pathogenicity on date palm leaves (Al-Nadabi et al. Citation2018).
The genus Curvularia is common on grasses and many other crops (Manamgoda et al. Citation2012; Ghosh et al. Citation2018; Jayawardena et al. Citation2019; Khiralla et al. Citation2019; Qostal et al. Citation2019). Curvularia verruculosa has been reported as a causal agent of leaf spot or blight diseases on Cynodon sp. in China (Huang et al. Citation2005), cotton in India (Shirsath et al. Citation2018), Zoysia japonica in Japan (Choi et al. Citation2018), and Eleusine indica in Thailand (Marin-Felix et al. Citation2017). This is the first report of association of C. verruculosa with leaf spot disease on date palms.
Didymella microchlamydospora belongs to the family Didymellaceae (de Gruyter et al. Citation2013). It used to be formerly known as Phoma microchlamydospora, where it has been described from Eucalyptus sp. leaves and an unknown vegetable crop (Aveskamp et al. Citation2009). D. microchlamydospora was detected in two areas in Oman and was found to result in leaf spot symptoms in date palms. It has been previously reported as a pathogen of Morus nigra in Iran (Akram Ahmadpour et al. Citation2017). This is the first report of D. microchlamydospora causing leaf spot on date palms.
Species of Nigrospora are a cosmopolitan in their distribution and frequently occur as plant pathogens, endophytes or saprobes (Cui et al. Citation2018; Raza et al. Citation2019). Several species of Nigrospora have been reported as leaf spot-inducing fungi, including N. sphaerica in cladode in Brazil (Conforto et al. Citation2019), date palms (Alam et al. Citation2019) and mandarin (Alam et al. Citation2017a) in Pakistan, and mulberry in India (Arunakumar et al. Citation2019), and N. oryzae on watermelon, Kiwifruit and lotus in China (Alam et al. Citation2017b; Li et al. Citation2018; Zhang et al. Citation2018) and Aloe in Bangladesh and Pakistan (Alam et al. Citation2017b; Begum et al. Citation2018). N. lacticolonia was first described by Wang et al. (Citation2017) on Camellia sinensis and Musa paradisiaca from China. N. lacticolonia has been reported as the causal agent of reddish brown spot disease of red-fleshed dragon fruit in Malaysia (Kee et al. Citation2019). Our study is the first to demonstrate that N. lacticolonia is a foliar pathogen of date palm.
Our findings showed that Fusarium brachygibbosum resulted in limited leaf spot symptoms on date palms, while F. incarnatum-equiseti could not cause symptoms on the non-injured leaves. F. brachygibbosum has been reported as a pathogen associated with marijuana wilt in California (Punja et al. Citation2018), sugar beet root rot in China (Cao et al. Citation2018), crown rot of wheat in Turkey (Shikur Gebremariam et al. Citation2018) and maize stalk rot in China (Shan et al. Citation2017). F. incarnatum-equiseti is generally a weak fungal species (Al-Sadi et al. Citation2012).
Fungal pathogens isolated from date palm resulted in almost similar leaf spot symptoms. Symptoms started light brown, then turned darker with time. Brown/black leaf spot symptoms usually develop in response to infection by necrotrophic pathogens (Sabburg et al. Citation2015; Zhu et al. Citation2018; Al-Daoude et al. Citation2019), which is the case with the pathogens isolated from date palms.
Characterization based on morphological characteristics and molecular techniques helped identify fungal isolates from leaf spot disease of date palm to the species level. The study showed the association of Alternaria burnsii, A. alternata, A. tomato, Curvularia verruculosa, Didymella microchlamydospora, Fusarium brachygibbosum, and Nigrospora lacticolonia with leaf spot on date palm. It is the first study to report C. verruculosa, D. microchlamydospora, and Nigrospora lacticolonia as leaf spot pathogens of date palm. Future studies should focus on investigating potential management options for these pathogens.
Compliance with ethical standards
Ethical approval
This article is original and not published elsewhere. The authors confirm that there are no ethical issues in the publication of the manuscript.
Acknowledgements
Thanks to Sultan Qaboos University, OAPGRC and VALE Oman for financial support of the study.
AMA designed the study, HA, ZA and AA conducted lab experiments, AMA and RV supervised the research project, AMA, HA, SM and RV wrote the manuscript, all authors approved the final version.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
Additional information
Funding
References
- Abass MH, Hameed MA, Ahmed AN. 2013. First report of Nigrospora sphaerica (Sacc.) Mason as a potential pathogen on date palm (Phoenix dactylifera L.). Can J Plant Pathol. 35:75–80. doi: https://doi.org/10.1080/07060661.2012.732612
- Abdalla MY, Al-Rokibah A, Moretti A, Mulè G. 2000. Pathogenicity of toxigenic Fusarium proliferatum from date palm in Saudi Arabia. Plant Dis. 84:321–324. doi: https://doi.org/10.1094/PDIS.2000.84.3.321
- Abdul Sattar MH, Rashid Yassin Ibrahim A, Aulaqi WA. 2012. Occurrence of false smut on date palm (Phoenix dactylifera L.) in the southern coastal plains of Yemen. J Appl Hortic. 14:144–145. doi: https://doi.org/10.37855/jah.2012.v14i02.27
- Abedalred EM, Ismail WMH, Abdulmoohsin RG, Al-Karhi MAJ. 2019. First molecular identification of Fusarium fujikuroi causing pollen rot of palm trees (Phoenix dactylifera L.) in Iraq and evaluation efficacy of some nanoparticles against it. IOP Conference Series: Earth and Environmental Science; 2019; Kerbala, Iraq.
- Akram Ahmadpour S, Farokhinejad R, Mehrabi-Koushki M. 2017. Further characterization and pathogenicity of Didymella microchlamydospora causing stem necrosis of Morus nigra in Iran. Mycosphere. 8:835–852. doi: https://doi.org/10.5943/mycosphere/8/7/3
- Al-Daoude A, Al-Shehadah E, Shoaib A, Jawhar M, Arabi MIE. 2019. Salicylic acid pathway changes in barley plants challenged with either a biotrophic or a necrotrophic pathogen. Cereal Res Commun. 47:324–333. doi: https://doi.org/10.1556/0806.47.2019.04
- Al-Jaradi A, Al-Mahmooli I, Janke R, Maharachchikumbura S, Al-Saady N, Al-Sadi AM. 2018. Isolation and identification of pathogenic fungi and oomycetes associated with beans and cowpea root diseases in Oman. PeerJ. 2018:e6064. doi: https://doi.org/10.7717/peerj.6064
- Al-Nadabi HH, Maharachchikumbura SSN, Agrama H, Al-Azri M, Nasehi A, Al-Sadi AM. 2018. Molecular characterization and pathogenicity of Alternaria species on wheat and date palms in Oman. Eur J Plant Pathol. 152:577–588. doi: https://doi.org/10.1007/s10658-018-1550-4
- Al-Sadi AM. 2013. Phylogenetic and population genetic analysis of Ceratocystis radicicola infecting date palms. J Plant Pathol. 95:49–57.
- Al-Sadi AM. 2016. Variation in resistance to spot blotch and the aggressiveness of Bipolaris sorokiniana on barley and wheat cultivars. J Plant Pathol. 98:97–103.
- Al-Sadi AM, Al-Jabri AH, Al-Mazroui SS, Al-Mahmooli IH. 2012. Characterization and pathogenicity of fungi and oomycetes associated with root diseases of date palms in Oman. Crop Prot. 37:1–6. doi: https://doi.org/10.1016/j.cropro.2012.02.011
- Al-Sadi AM, Al-Said FA, Al-Jabri AH, Al-Mahmooli IH, Al-Hinai AH, de Cock AWAM. 2011. Occurrence and characterization of fungi and oomycetes transmitted via potting mixtures and organic manures. Crop Prot. 30:38–44.
- Al-Yahyai R, Khan MM. 2015. Date palm status and perspective in Oman. In: Al-Khayri JM, Jain SM, Johnson DV, editors. Date palm Genetic Resources and Utilization. Vol. 2. Dordrecht, The Netherlands, Asia and Europe: Springer; p. 207–240.
- Alam MW, Rehman A, Ahmad S, Sarwar M, Nawaz A, Khan SM, Ali S, Aslam S, Mannan A. 2019. First report of Nigrospora sphaerica causing leaf spot of date palm in Pakistan. J Plant Pathol. 102:223.
- Alam MW, Rehman A, Gleason ML, Riaz K, Saira M, Aslam S, Rosli H, Muhammad S. 2017a. First report of Nigrospora sphaerica causing leaf spot of Kinnow mandarin in Pakistan. J Plant Pathol. 99:295.
- Alam MW, Rehman A, Saira M, Kan NA, Aslam S, Fiaz M, Muhammad S. 2017b. First report of leaf spots in Aloe vera caused by Nigrospora oryzae in Pakistan. Plant Dis. 101:841.
- Ali Maitlo W, Markhand GS, Abul-Soad AA, Lodhi AM, Jatoi MA. 2013. Chemical control of sudden decline disease of date palm (Phoenix dactylifera L.) in Sindh, Pakistan. Pak J Bot. 45:7–11.
- Arunakumar GS, Gnanesh BN, Supriya M, Sivaprasad V. 2019. First report of Nigrospora sphaerica causing shot hole disease on mulberry in India. Plant Dis. 103:1783.
- Aveskamp MM, Verkley GJM, De Gruyter J, Murace MA, Perelló A, Woudenberg JHC, Groenewald JZ, Crous PW. 2009. DNA phylogeny reveals polyphyly of Phoma section Peyronellaea and multiple taxonomic novelties. Mycologia. 101:363–382.
- Begum M, Hamza A, Tanny T, Das KC, Mahmud MT, Salimullah M, Alam I. 2018. First report of leaf spot disease in Aloe vera caused by Nigrospora oryzae in Bangladesh. Plant Dis. 102:1461.
- Berbee ML, Pirseyedi M, Hubbard S. 1999. Cochliobolus phylogenetics and the origin of known, highly virulent pathogens, inferred from ITS and glyceraldehyde-3-phosphate dehydrogenase gene sequences. Mycologia. 91:964–977.
- Cao S, Yang N, Zhao C, Liu J, Han C, Wu X. 2018. Diversity of Fusarium species associated with root rot of sugar beet in China. J Gen Plant Pathol. 84:321–329.
- Choi YJ, Lee JS, Lee HB, Kim HJ. 2018. First report of leaf blight caused by Curvularia verruculosa on zoysiagrass (Zoysia japonica) in Korea. Plant Dis. 102:1173.
- Conforto C, Lima NB, Silva FJA, Câmara MPS, Maharachchikumbura S, Michereff SJ. 2019. Characterization of fungal species associated with cladode brown spot on Nopalea cochenillifera in Brazil. Eur J Plant Pathol. 155:1179–1194.
- Cui YP, Wu B, Peng AT, Li ZL, Lin JF, Song XB. 2018. First report of nigrospora leaf blight on sugarcane caused by Nigrospora sphaerica in China. Plant Dis. 102:824.
- de Gruyter H, Woudenberg JHC, Aveskamp MM, Verkley GJM, Groenewald JZ, Crous PW. 2013. Redisposition of Phoma-like anamorphs in pleosporales. Stud Mycol. 75:1–36.
- El-Modafar C. 2010. Mechanisms of date palm resistance to Bayoud disease: current state of knowledge and research prospects. Physiol Mol Plant Pathol. 74:287–294.
- FAOSTAT. 2019. 2019 ed.: FAO. Available from http://www.fao.org/faostat/en/#data/QC/visualize.
- Fröhlich J, Hyde KD, Guest DI. 1997. Fungi associated with leaf spots of palms in north Queensland, Australia. Mycol Res. 101:721–732.
- Ghosh T, Biswas MK, Guin C, Roy P, Aikat K. 2018. A review on seed borne mycoflora associated with different cereal crop seeds and their management. Plant Cell Biotechnol Mol Biol. 19:107–117.
- Glass N, Donaldson G. 1995. Development of premier sets designed for use with the PCR to amplify conserved genes from filamentous Ascomycetes. Appl Environ Microbiol. 61:1323–1330.
- Huang J, Zheng L, Hsiang T. 2005. First report of leaf spot caused by Curvularia verruculosa on Cynodon sp. in Hubei, China. Plant Pathol. 54:253.
- Jayawardena RS, Hyde KD, Jeewon R, Ghobad-Nejhad M, Wanasinghe DN, Liu NG, Phillips AJL, Oliveira-Filho JRC, da Silva GA, Gibertoni TB, et al. 2019. One stop shop II: taxonomic update with molecular phylogeny for important phytopathogenic genera: 26–50 (2019). Fungal Divers. 94:41–129.
- Kee YJ, Hafifi ABM, Huda-Shakirah AR, Wong KL, Jin XL, Siti Nordahliawate MS, Zakaria L, Mohd MH. 2019. First report of reddish brown spot disease of red-fleshed dragon fruit (Hylocereus polyrhizus) caused by Nigrospora lacticolonia and Nigrospora sphaerica in Malaysia. Crop Prot. 122:165–170.
- Khairi MMA. 2015. Date palm status and perspective in Sudan. In: Al-Khayri JM, Jain SM, Johnson DV, editors. Date palm genetic resources and utilization. Vol. 1. Dordrecht, The Netherlands, Africa and the Americas: Springer; p. 169–191.
- Khairi MMA, Elhassan MI, Bashab FA. 2010. The status of date palm cultivation and date production in Sudan. Acta Hortic: International Society for Horticultural Science. p. 37–42.
- Khierallah HSM, Bader SM, Ibrahim KM, Al-Jboory IJ. 2015. Date palm status and perspective in Iraq. In: Al-Khayri JM, Jain SM, Johnson DV, editors. Date palm genetic resources and utilization. Vol. 2. Dordrecht, The Netherlands, Asia and Europe: Springer; p. 97–152.
- Khiralla A, Spina R, Saliba S, Laurain-Mattar D. 2019. Diversity of natural products of the genera Curvularia and Bipolaris. Fungal Biol Rev. 33:101–122.
- Li L, Pan H, Chen MY, Zhang SJ, Zhong CH. 2018. First report of Nigrospora oryzae causing brown/black spot disease of kiwifruit in China. Plant Dis. 102:243.
- Manamgoda DS, Cai L, McKenzie EHC, Crous PW, Madrid H, Chukeatirote E, Shivas RG, Tan YP, Hyde KD. 2012. A phylogenetic and taxonomic re-evaluation of the Bipolaris - Cochliobolus - Curvularia Complex. Fungal Divers. 56:131–144.
- Mansoori B. 2012. Fusarium proliferatum induces gum in xylem vessels as the cause of date bunch fading in Iran. J Agr Sc Tech. 14:1133–1140.
- Marin-Felix Y, Senwanna C, Cheewangkoon R, Crous PW. 2017. New species and records of Bipolaris and Curvularia from Thailand. Mycosphere. 8:11.
- Mirzaee MR, Tajali H, Javadmosavi SA. 2014. Thielaviopsis paradoxa causing neck bending disease of date palm in Iran. J Plant Pathol. 96:S4.122.
- Moslemi A, Ades PK, Groom T, Nicolas ME, Taylor PWJ. 2017. Alternaria infectoria and Stemphylium herbarum, two new pathogens of pyrethrum (Tanacetum cinerariifolium) in Australia. Australas Plant Pathol. 46:91–101.
- O’Donnell K, Cigelnik E. 1997. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol Phylogenet Evol. 7:103–116.
- Osorio JA, Crous CJ, de Beer ZW, Wingfield MJ, Roux J. 2017. Endophytic Botryosphaeriaceae, including five new species, associated with mangrove trees in South Africa. Fungal Biol. 121:361–393.
- Punja ZK, Scott C, Chen S. 2018. Root and crown rot pathogens causing wilt symptoms on field-grown marijuana (Cannabis sativa L.) plants. Can J Plant Pathol. 40:528–541.
- Qostal S, Kribel S, Chliyeh M, Serghat S, KarimaSelmaoui AOT, Zaarati H, Benkirane R, Douira A. 2019. Study of the fungal complex responsible for root rot of wheat and barley in the north-west of Morocco. Plant Archives. 19:2143–2157.
- Raspé O, Vadthanarat S, De Kesel A, Degreef J, Hyde KD, Lumyong S. 2016. Pulveroboletus fragrans, a new Boletaceae species from Northern Thailand, with a remarkable aromatic odor. Mycol Prog. 15:38.
- Raza M, Zhang ZF, Hyde KD, Diao YZ, Cai L. 2019. Culturable plant pathogenic fungi associated with sugarcane in southern China. Fungal Divers. 99:1–104.
- Sabburg R, Obanor F, Aitken E, Chakraborty S. 2015. Changing fitness of a necrotrophic plant pathogen under increasing temperature. Glob Change Biol. 21:3126–3137.
- Saeed EE, Sham A, El-Tarabily K, Elsamen FA, Iratni R, Abuqamar SF. 2016. Chemical control of black scorch disease on date palm caused by the fungal pathogen Thielaviopsis punctulata in United Arab Emirates. Plant Dis. 100:2370–2376.
- Saleh AA, Sharafaddin AH, El-Komy MH, Ibrahim YE, Hamad YK, Molan YY. 2017. Fusarium species associated with date palm in Saudi Arabia. Eur J Plant Pathol. 148:367–377.
- Samson RA, Visagie CM, Houbraken J, Hong S-B, Hubka V, Klaasen CHW, Perrone G, Seifert KA, Susca A, Tanney JB, et al. 2014. Phylogeny, identification and nomenclature of the genus Aspergillus. Stud Mycol. 78:141–173.
- Sarr MP, Ndiaye M, Groenewald JZ, Crous PW. 2014. Genetic diversity in Macrophomina phaseolina, the causal agent of charcoal rot. Phytopathol Mediterr. 53:250–268.
- Senanayake IC, Maharachchikumbura SSN, Jeewon R, Promputtha I, Al-Sadi AM, Camporesi E, Hyde KD. 2017. Morphophylogenetic study of Sydowiellaceae reveals several new genera. Mycosphere. 8:15.
- Shan LY, Cui WY, Zhang DD, Zhang J, Ma NN, Bao YM, Dai XF, Guo W. 2017. First report of Fusarium brachygibbosum causing maize stalk rot in China. Plant Dis. 101:837.
- Shikur Gebremariam E, Sharma-Poudyal D, Paulitz TC, Erginbas-Orakci G, Karakaya A, Dababat AA. 2018. Identity and pathogenicity of Fusarium species associated with crown rot on wheat (Triticum spp.) in Turkey. Eur J Plant Pathol. 150:387–399.
- Shirsath LP, Patil SP, Patil UK. 2018. Incidence of leaf spot disease on cotton caused by Curvularia verruculosa and role of its hydrolytic enzymes in pathogenesis. Physiol Mol Biol Pla. 24:711–714.
- Silvestro D, Michalak I. 2012. RaxmlGUI: a graphical front-end for RAxML. Org Divers Evol. 12:335–337.
- Wang M, Liu F, Crous PW, Cai L. 2017. Phylogenetic reassessment of Nigrospora: ubiquitous endophytes, plant and human pathogens. Persoonia: Mol Phyl Evol Fungi. 39:118–142.
- Woudenberg JHC, Groenewald JZ, Binder M, Crous PW. 2013. Alternaria redefined. Stud Mycol. 75:171–212.
- Woudenberg JHC, Seidl MF, Groenewald JZ, de Vries M, Stielow JB, Thomma BPHJ, Crous PW. 2015. Alternaria section Alternaria: species, formae speciales or pathotypes? Stud Mycol. 82:1–21.
- Zhang QH, Huang LL, Liu YJ, Ai Y, Peng DH. 2018. First report of leaf spot of lotus (Nelumbo nucifera) caused by Nigrospora oryzae in China. Plant Dis. 102:1038.
- Zhu X, Zhao J, Abbas HMK, Liu Y, Cheng M, Huang J, Cheng W, Wang B, Bai C, Wang G, et al. 2018. Pyramiding of nine transgenes in maize generates high-level resistance against necrotrophic maize pathogens. Theor Appl Genet. 131:2145–2156.