ABSTRACT
In this study, an entomopathogenic fungus was isolated from whitefly (Bemisia tabaci) cadaver samples collected from eggplant fields in Barka, Oman. The fungus was identified as Cordyceps javanica based on sequence analysis of ribosomal DNA internal transcribed spacer (ITS) regions. Under in vitro conditions, the fungus was able to grow between 20°C and 30°C, with a maximum growth at 30°C. No growth occurred at 35°C. Studies on the efficacy of different concentrations of conidia of C. javanica against whitefly nymphs revealed that the mortality rate increased with the increase in concentrations of inoculum. About 95.6 ± 2.1% corrected mortality was recorded at a concentration of 107 conidia ml−1 after 7 days of treatment. The culture filtrate of C. javanica exhibited toxicity against whitefly nymphs and recorded 61.3 ± 9.5% corrected mortality after 48 h of treatment. There was no significant difference between the mortality caused by conidial suspension and culture filtrate. Gas chromatography-mass spectrometry (GC-MS) analysis revealed the presence of 17 compounds in the culture filtrate of C. javanica. Lauric acid was present in the largest amount (28.7%) and the long chain (C22-25) hydrocarbons contributed 38.3%. This is the first report on the isolation and characterization of C. javanica from B. tabaci from Oman.
1. Introduction
Whitefly, Bemisia tabaci (Genn.), has more than 600 host plant species worldwide and cause economic damage to many crops (De Barro et al. Citation2011; Queiroz et al. Citation2017; Misaka et al. Citation2020; Sani et al. Citation2020). It is an important pest of tomato, eggplant, cucumber and melon in Oman (Al-Shehi and Khan Citation2013; Shah and Scott Citation2020). Bemisia tabaci can cause damage to crop plants directly or indirectly. The direct damage is through feeding on plant sap, which causes withering of leaves and reduction in the growth and yield. Indirect damage is due to secretion of honeydew, which promotes the growth of sooty mold (Capnodium spp.). As a result, the rate of photosynthesis and the quality and quantity of produce are affected. In addition, B. tabaci can transmit about 200 plant disease virus species including Tomato Yellow Leaf Curl Virus (TYLCV) in tomato (Sani et al. Citation2020). Since TYLCV is a serious problem on tomato, insecticides are used more frequently for control of B. tabaci in a single cropping season. Consequently, B. tabaci has developed resistance to 64 active ingredients worldwide (www.pesticideresistance.org). In Oman, resistance of B. tabaci to organophosphates and pyrethroids has been reported (Shah and Scott Citation2020).
Biological control method is widely adopted for the control of whitefly in order to avoid environmental pollution and development of resistance in the insect populations that are generally associated with the use of synthetic chemical pesticides (Faria and Wraight Citation2001; Sani et al. Citation2020). Several entomopathogenic fungi, including Beauveria bassiana (Xia et al. Citation2013), Verticillium lecanii (Xie et al. Citation2019), Metarhizium anisopliae (Norhelina et al. Citation2013), Isaria fumosorosea (Paecilomyces fumosoroseus) (Mascarin et al. Citation2013), Lecanicillium muscarium (Ali et al. Citation2017) and Cordyceps javanica (Xie et al. Citation2016), have been reported to be efficacious against B. tabaci. The toxic metabolites produced by V. lecanii (Gindin et al. Citation1994; Wang et al. Citation2007) and Metarhizium anisopliae (Zhang et al. Citation2017) have been reported to play an important role in the mortality of B. tabaci. Few commercial biocontrol formulations based on entomopathogenic fungal species including Botanigard (B. bassiana), PreFeRal (P. fumosoroseus) and Mycotal (V. lecanii) are available in the market for the control of whitefly (Xia et al. Citation2013). Entomopathogenic fungi, if available, can be a significant component of any integrated pest management program. They are efficacious, inexpensive and have broad-spectrum activity. Furthermore, they are safe for humans and many non-target organisms (Lacey et al. Citation2009). In this study, a local C. javanica strain SQUVR-A1 was isolated from infected whitefly (B. tabaci) adults, and the pathogenicity of the fungus conidia and toxicity of its culture filtrate against B. tabaci 2nd instar nymphs was evaluated under laboratory conditions. Further, the metabolites of C. javanica were identified using gas chromatography-mass spectrometry (GC-MS).
2. Materials and methods
2.1. Collection of infected whiteflies
Whitefly cadaver adults (Figure ) were collected from field-grown eggplants from Barka (23°41′47.1″N 57°53′16.0″E), Al-Batinah, Oman, during January 2019. The temperature at the time of collection of samples was in the range of 23–27°C.
2.2. Isolation of fungus
The white cottony fungal mycelium from the infected B. tabaci adults was transferred to potato dextrose agar (PDA) (Oxoid Ltd., UK) under aseptic conditions. The plates were incubated at 27°C for 3–5 days. Hyphal tip culture method was used for obtaining pure culture of the fungus.
2.3. Molecular identification
Mycelium was harvested from 7-day-old PDA plate culture under aseptic condition. About 80 mg of mycelium was used for DNA extraction following the procedure of Lee and Taylor (Citation1990). PCR was performed using a puReTaq Ready-to-Go PCR bead (GE Healthcare, UK) and the universal ITS4 and ITS5 primers (White et al. Citation1990). The reaction mixture and conditions were as per Al-Rashdi et al. (Citation2020). Five µl of PCR amplified product was analyzed in 1.2% agarose gel in TBE buffer. The electrophoresis was carried out at a constant voltage of 80 V for 45 min and visualized and documented using a gel documentation system. A 1-kb DNA ladder (Fermentas) was used as a maker. Sequencing was carried out at Macrogen Inc. (Seoul, Korea). The fungal sequences were compared with sequences of reference fungal species using BLAST search (www.ncbi.nlm.nih.gov).
2.4. Effect of temperature on growth of C. javanica
The experiments were conducted in 250 ml conical flasks containing 100 ml of potato dextrose broth medium. Each flask was inoculated with 100 µl of spore suspension (4 × 106 conidia ml−1) of C. javanica and incubated at 20°C, 25°C, 30°C, 35°C, and 40°C under static conditions for 10 days. After incubation, the mycelia were harvested by filtering through pre-weighed Whatman No. 1 filter paper and the mycelial dry weight was measured after 24 h of drying at 65°C. Three flasks were kept for each temperature.
2.5. Efficacy of conidial suspension and culture filtrate of C. javanica against B. tabaci nymphs
2.5.1. Bioassay of C. javanica conidial suspension against B. tabaci nymphs
Bemisia tabaci-infected eggplant leaves were collected from Agricultural Experiment Station, Sultan Qaboos University. The leaves were trimmed and placed with abaxial surface up in a Petri dish (90-mm diameter) containing 1.5% agarose. Under a dissecting microscope, third instar nymphs and pupae were removed from the leaves using a needle and the number of second instar nymphs per leaf was recorded before spray. The second instar nymphs were identified using a pictorial guide (Naranjo and Ellsworth Citation2017). Three different concentrations (105, 106, 107 conidia ml−1) of conidia were prepared in sterile water. No adjuvant was used. After vigorous shaking, 1 ml of the conidial suspension was sprayed over the leaves containing nymphs using a Potter spray tower (Burkard Scientific, UK) at 10 PSI. In control, the leaf discs were sprayed with distilled water. Three replicated leaf discs with nymphs were used for each treatment (concentration). The plates were kept at room temperature (25 ± 2°C). Mortality of nymphs was observed after 4 and 7 days of treatment.
2.5.2. Bioassay of culture filtrate of C. javanica against B. tabaci nymphs
2.5.2.1. Preparation of fungal culture filtrate
The fungus was grown for 2 weeks at room temperature (25 ± 2°C) in 500 ml conical flask containing 200 ml of synthetic Czapek Dox broth (HiMedia, India). The culture filtrate was obtained by filtering the fungal culture through two layers of sterile cheesecloth.
2.5.2.2. Bioassay
Leaves of eggplant containing second instar B. tabaci nymphs were trimmed and placed upside down in Petri plates (90-mm diameter) containing 1.5% agarose. One ml of fungal culture filtrate, after vigorous shaking, was sprayed on the nymphs using a Potter spray tower as described above. Control plates consisted of nymphs sprayed with distilled water. The treated leaves were air dried for 1 hour. A single layer of Whatman No.1 filter paper was placed in such a manner that the surface of leaves with nymphs was in contact with the filter paper, and plates were kept at room temperature (25 ± 2°C). After different times of incubation (1, 24 and 48 h), the leaves were removed and the filter papers were sprayed with 1 ml of 5% iodine solution using a Potter spray tower. The number of blue coloured spots developed on the filter paper upon treatment with iodine indicated the presence of live nymphs on the corresponding leaf (Azam et al. Citation2002). The mortality of nymphs was recorded 1, 24 and 48 h after treatment.
2.6. Analysis of metabolites in C. javanica culture filtrate
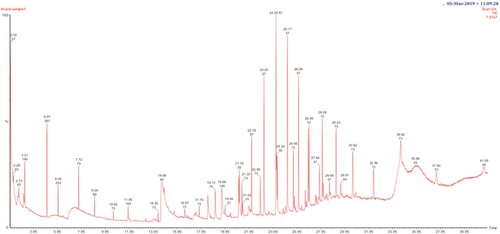
The fungal culture filtrate obtained in the previous step was extracted with ethyl acetate (1:1, v/v) twice by using a separating funnel. The solvent fractions were pooled, dried under vacuum at 50°C using a Buchi rotavapor R-215. The residue was weighed, dissolved in spectrophotometric grade methanol and analyzed by GC-MS. A PerkinElmer – Clarus 600 GC/MS, fitted with a Rtx-5MS capillary column (30 m × 0.25 mm i.d. × 0.25 µm film thickness) and quadrupole mass analyzer was used for the identification of compounds in metabolites. Flow rate of Helium gas (grade 6.0) was set at 1.0 ml/min. One µl of the sample with a split ratio of 10:1 was injected into the column. The temperatures of injection port, transfer line and ion source were 280, 270 and 270°C, respectively. The ionization electron energy was 70 eV. Electron multiplier (EM) voltage was obtained from standard autotune. The mass spectral scan range was set at 33–550 amu. The oven temperature started with 60°C, then increased at 8°C/min till 280°C. The total run time was 53.5 min. The mass spectrum libraries viz., NIST v.2.3 and Wiley 9th edition were used to identify the compounds.
2.7. Data analysis
Data on mycelial growth and efficacy of C. javanica conidial suspension at different concentrations were analyzed separately by one-way ANOVA while data on culture filtrate with three observation intervals were analyzed by two-way ANOVA, and Tukey’s post-hoc test in PAleontological STatistics (PAST) software Version 4.02 (https://folk.uio.no/ohammer/past/). Percent mortalities were corrected with Abbott formula (Abbott Citation1925).
3. Results and discussion
In this study, the internal transcribed spacer (ITS) regions of ribosomal DNA (rDNA) were amplified from the DNA extracted from the fungus isolated from the dead B. tabaci adults. The BLASTN analysis of the obtained nucleotide sequences showed 100% similarity with different isolates of C. javanica (accession numbers MH854836, MG742216, KM234218, JN204422) in the NCBI GenBank database. Based on the ITS sequence similarity the fungus isolated from B. tabaci was identified as C. javanica (Bally) Kepler, B. Shrestha & Spatafora (Ascomycota, Sordariomycetes, Hypocreales, Cordycipitaceae). The nucleotide sequence of C. javanica isolate SQUVR-A1 was deposited in the GenBank (accession number MK503773). Several strains of C. javanica (Syn = Spicaria javanica/ Paecilomyces javanica/Isaria javanica) have been described as a potential entomopathogenic fungus against many agricultural insect pests (Hu et al. Citation2007). Cordyceps javanica isolates have been used against Asian citrus psyllid (Ou et al. Citation2019b), green peach aphid (Myzus persicae) (Kang et al. Citation2018), Thrips palmi (Park et al. Citation2018) and B. tabaci (Zhu and Kim Citation2011). The potential of C. javanica to control both the insect pests and plant pathogens has also been reported. For instance, Kang et al. (Citation2018) demonstrated that I. javanica pf185 had aphicidal activity against Myzus persicae and Aphis gossypii and antioomycete activity against Pythium ultimum. Ou et al. (Citation2019a) reported that combined application of C. javanica and the whitefly parasitoid, Eretmocerus hayati, was more effective in the biological control of B. tabaci than using either of them individually. This is the first report on isolation and characterization of entomopathogenic fungus C. javanica from B. tabaci from Oman.
The growth response of C. javanica to changes in temperature was studied under in vitro conditions. The fungus showed mycelial growth between 20°C and 30°C, but failed to grow at 35°C (Table ). The maximum mycelial growth was recorded at 30°C which was significantly (F3,12 = 84.3, p < 0.001) higher than the growth at 20°C but not at 25°C. The lowest growth was recorded at 20°C. A strong positive correlation (r = 0.987) was observed between the temperature and mycelial weight of C. javanica. Shimazu and Takatsuka (Citation2010) reported that Isaria javanica isolated from gypsy moth (Lymantria dispar) was able to grow at 10–30°C, with optimal growth at 25°C. At 30°C, the fungal growth was disrupted and deformed and at 32.5°C, no visible growth was recorded. The strain of C. javanica isolated in the present study seems to be tolerant to higher temperature (30°C), which makes it an ideal candidate for the biocontrol of whitefly in controlled environment agriculture (CEA).
Table 1. Effects of temperature on in vitro growth of Cordyceps javanica after 10 days of incubation.
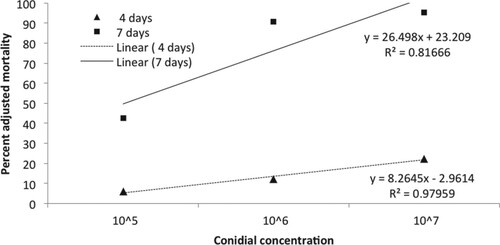
Studies on the efficacy of different concentrations of conidia of C. javanica (105 ml−1–107 ml−1) on mortality of second instar B. tabaci nymphs indicated that the corrected mortality rate (adjusted with Abbott’s formulae for control) (Abbott Citation1925) after 7 days of treatment significantly (F3,8 = 146.0, p < 0.001) increased with increase in the concentration of conidia (Figure ). At the highest tested concentration (107 conidia ml−1) 95.6 ± 2.1% mortality of B. tabaci nymphs was recorded after 7 days of treatment. The highest corrected mortality, after 4 days, with the highest concentration was 22.5%. Sain et al. (Citation2019) reported that the conidial concentration of C. javanica strains at 107 ml−1 caused 77.6–81.1% mortality in second to third instars of B. tabaci nymphs. An LC50 value of 107 spores ml−1 after 4 days of inoculation has been reported against nymphs of the green peach aphid (Myzus persicae) (Kang et al. Citation2018). The positive correlations between spore concentrations and time after treatment in this study indicated that prolonged exposure and high inoculum densities of C. javanica are required for effective insect pests control.
Figure 2. Effect of C. javanica spore concentrations on mortality of second instar B. tabaci nymphs after 4 and 7 days of treatment.
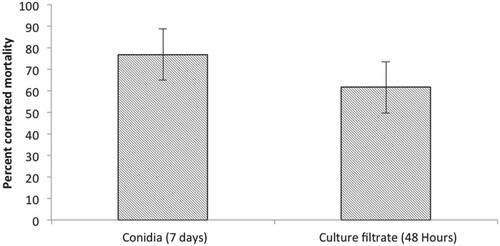
It is well known that entomopathogenic fungi produce various metabolites such as antibiotics, bioactive volatile compounds and enzymes that are toxic to insects (Quesada-Moraga et al. Citation2006; Kim et al. Citation2010; Wang and Xu Citation2012; Zhang et al. Citation2017). Hence the insecticidal activity of the culture filtrate of C. javanica was tested against B. tabaci nymphs in this study. The results indicated that the culture filtrate of C. javanica showed significant (F2,12 = 15.14, p < 0.001) increase in mortality of second instar B. tabaci nymphs after 48 h of treatment when 61.3 ± 9.5% corrected mortality of B. tabaci nymphs was recorded (Figure ). There was a significant (F1,12 = 71.8, p < 0.001) difference between the treatment and control. A direct correlation was observed between mortality and time after treatment. However, the difference in the mortality between those caused by conidial suspension and culture filtrate of C. javanica was non-significant (F2,8 = 1.57, p < 0.241) (Figure ). These findings are similar to those reported by Javed et al. (Citation2019) who observed no significant difference between conidial suspension and culture filtrate of V. lecanii in their efficacy against Myzus persicae. These results suggest that the culture filtrate of C. javanica has potential for the development of mycoinsecticide for the control of whitefly.
Figure 3. Effect of culture filtrate of C. javanica on mortality of second instar B. tabaci nymphs at different time intervals after treatment.
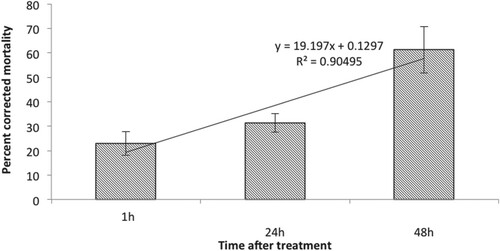
Figure 4. Overall effects of conidia and culture filtrate of C. javanica on mortality of second instar B. tabaci nymphs.
GC-MS analysis of the ethyl acetate fraction of the culture filtrate of C. javanica revealed the presence of 17 compounds including Dodecanoic acid, Eicosane, (Z)-7-Hexadecenoic acid, Henicosane, Docosane, Tricosane, Tetracosane, Pentacosane, Hexacosane, Heptacosane and Squalene (Table and Figure ). Dodecanoic acid (Lauric acid), a saturated fatty acid with 12-carbon atom, was present in the highest amount and occupied 28.7% of total area. The C22-25 hydrocarbons contributed 38.3%. Lee et al. (Citation2019) reported the production of Dibutyl succinate (Dibutyl butanedioate) by I. javanica that showed insecticidal activity against Myzus persicae and inhibitory activity against a fungal pathogen Colletotrichum acutatum. Wagan et al. (Citation2018) reported that squalene showed repellent activity against B. tabaci adults and insecticidal activity against B. tabaci nymphs and Tetranychus urticae (two-spotted spider mite). The production of lauric acid as the major compound by an entomopathogenic strain of Aspergillus flavus which is effective against teak defoliator (Hyblaea purea) has been reported (Senthilkumar et al. Citation2014). Siti Hajar et al. (Citation2016) demonstrated that lauric acid affected the reproductive system, growth rate and feeding activity of Aphis gossypii. To our knowledge, this is the first study reporting the production of lauric acid by C. javanica. The volatile compounds, especially lauric acid, produced by C. javanica could be involved in the insecticidal activity against B. tabaci. Further studies are needed to evaluate the effect of lauric acid on B. tabaci nymphs and adults and to assess the efficacy of C. javanica as a biocontrol agent against B. tabaci under greenhouse and field conditions in comparison with chemical pesticides.
Table 2. Compounds identified in the ethyl acetate extract of the culture filtrate of C. javanica by GC-MS.
Authors’ contributions
RV, RS, AMA designed the study, JNA, AAA, KKSA, ASA, SFMA conducted lab experiments, RV, RS, AMA supervised the research project, RV, RS, AMA, JNA wrote the manuscript.
Compliance with ethical standards
Ethical approval
This article is original and not published elsewhere. All authors discussed the results, read and approved the final manuscript. The authors confirm that there are no ethical issues in the publication of the manuscript.
Acknowledgement
We thank Ms. Houda Khlafan Al-Ruqaishi, CAMS, SQU for helping in GC-MS analysis.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability
The data supporting the findings of this study are available within the article. The nucleotide sequence of Cordyceps javanica isolate SQUVR-A1 has been deposited in the NCBI database (www.ncbi.nlm.nih.gov) under GenBank accession number MK503773.
Additional information
Funding
References
- Abbott WS. 1925. A method of computing the effectiveness of an insecticide. J Econ Entomol. 18:265–267. doi: https://doi.org/10.1093/jee/18.2.265a
- Al-Rashdi FKH, Al-Sadi AM, Al-Riyamy BZ, Maharachchikumbura SSN, Al-Ruqaishi HK, Velazhahan R. 2020. Alternaria alternata and Neocosmospora sp. from the medicinal plant Euphorbia larica exhibit antagonistic activity against Fusarium sp., a plant pathogenic fungus. All Life. 13:223–232. doi: https://doi.org/10.1080/26895293.2020.1759702
- Al-Shehi AA, Khan AJ. 2013. Identification of whitefly (Bemicia tabaci Genn.) biotypes and associated bacterial symbionts in Oman. J Plant Sci. 8:39–44. doi: https://doi.org/10.3923/jps.2013.39.44
- Ali S, Zhang C, Wang Z, Wang XM, Wu JH, Cuthbertson AGS, Shao Z, Qiu BL. 2017. Toxicological and biochemical basis of synergism between the entomopathogenic fungus Lecanicillium muscarium and the insecticide matrine against Bemisia tabaci (Gennadius). Sci Rep. 7:46558. doi: https://doi.org/10.1038/srep46558
- Azam KM, Bowers WS, Srikandakumar A, Al-Mahmuli IH, Al-Raeesi AA. 2002. Insecticidal action of plant extracts against nymphs of whitefly, Bemisia tabaci Gennadius. Crop Res. (Hisar). 24:390–393.
- De Barro PJ, Liu SS, Boykin LM, Dinsdale AB. 2011. Bemisia tabaci: a statement of species status. Annu Rev Entomol. 56:1–19. doi: https://doi.org/10.1146/annurev-ento-112408-085504
- Faria M, Wraight SP. 2001. Biological control of Bemisia tabaci with fungi. Crop Prot. 20:767–778. doi: https://doi.org/10.1016/S0261-2194(01)00110-7
- Gindin G, Barash I, Harari N, Raccah B. 1994. Effect of endotoxic compounds isolated from Verticillium lecanii on the sweetpotato whitefly, Bemisia tabaci. Phytoparasitica. 22:189–196. doi: https://doi.org/10.1007/BF02980318
- Hu QB, Ren SX, An XC, Qian MH. 2007. Insecticidal activity influence of destruxins on the pathogenicity of Paecilomyces javanicus against Spodoptera litura. J Appl Entomol. 131:262–268. doi: https://doi.org/10.1111/j.1439-0418.2007.01159.x
- Javed K, Javed H, Mukhtar T, Qiu D. 2019. Efficacy of Beauveria bassiana and Verticillium lecanii for the management of whitefly and aphid. Pak J Agri Sci. 56:669–674.
- Kang BR, Han JH, Kim JJ, Kim YC. 2018. Dual biocontrol potential of the entomopathogenic fungus, Isaria javanica, for both aphids and plant fungal pathogens. Mycobiology. 46:440–447. doi: https://doi.org/10.1080/12298093.2018.1538073
- Kim JS, Roh JY, Choi JY, Wang Y, Shim HJ, Je YH. 2010. Correlation of the aphicidal activity of Beauveria bassiana SFB-205 supernatant with enzymes. Fungal Biol. 114:120–128. doi: https://doi.org/10.1016/j.mycres.2009.10.011
- Lacey LA, De La Rosa F, Horton DR. 2009. Insecticidal activity of entomopathogenic fungi (Hypocreales) for potato psyllid, Bactericera cockerelli (Hemiptera: Triozidae): development of bioassay techniques, effect of fungal species and stage of the psyllid. Biocontrol Sci Technol. 19:957–970. doi: https://doi.org/10.1080/09583150903243904
- Lee YS, Han JH, Kang BR, Kim YC. 2019. Dibutyl succinate, produced by an insect pathogenic fungus, Isaria javanica pf185, is a metabolite that controls of aphids and a fungal disease, anthracnose. Pest Manag Sci. 75:852–858. doi: https://doi.org/10.1002/ps.5191
- Lee SB, Taylor JW. 1990. Isolation of DNA from fungal mycelia and single spores. In: Innis M, Gelfand D, Sninsky J, White T, editor. PCR protocols: a guide to methods and applications. Orlando: Academic Press; p. 282–287.
- Mascarin GM, Kobori NN, Quintela ED, Delalibera Jr I. 2013. The virulence of entomopathogenic fungi against Bemisia tabaci biotype B (Hemiptera: Aleyrodidae) and their conidial production using solid substrate fermentation. Biol Control. 66:209–218. doi: https://doi.org/10.1016/j.biocontrol.2013.05.001
- Misaka BC, Wosula EN, Marchelo-d’Ragga PW, Hvoslef-Eide T, Legg JP. 2020. Genetic diversity of Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) colonizing sweet potato and cassava in South Sudan. Insects. 11:58. doi: https://doi.org/10.3390/insects11010058
- Naranjo SE, Ellsworth PC. 2017. Methodology for developing life tables for sessile insects in the field using the whitefly, Bemisia tabaci, in cotton as a model system. J Vis Exp. 129:e56150.
- Norhelina L, Sajap AS, Mansour SA, Idris AB. 2013. Infectivity of five Metarhizium anisopliae (Deuteromycota: Hyphomycetales) strains on whitefly, Bemisia tabaci (Homoptera: Aleyrodidae) infesting brinjal, Solanum melongena (Solanaceae). Acad J Entomol. 6:127–132.
- Ou D, Ren LM, Liu Y, Ali S, Wang XM, Ahmed MZ, Qiu BL. 2019a. Compatibility and efficacy of the parasitoid Eretmocerus hayati and the entomopathogenic fungus Cordyceps javanica for biological control of whitefly Bemisia tabaci. Insects. 10:425. doi: https://doi.org/10.3390/insects10120425
- Ou D, Zhang LH, Guo CF, Chen XS, Ali S, Qiu BL. 2019b. Identification of a new Cordyceps javanica fungus isolate and its toxicity evaluation against Asian citrus psyllid. MicrobiologyOpen. 8:e00760. doi: https://doi.org/10.1002/mbo3.760
- Park SE, Kim JC, Lee SJ, Lee MR, Kim S, Li D, Baek S, Han JH, Kim JJ, Koo KB, Shin TY. 2018. Solid cultures of thrips-pathogenic fungi Isaria javanica strains for enhanced conidial productivity and thermotolerance. J Asia Pac Entomol. 21:1102–1109. doi: https://doi.org/10.1016/j.aspen.2018.08.005
- Queiroz PR, Lima LH, Martins ES, Sujii ER, Monnerat RG. 2017. Description of the molecular profiles of Bemisia tabaci (Hemiptera: Aleyrodidae) in different crops and locations in Brazil. J Entomol Nematol. 9:36–45. doi: https://doi.org/10.5897/JEN2017.0170
- Quesada-Moraga E, Carrasco-Diaz JA, Santiago-Alvarez C. 2006. Insecticidal and antifeedant activities of proteins secreted by entomopathogenic fungi against Spodoptera littoralis (Lep., Noctuidae). J Appl Entomol. 130:442–452. doi: https://doi.org/10.1111/j.1439-0418.2006.01079.x
- Sain SK, Monga D, Kumar R, Nagrale DT, Kranthi S, Kranthi KR. 2019. Comparative effectiveness of bioassay methods in identifying the most virulent entomopathogenic fungal strains to control Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae). Egypt J Biol Pest Control. 29:31. doi: https://doi.org/10.1186/s41938-019-0130-z
- Sani I, Ismail SI, Abdullah S, Jalinas J, Jamian S, Saad N. 2020. A review of the biology and control of whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae), with special reference to biological control using entomopathogenic fungi. Insects. 11:619. doi: https://doi.org/10.3390/insects11090619
- Senthilkumar N, Murugesan S, Babu DS. 2014. Metabolite profiling of the extracts of endophytic fungi of entomopathogenic significance, Aspergillus flavus and Nigrospora sphaerica isolated from tropical tree species of India, Tectona grandis L. J Agric Life Sci. 1:108–114.
- Shah R, Scott IM. 2020. Susceptibility of Bemisia tabaci (MEAM1) Gennadius (Hemiptera: Aleyrodidae) to deltamethrin, thiamethoxam and pyriproxyfen in Oman. Intl J Agric Biol. 24:279–284.
- Shimazu M, Takatsuka J. 2010. Isaria javanica (anamorphic Cordycipitaceae) isolated from gypsy moth larvae, Lymantria dispar (Lepidoptera: Lymantriidae), in Japan. Appl Entomol Zool. 45:497–504. doi: https://doi.org/10.1303/aez.2010.497
- Siti Hajar MS, Azila AA, Harisun YA, Khalidah P. 2016. Toxicological effect of lauric acid based insecticide on the reproduction system, growth development and feeding activity of aphids, Aphis gossypii. Int J Biotechnol Wellness Ind. 5:76–81. doi: https://doi.org/10.6000/1927-3037.2016.05.03.2
- Wagan TA, Cai W, Hua H. 2018. Repellency, toxicity, and anti-oviposition of essential oil of Gardenia jasminoides and its four major chemical components against whiteflies and mites. Sci Rep. 8:9375. doi: https://doi.org/10.1038/s41598-018-27366-5
- Wang L, Huang J, You M, Guan X, Liu B. 2007. Toxicity and feeding deterrence of crude toxin extracts of Lecanicillium (Verticillium) lecanii (Hyphomycetes) against sweet potato whitefly, Bemisia tabaci (Homoptera: Aleyrodidae). Pest Manag Sci. 63:381–387. doi: https://doi.org/10.1002/ps.1359
- Wang Q, Xu L. 2012. Beauvericin, a bioactive compound produced by fungi: a short review. Molecules. 17:2367–2377. doi: https://doi.org/10.3390/molecules17032367
- White TJ, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editor. PCR protocols: a guide to methods and applications. San Diego, USA: Academic Press; p. 315–322.
- Xia J, Zhang CR, Zhang S, Li FF, Feng MG, Wang XW, Liu SS. 2013. Analysis of whitefly transcriptional responses to Beauveria bassiana infection reveals new insights into insect-fungus interactions. PLoS One. 8:e68185. doi: https://doi.org/10.1371/journal.pone.0068185
- Xie L, Han JH, Kim JJ, Lee SY. 2016. Effects of culture conditions on conidial production of the sweet potato whitefly pathogenic fungus Isaria javanica. Mycoscience. 57:64–70. doi: https://doi.org/10.1016/j.myc.2015.09.002
- Xie T, Jiang L, Li J, Hong B, Wang X, Jia Y. 2019. Effects of Lecanicillium lecanii strain JMC-01 on the physiology, biochemistry, and mortality of Bemisia tabaci Q-biotype nymphs. PeerJ. 7:e7690. doi: https://doi.org/10.7717/peerj.7690
- Zhang C, Yan SQ, Shen BB, Ali S, Wang XM, Jin FL, Cuthbertson AG, Qiu BL. 2017. RNAi knock-down of the Bemisia tabaci Toll gene (BtToll) increases mortality after challenge with destruxin A. Mol Immunol. 88:164–173. doi: https://doi.org/10.1016/j.molimm.2017.06.031
- Zhu H, Kim JJ. 2011. Susceptibility of the tobacco whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae) biotype Q to entomopathogenic fungi. Biocontrol Sci Technol. 21:1471–1483. doi: https://doi.org/10.1080/09583157.2011.636482