Abstract
We aimed to explore the clinical efficacy and effects of albuterol combined with tiotropium bromide on pulmonary functions of patients with acute exacerbation of chronic obstructive pulmonary disease (AECOPD). 72 patients with AECOPD were divided into a combination treatment group (n = 38, albuterol + tiotropium bromide) and the control group (n = 34, routine treatment). Efficacy, incidence of adverse reactions, results of blood gas analysis, pulmonary function indices, time to improve clinical symptoms, St. George's respiratory questionnaire (SGRQ), and British Medical Research Council (MMRC) scores were compared between the two groups. The overall response rate was significantly higher, and the time to improve coughing was significantly shorter, in the combination treatment group compared with controls (p < 0.05). After treatment, both groups achieved a marked rise in PaO2, forced expiratory volume in 1 s (FEV1), FEV1/forced vital capacity (FVC) ratio, and peak expiratory flow (PEF) (p < 0.05), which was more dominant with combination therapy. The reduction in PaCO2 was more obvious in the combination therapy group. The combination of albuterol and tiotropium bromide is an effective treatment for patients with AECOPD and functions by improving conditions and pulmonary functions without raising the incidence of adverse reactions.
Introduction
Acute exacerbation of chronic obstructive pulmonary disease (AECOPD) is a common but progressively developing pulmonary disease characterized by airflow limitation and persistent decline in pulmonary function (PF). Patients with severe AECOPD are likely to experience respiratory failure and heart failure (Kiser and Vandivier Citation2015; Tan et al. Citation2017). With middle-aged and senior populations as the major targets, the disease is associated with a high incidence and case fatality rate as well as difficulties in treatment (Jing et al. Citation2016; Liu et al. Citation2017). So far, the clinical treatments for AECOPD have focused on improving PF, blood oxygen levels, and mitigating clinical symptoms (Tan et al. Citation2017; Morantes-Caballero and Fajardo Rodriguez Citation2019). Therefore, to find an appropriate clinical treatment to improve PF, pneumodynamics, and blood gas indices of AECOPD patients has become a major focus of pulmonary research (Yohannes et al. Citation2017; Heys et al. Citation2018).
Glucocorticoids, oxygen therapy, and bronchiectasia are usually adopted in the clinical treatment of AECOPD (Yao et al. Citation2017). Albuterol is a selective receptor agonist with some effect on preventing and improving symptoms caused by airflow limitation (Allen et al. Citation2016). When used alone, however, the drug is not likely to be effective in patients with chronic obstructive pulmonary disease (COPD): in particular, AECOPD (Wolfe and Silvestri Citation2016; Jacobson et al. Citation2017). Tiotropium bromide, an anticholinergic bronchiectasia, may mitigate disease exacerbations and enhance a patient's activity level when applied alone, but it does not improve PF (Panahi et al. Citation2016; Ohbayashi Citation2017).
Given the complicated onset mechanisms of AECOPD, single-drug therapies in the past have often failed to achieve marked effects when recovering patients’ PF indices, whereas some drug combinations used together achieve synergistic effectiveness for enhanced efficacy, PF, and ventilatory capacity (VC) (Bourbeau et al. Citation2016; Kaufman Citation2017; Rajala et al. Citation2017). Therefore, in order to provide more strategies for treating AECOPD patients, this study compared the clinical efficacies of combination therapy with albuterol and tiotropium bromide with routine treatment by observing and comparing PF changes.
Materials and methods
General materials
A total of 72 patients with AECOPD were admitted to our hospital from September 2016 to June 2018 and were divided into the observational group (OG; n = 38; albuterol + tiotropium bromide) and the control group (CG; n = 34; routine treatment). There were 20 males and 18 females in the OG, with an average age of 56.67 (±7.58) years, while the CG consisted of 19 males and 15 females aged 55.52 (±5.49) years. Inclusion criteria consisted of the following: compliance with the diagnosis criteria for AECOPD and requirements of this study; age < 80; worsening syndromes such as dyspnea, fever, cough, and expectoration; onset in the past two weeks; and demanding hospital treatment. Exclusion criteria were: severe heart, liver, or kidney dysfunctions; mental disorder; psychological block; diseases of the heart, liver, kidney, hematopoietic, or immune systems; hyperthyrea; acute heart failure; pulmonary interstitial fibrosis; lung cancer; tuberculosis; pulmonary embolism; bronchiectasia and rhinallergosis; incomplete medical records; allergy to the drugs studied; taking medicines affecting the study results; poor drug tolerance; drug contraindications; and non-compliance with the doctor's advice on medication. This study was approved by the Ethics Committee of Linyi People's Hospital. The research objects and their families were informed and they signed a fully-informed consent form.
Methods
After basic treatments including apophlegmatisant (a medicine for treating cough and asthma), low-flow oxygen uptake, and anti-infection medication in the hospital, patients in the CG were given tiotropium bromide (Nanchang Hongyi Pharmaceuticals Co., Ltd., H20130110) at a dose of 18 μg per day; in addition to receiving all treatments of the CG, patients in the OG additionally inhaled 2 mL of albuterol sulfate (Qilu Pharmaceuticals Co., Ltd., H37021518) in the morning and at night. This treatment lasted for three months.
Observation indices
Before and after four courses of treatment, blood gas and PF indexes were measured with a blood gas analyzer (Shanghai Radiometer Medical Appliance Co., Ltd., ABL90), and a photoelectric PF detector (Anhui Hongzhong Medical Devices Co., Ltd., Microspiro Hl-205), respectively. PF indices included forced vital capacity (FVC), forced expiratory volume in one second (FFV1%), (FEVI/FVC), and peak expiratory flow (PEF); the degree of dyspnea was measured with the modified British Medical Research Council (MMRC) scale before and after treatment (Make et al. Citation2015). On this scale, a higher score indicates more severe dyspnea. Quality of life was evaluated by the St. George's respiratory questionnaire (SGRQ) (Tee Citation2017) and indicated with a mark between 0 (minimum damage) and 100. 0 (severe damage).
Efficacy criteria
‘Markedly effective’ was defined as symptoms such as cough, expectoration, gasping, palpitations, cyanosis, edema, and lung rales were basically mitigated in tranquility. An ‘effective’ status demonstrated that symptoms such as cough, expectoration, gasping, and palpitations were obviously mitigated, and lung rales were reduced. Ineffective was defined as these symptoms and vital signs were neither improved nor deteriorated. OR = Markedly Effective Rate + Effective Rate.
Statistical analysis
Statistical analysis was performed with SPSS22.0 (IBMCorp, Armonk, NY, USA). In cases of numerical data expressed as means ± standard deviation, comparison studies were carried out through t test; in the case of nominal data expressed as (%), comparison studies were carried out through the χ2 test. For all statistical comparisons, significance was defined as p < 0.05.
Results
Patients
No significant difference was found between the two groups in terms of age, BMI, course of disease, gender, history of smoking and drinking alcohol, domicile, and grading (p > 0.05, Table ).
Table 1. Intergroup comparison of basic materials.
Intergroup comparison of blood gas analysis
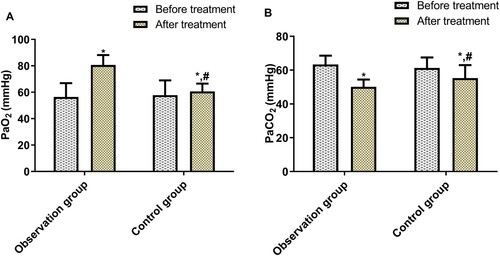
Before treatment, the two groups demonstrated no significant difference in PaO2 and PaCO2; after treatment, PaO2 rose and PaCO2 dropped in both groups: more significantly in the OG (p < 0.05, Figure ).
Intergroup comparison of PF indices
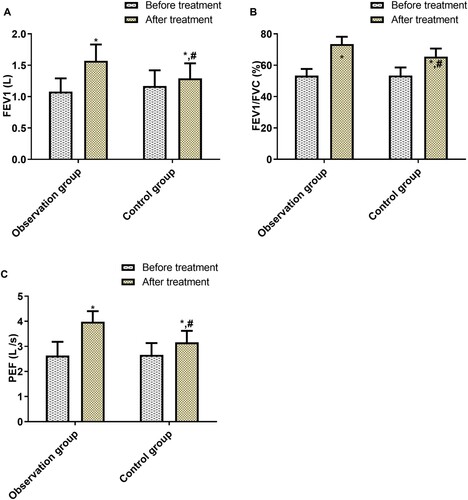
Before treatment, the FEV1, FEV1/FVC, and PEF were not statistically different between the two groups; after treatment, these three indices rose sharply in both groups, and more significantly in the OG (p < 0.05, Figure ).
Intergroup comparison of efficacy
The OG contained a greater percentage of marked effective cases and effective cases than did the CG, whereas the CG had a greater number of ineffective cases (p < 0.05, Table ).
Table 2. Intergroup comparison of efficacy.
Intergroup comparison of incidence of adverse reactions
Adverse reactions before and after treatment were compared between the two groups. The OG reported one case of dry mouth (2.63%), one of palpitation (2.63%), two of nausea (5.26%), and one of constipation (2.63%) with a total incidence of 10.53%. In the CG, adverse reactions found were dry mouth (two cases; 5.88%) and constipation (one case; 2.94%), leading to a total incidence of 8.82% (not significant; Table ).
Table 3. Intergroup comparison of incidences of adverse reactions.
Intergroup comparison of SGRQ scores before and after treatment
Before treatment, the two groups did not have statistically different SGRQ scores; after treatment, however, both groups experienced a sharp score reduction (p < 0.05), which has greater significant in the OG (p < 0.05, Table ).
Table 4. Intergroup comparison of SGRQ scores before and after treatment.
Intergroup comparison of time to improve clinical symptoms
The time to improve clinical symptoms including cough, gasping, and lung rales, was significantly shorter in the OG than in the CG (p < 0.05, Table ).
Table 5. Intergroup comparison of time to improve clinical symptoms (d).
Intergroup comparison of MMRC scores before and after treatment
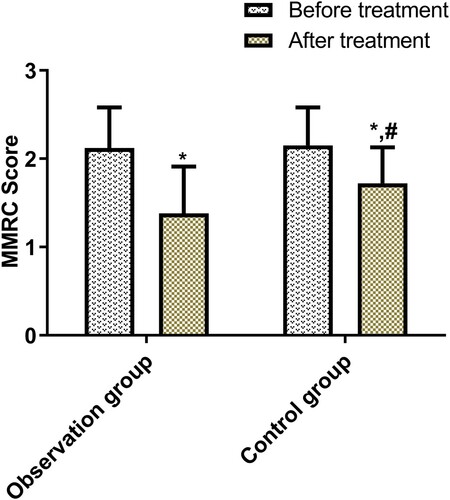
Before treatment, the two groups were not statistically different in MMRC scores, but after treatment, the index reduced sharply in both groups (p < 0.05), and more significantly in the OG (p < 0.05, Figure ).
Discussion
COPD is a disease characterized by airflow limitation, long course, and easy onset, and it is accompanied by high airway reactivity, causing irreversible damage to PF. AECOPD is a phenomenon of serious deterioration as a result of acute onset of COPD in the consequence of severe lung infection. If AECOPD is not carefully and timely treated, it can further develop into pulmonary heart disease and respiratory failure, which seriously affect the quality of life and safety of patients (Adeloye et al. Citation2015; Lipson et al. Citation2018; Wei et al. Citation2018).
The key to clinical COPD treatment lies in effectively relieving patients from airway resistance and respiratory muscle fatigue, maintaining clear respiratory tract, and actively improving dyspnea-related syndromes to enhance patient PF. Throughout long-term clinical practice, single-drug therapies may have limited effects on COPD patients in terms of mitigating respiratory tract obstruction and respiratory muscle fatigue, but some drugs are capable of mitigating syndromes, improving PF, and slowing the progress of COPD (Rabe Citation2007). Therefore, medication still plays a vital role in the clinical treatment of COPD, and aerosol rebreathing method (ARM) is widely recognized for its effectiveness with AECOPD (Xiong et al. Citation2008). Ordinary drugs for ARM include receptor agonists and glucocorticoids (Hartman et al. Citation2015). As a selective β2 adrenergic receptor agonist, the aerosol particles of albuterol are driven by high-flow oxygen to reach the bronchioli terminals rapidly, where they inhibit the cholinergic nerve form releasing acetylcholine in large amounts and block the M receptors in the air tract, thus relaxing and humidifying bronchial smooth muscle, reducing mucus secretion and airway resistance, and increasing airway diameter. This results in better PF and respiratory mechanics and higher blood oxygen level in patients (Lötvall et al. Citation1998; Cazzola et al. Citation2015). Tiotropium bromide is a specific long-lasting antimuscarinic drug that can competitively inhibit M1 and M3 cholinergic receptors and reduce the tension of the cholinergic nerve, relax smooth muscle, and dilate the bronchus (Cazzola et al. Citation2015; Trudo et al. Citation2015). Relevant studies have revealed that the efficacy of tiotropium bromide is dose-dependent and may last 24 h to effectively mitigate bronchial spasms during sleep (Alvarado-Gonzalez and Arce Citation2015). Therefore, tiotropium bromide can maintain long-term PF improvement, bronchiectasia, and dyspnea mitigation. Panahi et al. (Panahi et al. Citation2016) found in their study that tiotropium bromide effectively mitigated major syndromes found in COPD patients and their overall SGRQ score, while Santus et al. (Santus et al. Citation2012) demonstrated the more ideal anti-inflammatory activity of tiotropium bromide when compared with formoterol by reducing the hyperoxides and LTB4 in the peripheral neutrophils of COPD patients. In a study designed by Vogelmeier et al. (Vogelmeier et al. Citation2013), a combined therapy significantly and consistently improved the PF of COPD patients, while in the present study, the combination of albuterol and tiotropium bromide, when compared with routine therapy, yielded a high OR and consumed less time to improve clinical symptoms, although no statistical difference arose in the total incidence of adverse reactions. These data suggest that the combination is an effective and feasible therapy for COPDs.
The alveolar wall and airway of COPD patients has different degrees of damage cumulatively resulting in a decrease in the lung's elastic resilience, FEV1, FEV1/FVC, and PEF and resulting in a significant increase in residual gas volume. A test of PF indexes before and after treatment showed that after treatment, both groups experienced a marked increase in FEV1, FEV1/FVC, and PEF (p < 0.05), which was more significant in the OG. Moreover, the results of blood gas analysis showed that the reductions in PaO2 and PaCO2 were more obvious in the OG after treatment, indicating the combination of albuterol and tropium bromide improves the PF and quality of life of these patients.
Although the study subjects were selected in strict accordance with the inclusion and exclusion criteria, the study failed to compare the long-term efficacy and the hazards related to AECOPD patients, which shall be ameliorated in future studies.
In conclusion, the combination of albuterol and tiotropium bromide is an effective treatment for patients with AECOPD as it can improve their conditions and PF without raising the incidence of adverse reactions.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Adeloye D, Chua S, Lee C, Basquill C, Papana A, Theodoratou E, Nair H, Gasevic D, Sridhar D, Campbell H, et al. 2015. Global and regional estimates of COPD prevalence: systematic review and meta-analysis. J Glob Health. 5(2):020415–020415. https://doi.org/https://doi.org/10.7189/jogh.05-020415.
- Allen NM, Hacohen Y, Palace J, Beeson D, Vincent A, Jungbluth H. 2016. Salbutamol-responsive fetal acetylcholine receptor inactivation syndrome. Neurology. 86(7):692–694. https://doi.org/https://doi.org/10.1212/WNL.0000000000002382.
- Alvarado-Gonzalez A, Arce I. 2015. Tiotropium bromide in chronic obstructive pulmonary disease and bronchial asthma. J Clin Med Res. 7(11):831–839. https://doi.org/https://doi.org/10.14740/jocmr2305w.
- Bourbeau J, Sedeno MF, Metz K, Li PZ, Pinto L. 2016. Early COPD exacerbation treatment with combination of ICS and LABA for patients presenting with mild-to-moderate worsening of dyspnea. COPD. 13(4):439–447. https://doi.org/https://doi.org/10.3109/15412555.2015.1101435.
- Cazzola M, Rogliani P, Ora J, Matera MG. 2015. Olodaterol + tiotropium bromide for the treatment of chronic obstructive pulmonary disease. Expert Rev Clin Pharmacol. 8(5):529–539. https://doi.org/https://doi.org/10.1586/17512433.2015.1075389.
- Hartman S, Merkus P, Maseland M, Roovers L, van Setten P. 2015. Hypokalaemia in children with asthma treated with nebulised salbutamol. Arch Dis Child. 100(10):970–972. https://doi.org/https://doi.org/10.1136/archdischild-2015-308427.
- Heys D, Swain A, Knowles S, Waugh A, Bailey M. 2018. An audit of change in clinical practice: from oxygen-driven to air-driven nebulisers for prehospital patients with acute exacerbations of chronic obstructive pulmonary disease (AECOPD). Intern Med J. 48(6):668–673. https://doi.org/https://doi.org/10.1111/imj.13684.
- Jacobson GA, Raidal S, Robson K, Narkowicz CK, Nichols DS, Haydn Walters E. 2017. Bronchopulmonary pharmacokinetics of (R)-salbutamol and (S)-salbutamol enantiomers in pulmonary epithelial lining fluid and lung tissue of horses. Br J Clin Pharmacol. 83(7):1436–1445. https://doi.org/https://doi.org/10.1111/bcp.13228.
- Jing Z, Chun C, Ning S, Hong Z, Bei H, Wan-Zhen Y. 2016. Systemic inflammatory marker CRP Was better predictor of readmission for AECOPD than sputum inflammatory markers. Arch Bronconeumol. 52(3):138–144. https://doi.org/https://doi.org/10.1016/j.arbres.2015.01.011.
- Kaufman JS. 2017. Acute exacerbation of COPD: diagnosis and management. Nurse Pract. 42(6):1–7. https://doi.org/https://doi.org/10.1097/01.NPR.0000515997.35046.b8.
- Kiser TH, Vandivier RW. 2015. Severe acute exacerbations of chronic obstructive pulmonary disease: does the dosage of corticosteroids and type of antibiotic matter? Curr Opin Pulm Med. 21(2):142–148. https://doi.org/https://doi.org/10.1097/MCP.0000000000000142.
- Lipson DA, Barnhart F, Brealey N, Brooks J, Criner GJ, Day NC, Dransfield MT, Halpin DMG, Han MK, Jones CE, et al. 2018. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 378(18):1671–1680. https://doi.org/https://doi.org/10.1056/NEJMoa1713901.
- Liu S, Chen J, He Y, Wu L, Lai J, Zuo J, Yang L, Guo X. 2017. Comparative effectiveness of six Chinese herb formulas for acute exacerbation of chronic obstructive pulmonary disease: protocol for systematic review and network meta-analysis. BMJ Open. 7(8):e017099–e017099. https://doi.org/https://doi.org/10.1136/bmjopen-2017-017099.
- Lötvall J, Lunde H, Svedmyr N. 1998. Onset of bronchodilation and finger tremor induced by salmeterol and salbutamol in asthmatic patients. Can Respir J. 5(3):191–194. https://doi.org/https://doi.org/10.1155/1998/364639.
- Make BJ, Eriksson G, Calverley PM, Jenkins CR, Postma DS, Peterson S, Östlund O, Anzueto A. 2015. A score to predict short-term risk of COPD exacerbations (SCOPEX). Int J Chron Obstruct Pulmon Dis. 10:201–209. https://doi.org/https://doi.org/10.2147/COPD.S69589.
- Morantes-Caballero JA, Fajardo Rodriguez HA. 2019. Effects of air pollution on acute exacerbation of chronic obstructive pulmonary disease: a descriptive retrospective study (pol-AECOPD). Int J Chron Obstruct Pulmon Dis. 14:1549–1557. https://doi.org/https://doi.org/10.2147/COPD.S192047.
- Ohbayashi H. 2017. Comparison of the rapid effects of single inhalations of formoterol and tiotropium bromide on respiratory function and COPD symptoms in a randomized crossover study. Respir Investig. 55(6):348–356. https://doi.org/https://doi.org/10.1016/j.resinv.2017.07.004.
- Panahi Y, Ghanei M, Behzadi M, Salehi M, Soflaei SS, Sahebkar A. 2016. Investigation of the efficacy of generic and brand-name tiotropium bromide in the management of chronic obstructive pulmonary disease: A randomized comparative trial. Saudi Pharm J. 24(2):147–152. https://doi.org/https://doi.org/10.1016/j.jsps.2015.01.005.
- Rabe KF. 2007. Global initiative for chronic obstructive lung disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. GOLD executive summary. Am J Respir Crit Care Med. 176:532–555.
- Rajala K, Lehto JT, Sutinen E, Kautiainen H, Myllärniemi M, Saarto T. 2017. mMRC dyspnoea scale indicates impaired quality of life and increased pain in patients with idiopathic pulmonary fibrosis. ERJ Open Res. 3(4):00084–2017. https://doi.org/https://doi.org/10.1183/23120541.00084-2017.
- Santus P, Buccellati C, Centanni S, Fumagalli F, Busatto P, Blasi F, Sala A. 2012. Bronchodilators modulate inflammation in chronic obstructive pulmonary disease subjects. Pharmacol Res. 66(4):343–348. https://doi.org/https://doi.org/10.1016/j.phrs.2012.05.007.
- Tan DBA, Armitage J, Teo T-H, Ong NE, Shin H, Moodley YP. 2017. Elevated levels of circulating exosome in COPD patients are associated with systemic inflammation. Respir Med. 132:261–264. https://doi.org/https://doi.org/10.1016/j.rmed.2017.04.014.
- Tee AK. 2017. Chronic obstructive pulmonary disease (COPD): “not a cigarette only pulmonary disease”. Ann Acad Med Singapore. 46(11):415–416.
- Trudo F, Kern DM, Davis JR, Tunceli O, Zhou S, Graham EL, Strange C, Williams SA. 2015. Comparative effectiveness of budesonide/formoterol combination and tiotropium bromide among COPD patients new to these controller treatments. Int J Chron Obstruct Pulmon Dis. 10:2055–2066. https://doi.org/https://doi.org/10.2147/COPD.S90658.
- Vogelmeier CF, Bateman ED, Pallante J, Alagappan VKT, D'Andrea P, Chen H, Banerji D. 2013. Efficacy and safety of once-daily QVA149 compared with twice-daily salmeterol-fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE): a randomised, double-blind, parallel group study. Lancet Respir Med. 1(1):51–60. https://doi.org/https://doi.org/10.1016/S2213-2600(12)70052-8.
- Wei X, Yu N, Ding Q, Ren J, Mi J, Bai L, Li J, Qi M, Guo Y. 2018. The features of AECOPD with carbon dioxide retention. BMC Pulm Med. 18(1):124–124. https://doi.org/https://doi.org/10.1186/s12890-018-0691-8.
- Wolfe GI, Silvestri NJ. 2016. Comment: Salbutamol—a means to an endplate. Neurology. 86(7):693–693. https://doi.org/https://doi.org/10.1212/WNL.0000000000002385.
- Xiong G, Xu L, Wei L, Li X. 2008. Atomization inhalation of terbutaline and budesonide efficiently improved immunity and lung function of AECOPD patients. Cell Mol Immunol. 5(4):287–291. https://doi.org/https://doi.org/10.1038/cmi.2008.35.
- Yao C, Liu X, Tang Z. 2017. Prognostic role of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio for hospital mortality in patients with AECOPD. Int J Chron Obstruct Pulmon Dis. 12:2285–2290. https://doi.org/https://doi.org/10.2147/COPD.S141760.
- Yohannes AM, Mülerová H, Lavoie K, Vestbo J, Rennard SI, Wouters E, Hanania NA. 2017. The association of depressive symptoms with rates of acute exacerbations in patients with COPD: results from a 3-year longitudinal follow-up of the ECLIPSE cohort. J Am Med Dir Assoc. 18(11):955–959.e6. https://doi.org/https://doi.org/10.1016/j.jamda.2017.05.024.