?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Dexmedetomidine is a novel, highly selective α2 adrenoceptor agonist, whereas oxycodone serves as a μ- and ĸ-amboceptor agonist. To analyze the effects of dexmedetomidine combined with oxycodone in locally anesthetized patients undergoing transforaminal endoscopic resection of nucleus pulposus. 112 patients were evenly randomized into oxycodone group (OG), dexmedetomidine group (DG), oxycodone-dexmedetomidine combination group (ODG), and control group (CG). No significant difference among the four groups in terms of baseline information, recovery time, or atropine was found (p > 0.05). The mean arterial pressures (MAPs) and heart rates (HRs) in ODG reduced from the injection point and were dramatically lower over those in CG, and visual analog scale (VAS) scores in ODG at 1, 2, and 6 h following surgery were lower over those in other groups (p < 0.05). The combination of dexmedetomidine with oxycodone in the local anesthesia during analgesia and sedation of patients undergoing transforaminal endoscopic resection of the nucleus pulposus manifested tangible effects. Specifically, such improved perioperative inflammatory responses and controlled cellular immunity without promoting the onset of adverse reactions.
Introduction
The major causes of lumbar disk herniation include degeneration or rupture of the intervertebral disc and compression of the spinal cord or nerves by the kyphosis. Lumbar disk herniation is also one of the pathogenic factors for pain in the waist and lower extremities (Kopecky et al. Citation2017). With the growing aged population, more people than ever are being diagnosed with lumbar disk herniation. It has been reported that the incidence among adults in China is approximately 8% to 25%, while that in western world was 3.7% to 5.1% (Wu et al. Citation2019). Since the first successful resection of nucleus pulposus by Mixter in 1934, the application of this surgical method has been extended. At the same time, with the continuing development of minimally invasive techniques, transforaminal endoscopic resection of nucleus pulposus for the pain management of lumbar disc herniation has developed rapidly. More and more patients are willing to be treated with transforaminal endoscopic resection of nucleus pulposus, thanks to the evolution of minimally invasive spine operation techniques and the increased acceptance of minimally invasive surgery by people. Even though merely including local anesthesia, various factors involving those that are associated with anesthesia use, surgical wounds or surgical position (prone position) are able to result in more serious intraoperative elevated blood pressure and/or heart rate (HR) and other adverse reactions, increasing the risk of adverse consequence (Xie et al. Citation2017).
Dexmedetomidine is a novel, highly selective α2 adrenoceptor agonist, whereas oxycodone serves as a μ- and ĸ-amboceptor agonist. Studies have shown that preemptive analgesia in thoracoscopic surgery, laparoscopic cholecystectomy, orthopedic surgery using dexmedetomidine combined with oxycodone led to good results (Cui et al. Citation2017; Kibbe et al. Citation2018; Xiang et al. Citation2018). The combined use of dexmedetomidine and oxycodone might be an auxiliary method in transforaminal endoscopic nucleus pulposus excision through local anesthesia technique. However, few reports have discussed the use of such a combination in local anesthetized transforaminal endoscopic resection of nucleus pulposus. Tumor necrosis factor-α (TNF-α) serves as an active cytokine synthesized and released by activated macrophages, T-cells, astrocytes, and the like. Interleukin (IL)-6 is recognized as an important cytokine of acute inflammation as well as an early sensitive indicator of tissue damage that plays an important role in inflammatory responses. IL-2 is a multicellular derived cytokine that can promote the growth, proliferation and differentiation of lymphocytes and plays an important role in the immune response of the body. Surgery, as a stressor, induces stress responses leading to patients experiencing perioperative acute inflammation. Postoperative inflammation is tightly associated with the incidence of complications, involving death (Wang et al. Citation2016). This study was therefore designed to analyze the application of dexmedetomidine combined with oxycodone in patients undergoing transforaminal endoscopic resection of nucleus pulposus with a local anesthesia approach as well as its impact on TNF-α, IL-6 and IL-2.
Materials and methods
General information
A total of 112 patients who underwent transforaminal endoscopic resection of nucleus pulposus from June 2018 to June 2019 in our hospital were divided into four groups based on a random number table as follows: an oxycodone group (OG; n = 28), a dexmedetomidine group (DG; n = 28), an oxycodone–dexmedetomidine combination group (ODG; n = 28), and a control group (CG; n = 28). Study inclusion criteria were (1) age of less than 70 years; (2) body mass index of 18.5–28 kg/m2; (3) the American Society of Anesthesiologists (ASA) classification of II to III; (4) no other surgery in the past 3 months; and (5) no history of drug allergy. In contrast, exclusion criteria included (1) combined malignancy, (2) severe systemic diseases (3) coagulation disturbance (4) epilepsy or psychosis history (5) bradycardia (6) intracardiac conduction block (7) history spinal surgery history and (8) serious dystrophia. The study has been agreed by the Ethics Committee of the Second People's Hospital of Hefei, Hefei Hospital Affiliated to Medical University of Anhui. All subjects have signed informed consent.
Methods
After preoperative fasting for 8 h, the subjects were intramuscularly injected 0.5 mg of atropine + 0.1 g of Lumina 30 min before entering the operating room. In the operating room, patients underwent the establishment of a routine peripheral venous channel, coupled with electrocardiogram, oxygen saturation level, arterial blood pressure, and bispectral index monitoring. Then, 10 mL/kg/h of Ringer's solution was given through this channel. Patients in the OG were intravenously injected with 0.3 mg/kg of oxycodone (SFDA J20180003; Rafa Laboratories Ltd., Jerusalem, Israel) and then intravenously pumped with 0.2 mg/kg/h until the operation was finished. Patients in the DG were intravenously pumped first with 0.5 µg/kg of dexmedetomidine (SFDA H20163388; Cisen Pharmaceutical Co., Ltd., China) until the injection was exhausted for 10 min and then pumped with 0.5 µg/kg/h of dexmedetomidine until the operation was completed. Patients in the ODG were intravenously pumped with 0.3 µg/kg/h of dexmedetomidine + 0.1 mg/kg/h oxycodone until the operation was completed. Finally, the control group was intravenously pumped with the same amount of normal saline. For the surgical incision, 1% lidocaine was applied as the local infiltration anesthesia. When the intraoperative HR was fewer than 55 bpm, 0.15 mg of atropine was given as an intravenous injection. Postoperative analgesia was 1.0 µg/kg/d of sufentanil + 1.0 µg/kg/d of dexmedetomidine, with a single loading dose of 2 mL, continuous dose of 2 mL/h, and locking period of 15 min. The total capacity was 100 mL.
Evaluation criteria
Study evaluations included (1) the comparison of patients in the four groups with respect to recovery time and atropine use; (2) the comparison of HRs and mean arterial pressures (MAPs) at predose (T0), 10 min after injection (T1), skin incision (T2), the end of surgery (T3), and 12 h following surgery (T4); (3) the comparison of visual analog scale (VAS) scores at 1, 2, 6, and 12 h following surgery, in which a 10 cm blank scale plate was utilized where 10 equidistance marks represented 0∼10 points (0 = painless and 10 = unbearable pain); (4) the comparison of Ramsay scores at T0, T1, T2, and T3 (1 point = dysphoria, 2 points = conscious but quiet and cooperative, 3 points = drowsy but obedient to directions, 4 points = lightly asleep and may be woken up quickly, 5 points = slow response to arousal stimulus, and 6 points = deep sleep from which the patient may not be woken up from); (5) the comparison of TNF-α, IL-6 and IL-2 levels at T0, T3, T4, and 24 h following surgery (T5), in which 3 mL of venous blood was collected and centrifuged for 5 min at 3,000 r/min and subsequently preserved at −80°C prior to detection through ELISA as the guidance in the kit provided by R&D Systems (Minneapolis, MN, USA); (6) the comparison of levels of CD4+, CD8+, and natural killer (NK) cells at T0, T3, T4, and T5, where 2 mL of venous blood was collected into anticoagulation tubes subsequently subjected to direct immunofluorescence labeling with monoclonal antibodies and detected in a Beckman Coulter flow cytometer produced by Becton, Dickinson, and Company (Franklin Lakes, NJ, USA); and (7) the recording of perioperative adverse reactions.
Statistical analysis
Using the Statistical Package for the Social Sciences version 19.0 software program (IBM Corp., Armonk, NY, USA), measurement data were expressed by ± s and subjected to a t-test, whereas enumeration data were expressed as percentages followed with chi-squared test. A p-value of less than 0.05 was used to suggest statistical significance.
Results
Comparison of general information among the four groups
No significant differences among the four groups in terms of gender, age, body mass index, ASA classification, duration of operation, intraoperative blood loss, or intraoperative fluid infusion were found (p > 0.05) as shown in Table .
Table 1. General information among the four groups ( ± s, n).
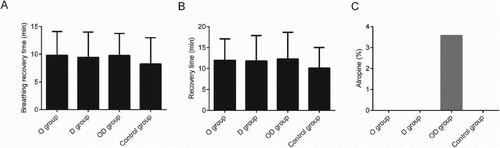
Comparison of recovery time and atropine among the four groups
No obvious distinctions in atropine or recovery time among four groups (p > 0.05) was detected, indicating that the combined use of dexmedetomidine and oxycodone exhibit little effect on recovery time or necessary atropine dosage (Figure ).
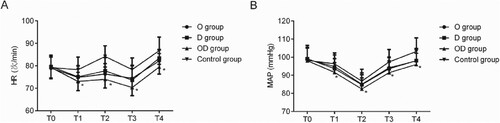
Comparison of perioperative HR and MAP among the four groups
Patients in all groups presented with HRs decreased at T1, increased at T2, decreased at T3, and increased again at T4 in comparison with T0, respectively, whereas MAPs were decreased at T1 and T2 but increased again at T3 and T4. The HRs and MAPs in ODG at T1, T2, T3, and T4 were lower over those in CG (p < 0.05), even though the HRs and MAPs at these time points among OG, DG and ODG didn’t exhibit notable distinctions (p > 0.05) (Figure ).
Comparison of VAS scores after surgery among four groups
VAS scores in ODG at 1, 2, and 6 h following surgery were the lowest relative to those in other groups (p < 0.05). In contrast, at 12 h following surgery, VAS scores in four groups exhibited no obvious distinctions (p > 0.05) (Table ).
Table 2. Comparison of postoperative VAS scores among the four groups ( ± s, points).
Comparison of perioperative Ramsay scores among the four groups
In the OG, DG, and ODG, Ramsay scores at T1 and T2 increased, whereas those at T3 were decreased, in contrast with those in the CG that simply continued to decrease from T0 onward. The ODG presented higher Ramsay scores at T2 and T3 than those in the OG, DG, and CG (p < 0.05) (Table ).
Table 3. Comparison of perioperative Ramsay scores among the four groups ( ± s, points).
Comparison of perioperative inflammatory indicators among the four groups
TNF-α and IL-6 levels at T3, T4, and T5 were increased in the four groups, with the levels in the ODG being lower as compared with those in the OG, DG, and CG (p < 0.05), while IL-2 levels at T3, T4, and T5 were decreased in the four groups, with the levels in the ODG being higher as compared with those in the OG, DG, and CG (p < 0.05), as shown in Table .
Table 4. Comparison of perioperative inflammatory indicators among the four groups ( ± s, pg/mL).
Comparison of perioperative cellular immunity indicators among the four groups
CD4+, CD8+, and NK cell levels at T3, T4, and T5 were decreased, whereas the ODG showed elevated CD4+ and NK cells when compared with the OG, DG, and CG (p < 0.05). Among the four groups, CD8+ levels at each temporal point did not show statistical significance (p > 0.05) as shown in Table .
Table 5. Comparison of perioperative cellular immunity indicators among the four groups ( ± s, %).
Comparison of the incidence of adverse reactions among four groups
The incidence of adverse reactions in four groups didn’t exhibit obvious distinctions (p > 0.05) (Table ).
Table 6. Comparison of incidence of adverse reactions among the four groups [n (%)].
Discussion
The commonly used analgesic and sedative drug dexmedetomidine mainly acts on brainstem vasomotor receptors to lower the release of norepinephrine (NE) and plasma NE, inhibiting sympathetic activity, without any respiratory depression. Of note, it applies analgesic and sedative effects in a dose-dependent manner. Besides, dexmedetomidine also suppresses systemic inflammatory responses in patients with sepsis and reduces serum TNF-α, IL-6, and IL-8 levels (Nagao et al. Citation2017). Separately, oxycodone is currently the only opioid amboceptor agonist available in clinical practice. As the basis for multimodal analgesia, it does not cause histamine release, bradycardia, or parasympathetic inhibition, and manifests the advantages of rapid onset and high safety. Further, oxycodone has a light effect on immunosuppression and reduces cortisol and testosterone levels while inhibiting excessive stress responses induced by trauma. Research (Toyama et al. Citation2017) has shown that oxycodone plays roles in the inhibition of inflammatory responses.
The study indicated that there existed obvious distinctions in recovery time and atropine use among four groups, because the combined use of dexmedetomidine and oxycodone exhibited less suppressive effects on the respiratory and central nervous systems, which could be confirmed by the fact that the patients were more easily woken up (Nonaka et al. Citation2016). Local anesthesia-based transforaminal endoscopic resection of nucleus pulposus is affected by multiple factors including those that are related to anesthesia, the operative wound, or patient positioning (prone position) that lead to intraoperative elevated blood pressure and/or myocardial oxygen consumption or an increase in the heartbeat, which are not conducive to surgery (Seddighi et al. Citation2016). In this paper, perioperative HRs and MAPs of the four groups were undulant. The HRs and MAPs in ODG reduced from the injection point (until the end of the surgery) and were dramatically lower over those in CG, dropped into the acceptable range (HR: 60–80 bpm, MAP: 70 mmHg). The HRs and MAPs in ODG were lower over those in OG and DG, and no statistically obvious distinctions were observed (Backonja et al. Citation2016; Kokki et al. Citation2017). These findings suggest that dexmedetomidine combined with oxycodone may lower the perioperative HR and blood pressure and the myocardial oxygen consumption of patients undergoing local anesthesia-based transforaminal endoscopic resection of nucleus pulposus. As compared with when using dexmedetomidine or oxycodone alone, the combination of both achieved a degree of superiority in decreasing the perioperative HR and blood pressure, although the effect was not strongly obvious. The reasons could be associated with the larger dexmedetomidine or oxycodone dose administered alone over that in combined use or the limited sample size. Such a slight amount of superiority may be associated with the integration of the two drugs offering multiapproach stress response inhibition.
Postoperative pain is a common stress reaction. VAS scores in ODG at 1, 2, 6, and 12 h following surgery were the lowest relative to those other groups. Besides, at 12 h following surgery, VAS scores in four groups exhibited no obvious distinction, which was in line with previous reports (Jiang et al. Citation2017). It was suggested that better early analgesic effects of dexmedetomidine combined with oxycodone were achieved as compared with those following just the use of dexmedetomidine or oxycodone alone. Possibly, dexmedetomidine combined with oxycodone enhances the potential analgesic effects via the route of synergistic interaction and the inhibition of stress responses in multiple pathways. The finding of comparative pain levels in the four groups at 12 h after surgery is likely to be related to the gradual fading of drug efficacy (Willey et al. Citation2016). In the OG, DG, and ODG, Ramsay scores at T1 and T2 increased, whereas those at T3 decreased, unlike in the CG where the Ramsay scores continued to decrease. The ODG presented higher Ramsay scores at T2 and T3 than did the OG, DG, and CG (p < 0.05). These results were consistent with those of other reports (Setnik et al. Citation2017). Perhaps, the sedative effects exerted from the time of injection onward increased the Ramsay scores. In the ODG, the noted superior sedation, especially with an average score (3.36 ± 0.32 points) at skin incision that evoked a state similar to normal sleep and which was conducive to stable hemodynamics, could be explained by that dexmedetomidine and oxycodone presented synergistic interactions by overlaying their respective analgesic and sedative effects. Conversely, in the CG, the normal saline used was not analgesic or sedative and lowered the Ramsay score.
In this study, TNF-α and IL-6 levels at T3, T4, and T5 were increased in the four groups, with the levels in the ODG being lower as compared with those in the OG, DG, and CG, while IL-2 levels at T3, T4, and T5 were decreased in the four groups, with the levels in the ODG being higher as compared with those in the OG, DG, and CG (p < 0.05), coinciding with prior reports (Mo and Qiu Citation2017). Among these records, the enhanced perioperative inflammation in patients receiving local anesthetized transforaminal endoscopic resection of nucleus pulposus was alleviated by the actions of dexmedetomidine combined with oxycodone, possibly through mechanisms related to dexmedetomidine suppressing stress reactions in such way that postsynaptic receptors were activated, sympathetic nerve activity was reduced, and the excitability in the sympathetic nervous system induced by the operative wound was blocked as well as related to oxycodone lowering the intracellular cyclic adenosine as well as disturbing the e synthesis, and release of inflammatory factors (Ok et al. Citation2016; Wong et al. Citation2018).
The T-cell subset is involved in cellular immunity and immunoregulation, in which CD4+ functions as the primary. CD8+ recognizes endogenous antigen-peptide T-cells presented by major histocompatibility complex class I molecules and applies cell-mediated cytotoxicity on target cells (Makhni et al. Citation2017). NK cells are an important part of innate immunity: they are involved in anti-infection, immune regulation, and, in some cases, in the killing of medium and target cells. The results of this study showed that CD4+, CD8+, and NK cell levels at T3, T4, and T5 were decreased, whereas the ODG presented elevated CD4+ and NK cells as compared with the OG, DG, and CG. Among the four groups, CD8+ levels at each temporal point did not show degrees of statistical significance. These findings indicated the occurrence of perioperative immunosuppression in participants and were consistent with prior reports (Chen et al. Citation2018). Dexmedetomidine combined with oxycodone alleviated immunosuppression likely by oxycodone elevating the IL-2 level, which activates and enhances immune responses, inducing Tc cell production, activating B-cell proliferation, and increasing the activity of T-cells and NK cells; at the same time, dexmedetomidine inhibited stress responses and reduced inflammation due to the peripheral immune system. Thus, these two medications had a synergistic interaction on immunosuppression (Sayed and Yassen Citation2016; Zhou et al. Citation2017). No obvious distinction existed in the incidence of adverse reactions among four groups; revealing that the combined use of dexmedetomidine and oxycodone didn’t elevate the incidence of adverse reactions.
Conclusions
Briefly, the combined use of dexmedetomidine and oxycodone exhibited noticeable sedation and analgesia effects in patients who receive local anesthesia-based transforaminal endoscopic nucleus pulposus excision. Of note, this medication approach ameliorated perioperative inflammations and relived immunosuppression without increasing adverse reactions. However, this study’s smaller sample size and bias in case selection make it necessary to perform further studies involving larger sample sizes so as to collect additional data.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data is not available.
References
- Backonja M, Webster LR, Setnik B, Bass A, Sommerville KW, Matschke K, Malhotra BK, Wolfram G. 2016. Intravenous abuse potential study of oxycodone alone or in combination with naltrexone in nondependent recreational opioid users. Am J Drug Alcohol Abuse. 42(5):539–549.
- Chen W, Jin N, Lin Y, Villani V, Shimizu A, Zhao X, Lu F, Li C, Chen K, Lin Z, et al. 2018. Immunomodulatory effects of fentanyl or dexmedetomidine hydrochloride infusion after allogeneic heart Transplantation in mice. Reg Anesth Pain Med. 43(5):509–515.
- Cui JH, Jiang WW, Liao YJ, Wang QH, Xu M, Li Y. 2017. Effects of oxycodone on immune function in patients undergoing radical resection of rectal cancer under general anesthesia. Medicine (Baltimore). 96(31):e7519.
- Jiang W, Wang Q, Xu M, Li Y, Yang R, Song X, Duan H, Zhang P. 2017. Assessment of different loading doses of dexmedetomidine hydrochloride in preventing adverse reaction after combined spinal-epidural anesthesia. Exp Ther Med. 13(6):2946–2950.
- Kibbe AH, Franko TS, Shah VM. 2018. Oxycodone hydrochloride immediate-release analgesic for managing severe pain: abuse-deterrent formulations. Ther Clin Risk Manag. 14:779–782.
- Kokki M, Heikkinen M, Valitalo P, Hautajarvi H, Hokkanen J, Pitkanen H, Sankilampi U, Ranta VP, Kokki H. 2017. Maturation of oxycodone pharmacokinetics in neonates and infants: oxycodone and its metabolites in plasma and urine. Br J Clin Pharmacol. 83(4):791–800.
- Kopecky EA, Vaughn B, Lagasse S, O'Connor M. 2017. Tolerability, safety, and effectiveness of oxycodone DETERx in elderly patients ≥65 years of age with chronic low back pain: A randomized controlled trial. Drugs Aging. 34(8):603–613.
- Makhni R, Attri JP, Jain P, Chatrath V. 2017. Comparison of dexmedetomidine and magnesium sulfate as adjuvants with ropivacaine for spinal anesthesia in infraumbilical surgeries and postoperative analgesia. Anesth Essays Res. 11(1):206–210.
- Mo Y, Qiu S. 2017. Effects of dexmedetomidine in reducing post-cesarean adverse reactions. Exp Ther Med. 14(3):2036–2039.
- Nagao S, Furihata M, Fukagawa K, Furihata T, Matsuhashi Y, Wada T. 2017. Premedication with fast-acting oxycodone hydrochloride hydrate effectively reduced oxaliplatin-induced severe vascular pain. J Infect Chemother. 23(7):493–497.
- Nonaka T, Inamori M, Miyashita T, Harada S, Inoh Y, Kanoshima K, Matsuura M, Higurashi T, Ohkubo H, Iida H, et al. 2016. Feasibility of deep sedation with a combination of propofol and dexmedetomidine hydrochloride for esophageal endoscopic submucosal dissection. Dig Endosc. 28(2):145–151.
- Ok SH, Kwon SC, Baik J, Hong JM, Oh J, Han JY, Sohn JT. 2016. Dexmedetomidine-induced contraction involves CPI-17 phosphorylation in isolated rat aortas. Int J Mol Sci. 17:10.
- Sayed E, Yassen KA. 2016. Intraoperative effect of dexmedetomidine infusion during living donor liver transplantation: A randomized control trial. Saudi J Anaesth. 10(3):288–294.
- Seddighi R, Odoi A, Doherty TJ. 2016. Effect of dexmedetomidine hydrochloride on tiletamine hydrochloride-zolazepam hydrochloride anesthesia in alpacas. Am J Vet Res. 77(10):1057–1063.
- Setnik B, Schoedel K, Bartlett C, Dick C, Hakim N, Geoffroy P. 2017. Intranasal abuse potential of an abuse-deterrent oxycodone formulation compared to oxycodone immediate release and placebo in nondependent, recreational opioid users. J Opioid Manag. 13(6):449–464.
- Toyama K, Furuie H, Kuroda K, Ishizuka H. 2017. Pharmacokinetic bioequivalence studies of an extended-release oxycodone hydrochloride tablet in healthy Japanese subjects under fasting and fed conditions without an opioid antagonist. Drugs R D. 17(3):363–370.
- Wang X, Wang K, Wang B, Jiang T, Xu Z, Wang F, Yu J. 2016. Effect of oxycodone combined with dexmedetomidine for intravenous patient-controlled analgesia after video-assisted thoracoscopic lobectomy. J Cardiothorac Vasc Anesth. 30(4):1015–1021.
- Willey JL, Julius TM, Claypool SP, Clare MC. 2016. Evaluation and comparison of xylazine hydrochloride and dexmedetomidine hydrochloride for the induction of emesis in cats: 47 cases (2007-2013). J Am Vet Med Assoc. 248(8):923–928.
- Wong ESW, Man RYK, Ng KFJ, Leung SWS, Vanhoutte PM. 2018. L-arginine and arginase products potentiate dexmedetomidine-induced contractions in the rat aorta. Anesthesiology. 128(3):564–573.
- Wu J, Lu Y, Cao X. 2019. Different effects of oxycodone and remifentanil in patients undergoing ultrasound-guided percutaneous radiofrequency ablation of hepatic cancer: a randomized trial. Drug Des Devel Ther. 13:365–372.
- Xiang X, Yuan X, Lian Y, Fang J, Wu Y. 2018. Effect of oxycodone hydrochloride combined with flurbiprofen axetil for intravenous patient-controlled analgesia in lower abdominal patients: A randomized trial. Medicine (Baltimore). 97(7):e9911.
- Xie K, Zhang W, Fang W, Lian Y, Lin S, Fang J. 2017. The analgesic efficacy of oxycodone hydrochloride versus fentanyl during outpatient artificial abortion operation: A randomized trial. Medicine (Baltimore). 96(26):e7376.
- Zhou HD, Jiang HF, Zhu YJ, Fang J. 2017. Clinical application of oxycodone combined with dexmedetomidine in percutaneous ultrasound-guided radiofrequency ablation of hepatocellular carcinomas. Zhonghua Yi Xue Za Zhi. 97(44):3480–3482.