Abstract
Dengue has become a global affliction despite continuous efforts to find cure for this age-old disease. Hopeful results for dengue vaccine are far-fetched as the effectiveness are tied to previous dengue virus (DENV) exposure and billions of dollars needed to be invested for vaccine development. Medicinal plants are ideal alternatives to combat DENV infection as it has long been utilized in traditional medicine besides showing virucidal properties. However, the mechanism of action by which the plant compounds inhibits the viral replication machinery remains to be discovered. This review provides existing knowledge on how DENV replicates within the host cells, recruiting more than 70 medicinal plants and some essential oils with anti-DENV properties, highlighting possible viral targets for DENV inhibition. The possible mechanism on how phytochemicals modulates host immune system in DENV infection by inhibiting cytokine storm and vascular leakage, were also discussed. Based on the in silico findings, NS2B/NS3 protease is the most distinctive DENV inhibitor target. Most anti-DENV plants research stood at their initial stages and efforts should be channeled into further in vitro characterization, in vivo studies by employing larger subsets of human subjects, and in silico studies to facilitate the understanding of inhibition mechanism.
Introduction
Dengue is an arthropod-borne viral disease carried by female Aedes mosquitoes. Dengue is transmitted to humans by Aedes aegypti (A. aegypti), to a lesser extent Aedes albopictus (A. albopictus) and Aedes polynesiensis (A. polynesiensis) (Cao-Lormeau Citation2009; Ferreira-De-Lima and Lima-Camara Citation2018). Dengue virus (DENV) infected individual suffers from headache, joint pain, rashes, low white blood cell count and may have mild asymptomatic dengue fever (DF) or severe dengue haemorrhagic fever (DHF) accompanied with shock known as dengue shock syndrome (DSS) (Murphy and Whitehead Citation2011). According to World Health Organization (WHO), the world population is at risk of contracting dengue as there are millions of infection occurring annually (https://www.who.int/denguecontrol/disease/en/).
Dengue is common in tropical and subtropical regions but air travel and unplanned urbanization have open ways to transmission of dengue into non-endemic countries (Salami et al. Citation2020). The Chinese encyclopedia written during the Jin Dynasty was the first record on dengue disease and its remedies (Gubler Citation1998). Recently, a tetravalent dengue vaccine, CYD-TDV (Dengvaxia, Sanofi Pasteur, Lyon, France), was approved in 20 endemic countries. CYD-TDV is recommended by WHO to only be used in dengue-infected individuals aged 9–45 years, as the vaccination of seronegative individuals may lead to severe dengue (WHO Citation2016). Due to the antibody dependent effect, dengue vaccine development is very challenging. Moreover, limited access of dengue vaccine to some countries suggested that effective next generation vaccine regardless of dengue exposure and other treatment options should be developed to combat this millennium old disease. Therefore, plant-based anti-DENV treatment could be a promising alternative in dengue treatment.
Plants have been traditionally used for centuries in Asia, Africa and Europe to treat various diseases including viral diseases (Herrmann et al. Citation2011; Ogbole et al. Citation2018). The antiviral properties were known to inhibit viral replication, endocytosis, viral genome transcription and protein synthesis (Ben-Shabat et al. Citation2020). Plant compounds could bind either to surface proteins, biomembrane, DNA or RNA of the viral particle (Herrmann et al. Citation2011). In order to understand the dengue viral machinery and to combat DENV effectively, in silico study in addition to in vitro and in vivo approaches has become a prominent strategy. The NS2B/NS3 protease of DENV-1, -2, -3 and -4 serotypes shared closely similar peptide substrate structure-activity relationship (Raut et al. Citation2015). The DENV infectivity decreases by 80% in cells treated with DENV protease inhibitors which corroborated that inhibition of viral protease could be the route to combat DENV pan-serotype (Raut et al. Citation2015). In silico studies of small molecules have suggested the potential anti-DENV activity which made further studies on its effectivity and mechanism worthwhile (refer in silico section).
The most recent comprehensive review of anti-DENV plants was reported by Kadir et al. (Citation2013). Despite the positive outcome of medicinal plants tested against DENV infections, efforts in understanding and mapping out the mechanisms of action is still ongoing. This review intended to provide an update on recent findings of anti-dengue medicinal plants around the world across in vitro, in vivo and in silico studies. This work also identifies the effective plant derivatives besides exploring likely mechanisms of medicinal plants in DENV pan-serotypes inhibition. This review is anticipated to offer a better understanding of the potential of medicinal plants to be developed into effective antiviral medication for dengue virus treatment.
Literature search and selection criteria
The literature search was carried out in March to April 2020. The following databases were searched electronically namely PubMed, ScienceDirect, BioMed Central, Frontiers and WHO’s library. The keywords used includes ‘dengue’ combined with ‘medicinal plants’, a combination of terms ‘DENV’, ‘DENV-1’, ‘DENV-2’, ‘DENV-3’, ‘DENV-4’, ‘dengue medicinal plants’ combined with ‘in silico’, ‘in vitro’ or ‘in vivo’, ‘anti-DENV plants’, ‘dengue mechanism’, ‘NS2B/NS3’ and ‘dengue and vascular leakage’. In addition, the reference list of all identified publications were checked to obtain more significant studies. Past review papers were also denoted to identify the plant names for related studies. Searches were restricted to papers written in English, however, one paper was written in Mandarin with English translations (paper by (Jiang et al. Citation2005)).
We removed duplicate papers collected from the databases. The inclusion criteria were; (i) studies (e.g. in silico, in vitro, in vivo) related to anti-dengue medicinal plants, (ii) case-reports and clinical studies. The exclusion criteria applied were; (i) letters, newspaper report and lectures conveying single expert opinion, (ii) papers published before 2015, however we also referred to older studies especially the initial study of the medicinal plant against dengue. We do not exclude articles based on the quality or reproducibility of evidence presented as we recognize the contribution of each study.
DENV structure, endocytosis and replication within host cells
The DENV particle is approximately 50 nm in diameter. An uninterrupted open reading frame (ORF) encoded the DENV RNA genome (10,723 nucleotides), is responsible for polyprotein precursor NH2-C-prM-E-NS1-NS2A-NS2B-NS3-NS4A-NS4B-NS5-COOH synthesis (Oliveira et al. Citation2017). Its polyprotein comprised of capsid protein (C protein), envelope protein (E protein), membrane-associated protein (M protein) and 7 non-structural (NS) proteins including NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5 (Chambers et al. Citation1990). Proteins NS3 and NS5 are fundamental for viral replication, RNA genome synthesis, and methylation of the 50 cap of the viral genome (Oliveira et al. Citation2017).
DENV is spread from one individual to another when an individual is bitten by an infected Aedes mosquito. DENV is a single stranded RNA virus with positive polarity, which belongs to the Flavivirus genus, Flaviviradae family of viral pathogens. Currently five different serotypes namely DENV-1, -2, -3, -4, and -5 have been identified, which poses challenges in vaccine development (Chambers et al. Citation1990; Mukhopadhyay et al. Citation2005; Cruz-Oliveira et al. Citation2015). Individuals only confer protective immunity against one serotype if previously contracted but not against other serotypes. Secondary dengue infection to other serotype however can be more severe (Soo et al. Citation2016).
DENV is inoculated into human skin during feeding and the target cell for dengue includes the dermal dendritic and mast cells that are localized under the epithelia (Navarro-Sánchez et al. Citation2005). Briefly, dengue infection started once the virus surface proteins attaches itself to the receptor molecules of the host cell (e.g. dendritic cells). The receptor-mediated endocytosis allows the internalization of virus particle. Once the virus enters the host cell, the E protein molecules on the viral particle surface shift to a trimeric conformation in the acidic compartment of an endosome. Then they protrude from the virus surface, and followed by membrane fusion which facilitates viral RNA release into the cytoplasm (Khetarpal and Khanna Citation2016). The viral genome acts as a messenger RNA and generates viral proteins. Newly generated RNA copies are subsequently incorporated into nascent organelles. DENV is assembled within the endoplasmic reticulum (ER) and the produced virions migrates through trans-Golgi network (TGN) and secretes into blood vessels, liver and spleen or migrate to regional lymph nodes along with their maturation process (Mukhopadhyay et al. Citation2005; Navarro-Sánchez et al. Citation2005). DENV may enter and replicate within white blood cells which in turn produces interferons that are responsible for fever, pain and flu-like symptoms (Kao et al. Citation2018).
Anti-DENV medicinal plants
This section highlighted the potential medicinal plants for dengue treatment including in vitro, in vivo and in silico studies worldwide. In silico findings regarding plant derivatives and small molecules that potentially target the proteins of DENV and the possible anti-dengue pathways will be discussed further towards the end of this paper. The anti-DENV properties from these medicinal plants are summarized according to the type of experiments as shown in Table 1.
Table 1. Summary of medicinal plants from around the world, their DENV inhibition activities and study approach, classified into type of experiments: (a) in vitro antiviral and cytotoxicity studies, (b) in vitro gene and/or protein expression studies, (c) in silico molecular docking studies, (d) animal studies, (e) human studies, and (f) isolation and structure analysis.
Acacia catechu
Acacia catechu (Family: Mimosaceae) is an indigenous plant found in India and Southeast Asia (Panya et al. Citation2019). In Thailand, the heartwood of Acacia catechu was used for anti-inflammatory and antidiarrheal purposes (Panya et al. Citation2019). The peptides extracted from Acacia catechu caused a reduction in intracellular envelope proteins across all DENV serotypes following a dose-dependent manner (Panya et al. Citation2019). The half maximal inhibitory concentration (IC50) of 0.18 µg ml−1 inhibited DENV foci formation effectively. From the four peptide tested, Peptide-2, -3 and -4 significantly reduced the foci formation (Panya et al. Citation2019). The crude peptide extract of 1.25 µg ml−1 could reduce virus production by approximately 100-fold with no observable cell toxicity (Panya et al. Citation2019).
Acorus calamus
Acorus calamus, Acorus odoratus or sweet flag (Family: Acoraceae), is a plant found in India, Sri Lanka, and margin of rivers in most Western countries (Chandra and Prasad Citation2017). It is a well-known traditional Chinese medicinal plant in which its roots were used to treat cholera and neurodegenerative diseases (Yao et al. Citation2018). Among the 12 compounds (α-asarone, β-asarone, acoramone, 2-hydroxyaconenone, butyric acid methyl ester, 2-acetoxyacorenone, acoramol, N-transferuloyl tyramine, tatanan A, tatarinoids A, tatarinoids B, acortatarin A) isolated from the root of Acorus calamus, tatanan A showed most significant anti-DENV-2 induced cytopathic effect which inhibited early stage of viral RNA replication, mRNA and protein (Yao et al. Citation2018). The Phase 2 glucokinase activator did not suppress DENV-2 replication suggesting that tatanan A inhibited flavivirus replication independent of glucokinase activation (Yao et al. Citation2018). Additionally, methanol leaf extracts of Acorus calamus inhibited DENV-2 in vitro by 96.5% at a dose of 20 µg ml−1 and 50% cytotoxic concentration (CC50) of 424.93 µg ml−1 (Rosmalena et al. Citation2019).
Allium sativum
Allium sativum or garlic (Family: Amaryllidaceae) originated from Central Asian countries had several therapeutic effects such as antioxidant, antibacterial, anticarcinogenic, reducing platelet aggregation, and antihyperlipidemia (Kusyati and Nyoman Citation2017). Garlic organosulfur compounds namely diallyl disulfide (DADS), diallyl sulfide (DAS) and alliin significantly reduced inflammatory cytokines in DENV-2 infection as observed in various cell culture study including: (i) Huh-7 cells – tumor necrosis factor α (TNF-α), interleukin 8 (IL-8), IL-10 and (ii) U937 cells – TNF-α, IL-8, IL-10 (Hall et al. Citation2017).
Alternanthera philoxeroides
Alternanthera philoxeroides (Family: Amaranthaceae) is a perennial herb, aquatic plant native to South Africa, China and South America (Pulipati et al. Citation2015). Alternanthera philoxeroides was reported to have therapeutic effects against influenza, human immunodeficiency virus (HIV) (Pulipati et al. Citation2015). A Mandarin article reported that all four petroleum ether, ethyl ether, ethyl acetate and coumarin extracts of Alternanthera philoxeroides inhibited DENV-2 with toxic dose (TD50) ratio as follows: 47.43, 86.67, 97.63 and 106.06 respectively (Jiang et al. Citation2005).
Anacolosa pervilleana
Anacolosa pervilleana (Family: Olacaceae) comprises of 17 species, found across Asia, Madagascar and Africa (Bourjot et al. Citation2012a). In Madagascar, the bark, leaves and young shoots of Anacolosa pervilleana called ‘Tanjaky’ were used for treating schistosomiasis, syphyllis, and general weakness (Bourjot et al. Citation2012a). Acetylenic acids extracted from Anacolosa pervilleana produced IC50 values around 3 µM in the DENV RNA-dependent RNA polymerase (RdRp) assay and inhibited DENV5 NS5 polymerase activity (Bourjot et al. Citation2012a).
Andrographis paniculata
Andrographis paniculata (Burm F.) Nees (Family: Acanthaceae) or known as king of bitters, is an herbaceous plant found in China, India and Southeast Asia (Jayakumar et al. Citation2013). It is traditionally used to treat sore throat, flu and respiratory infection while its major constituent, andrographolide, and its derivatives showed anticancer, anti-inflammatory, antibacterial, antidiabetic and antiviral activities (Jayakumar et al. Citation2013). Tang et al. found that methanolic extracts of Andrographis paniculata possessed inhibitory effects on serotype DENV-1 activity in in-vitro assays (Tang et al. Citation2012). The group compared the antiviral inhibitory effects of 6 plants (e.g. Andrographis paniculata, Citrus limon, Cymbopogon citratus, Momordica charantia, Ocimum sanctum, Pelargonium citrosum) and Andrographis paniculata showed the highest antiviral inhibitory effects (75%) in which the Andorgraphis paniculata treated cells showed least changes in cell morphologies without causing cytopathic effects (CPE) to the DENV-1 infected Vero E6 cells (Tang et al. Citation2012).
Panraksa et al. evaluated the antiviral effects of andrographolide against DENV-2 infected HepG2 and HeLa as well as DENV-4 with HepG2 cells (Panraksa et al. Citation2017). Andrographolide showed significant anti-DENV serotype 2 and 4 effects on both HepG2 and HeLa in which the cellular infection and virus output were all reduced with 50% effective concentration (EC50) of 21.304 and 22.739 µM for HepG2 and HeLa respectively (Panraksa et al. Citation2017). On the other hand, andrographolide was reported to significantly reduce viral production and DENV-4 genome copy number as well as no DENV-4 E protein was expressed in HepG2 cells from day 1 post infection (Panraksa et al. Citation2017). Time addition studies carried out by treating andrographolide to: (i) pre-treat cells before infection for 1 h, 30 min or 15 min, (ii) post treat cells infected cells at 0, 1, 2, 3, 4, 6, 9, 12 h after infection, showed that addition of andrographolide post-infection stage produced marked effects as compared to insignificant results showed in pre-treat cells (Panraksa et al. Citation2017).
It was evidenced that the disruptive effects of Andrographis paniculata extracts and andrographolide were via binding of DENV-2 and DENV-4 E protein to actin and several cellular pathways (Jitoboam et al. Citation2016; Panraksa et al. Citation2017). A follow up proteomic study carried out by Paemanee et al. found that the glucose regulated protein 78 (GRP78) was significantly down-regulated in DENV-2 infected andrographolide treated HepG2 cells as compared control (Paemanee et al. Citation2019). GRP78 functioned as a chaperone in protein folding in ER and a mediator of unfolded protein response which was manipulated by DENV to benefit its viral production (Pena and Harris Citation2011). This suggested that GRP78 and unfolded protein response played an important role in mediating andrographolide anti-DENV activity which may help in explaining the broad antiviral pathway of andrographolide.
Arrabidaea pulchra
Arrabidaea pulchra (Family: Bignoniaceae), are neotropical lianas occurring in Central America, Amazonia, Argentina, Bolivia, Brazil and Paraguay. Arrabidaea species were used as anti-inflammatory, astringent and anti-syphilitic agents (Brandão et al. Citation2013). Arrabidaea pulchra leaves ethanol extract called caffeoylcalleryanin showed anti-DENV-2 activity with EC50 of 46.8 ± 1.6 µg ml−1 (Brandão et al. Citation2013).
Azadarachta indica
Azadirachta indica or Neem (Family: Meliaceae) is a plant grown in Myanmar and Indian subcontinent countries. Azadirachta indica has therapeutic roles as anti-inflammatory, antiarthritic, antipyretic, antifungal, antibacterial and antitumor agent (Alzohairy Citation2016). Parida et al. reported that aqueous neem leaves extract at its maximum non-toxic dose of 1.897 mg ml−1 inhibited 100–10,000 TCID50 of DENV in vitro. In vivo results showed that neem leaves extract inhibited the virus replication in suckling mice at its maximum non-toxic dose 30–120 mg ml−1 (Parida et al. Citation2002). However, the pure neem compound (Azadirachtin) did not depict any inhibition on DENV-2 replication in both in vitro and in vivo studies (Parida et al. Citation2002).
Basilicum polystachyon
Basilicum polystachyon (Family: Lamiaceae) is a plant grown in Africa, Asia, Australia and India used to treat sprains, epilepsy and neuralgia (Madhavan et al. Citation2013). DENV plaque-reduction neutralization (PRNT) assay in Vero cells (African green monkey kidney) revealed that mictuxe and pure stachyonic acid A from Basilicum polystachyon showed identical inhibitory activity against DENV with IC50 of 1.4 ± 2/1 µM (Tan et al. Citation2019). The IC50 is expressed as normalized mean ± range from two independent experiments. Conjugated diene was found to be the active pharmacophore of stachyonic acids opening new pathway to anti-DENV drug discovery (Tan et al. Citation2019).
Boerhaavia diffusia
Boerhaavia diffusia (Family: Nyctaginaceae) is a medicinal plant used in traditional Indian medicine (Ayurveda) and also in South America and Africa (Mishra et al. Citation2014). Various parts and roots of Boerhaavia diffusia were used to treat gastrointestinal, hepatic and gynaecological illnesses (Mishra et al. Citation2014). Ayurvedic mixture of Tinospora cardifolia and Boerhaavia diffusia given with cow’s milk to dengue patients 2 or 3 times daily showed significant increase of patients’ platelet count and reduction in body temperatures (Bharati and Sinha Citation2012).
Boesenbergia rotunda
Boesenbergia rotunda (Family: Zingiberaceae) is a ginger species that grows in Southeast Asia, India, Sri Lanka, and Southern China (Eng-Chong et al. Citation2012). Boesenbergia rotunda was used traditionally to treat inflammation, fever, gout, flatulence, stomachache and dyspepsia (Eng-Chong et al. Citation2012). Among six compounds extracted from Boesenbergia rotunda namely pinostrobin, pinocembrin, alpinetin, cardamonin, panduratin A, and 4-hydroxypanduratin A that were tested for their inhibitory activities towards DENV-2 NS3 protease, panduratin A and 4-hydroxypanduratin A exhibited most prominent inhibition towards DENV-2 NS2B/3 protease cleavage by inhibiting 65% activity at the concentration of 80 ppm (parts per million) (Kiat et al. Citation2006). Pinocembrin and cardamonin showed weaker inhibitory activities at all concentrations tested but exhibited prominent inhibitory activity when both were combined suggesting synergistic effect against DENV-2 protease (Kiat et al. Citation2006). Further inhibitor constant (Ki) inhibition studies revealed that pinostrobin and cardomonin inhibited the NS3 protease via non-competitive mechanism with Ki (µM) value of 345 ± 70 and 377 ± 77 while panduratin A and 4-hydroxypanduratin A inhibition was via competitive inhibition with Ki (µM) value of 25 ± 8 and 21 ± 6 (Kiat et al. Citation2006).
Carica papaya
Carica papaya (Family: Caricaceae) are commonly grown in Central America, Africa and Asia (Kaur et al. Citation2019). All parts of Carica papaya had ethnomedicinal uses for instance; (i) leaves (to treat dengue, jaundice, obesity, fever, asthma, indigestion), (ii) flower (to treat jaundice, fever), (iii) fruit (to treat ulcer, impotency, heart attack, stroke, laxative), (iv) seeds (to treat intestinal worms, sickle cell, nasal congestion, detoxify liver and kidneys), (v) latex (to treat whooping cough, dyspepsia, diarrhea, burns pain, haemorrhoid), (vi) roots (to treat bronchitis, syphilis, respiratory and renal diseases) and (vii) stem bark (to treat jaundice, sexually transmitted diseases, anti-fungal and anti-haemolytic) (Kaur et al. Citation2019).
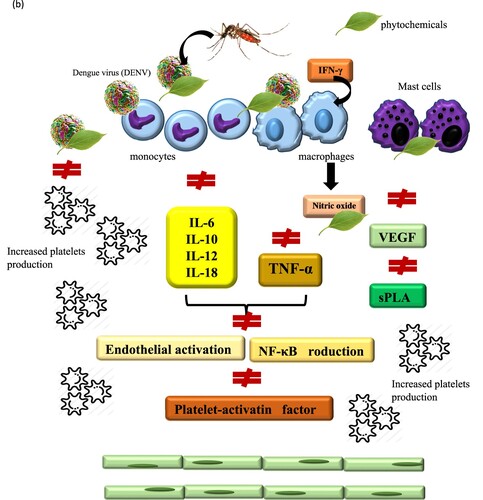
Carica papaya could control dengue at both, transmission and host level (Sarala and Paknikar Citation2014). Dengue patients who took two tablespoons of papaya leave juice three times daily at every 6-hour interval showed an increase in platelet count within 24 h (Kala Citation2012). Carica papaya capsules administered to dengue patients with thrombocytopenia for 7 days showed significant but fluctuating increment of platelet counts (Gadhwal et al. Citation2016). Carica papaya leaf extract (CPLE) treatment modulated DENV infection induced lower pro-inflammatory cytokine levels of TNF-α, interferon gamma (IFN-γ), interleukin 6 (IL-6), interleukin 4 (IL-4) and increased levels of helper T cells (Th2) cytokine IL-4 in CPLE treated group correlated with platelet count increment (Sathyapalan et al. Citation2020).
Despite studies that suggested anti-DENV properties of Carica papaya leaf juice, there are studies which reported that Carica papaya leaf juice or capsule administration actually showed insignificant increments in patients’ platelet counts and haematocrit after days of consumption (Assir et al. Citation2012; Yunita et al. Citation2012). It is worth noting that it is premature to recommend Carica papaya leaf juice to all patients with dengue fever as the actual mechanism are not known and that not all patients who consumed Carica papaya leaf juice showed an increment in platelet counts (Nuri and Ming Citation2016). Most of the aforementioned studies claimed the anti-dengue effects of Carica papaya based on patients’ symptoms reduction.
New Guinea C strain mouse model fed with 1000 mg/kg freeze-dried Carica papaya leaf juice (FCPLJ) from day 1 to day 3 post infection showed an alteration in its gene expression (Mohd Abd Razak et al. Citation2019). The altered genes include genes involved in regulation of endothelial permeability during DENV infection: (i) chemokine ligand 2 or monocyte chemoattractant protein-1 (CCL-2/MCP-1) was upregulated, (ii) Integrin beta-3 (ITGB3), Intercellular Adhesion Molecule 1 (ICAM1) and Fibronectin 1 (FN1) was downregulated (Mohd Abd Razak et al. Citation2019). Inflammatory cytokine genes namely chemokine ligand 6 (CCL6 or MRP-1), chemokine ligand 8 (CCL8 or MCP-2), chemokine ligand 12 (CCL12 or MCP-5), chemokine Ligand 17 (CCL17 or TARC), interleukin 1 receptor type I (IL1R1), interleukin-1 receptor antagonist (IL1RN or IL1Ra), nicotinamide phosphoribosyltransferase (NAMPT or PBEF1) and platelet factor 4 (PF4 or CXCL4) were downregulated in the liver of DENV-2 infected AG129 mice after treated with freeze-dried Carica papaya leaf juice. These data suggested that Carica papaya leaf juice could be used to reduce severe inflammatory processes in dengue infection (Norahmad et al. Citation2019). DENV-2 infected Sprague Dawley (SD) rats on the other hand showed significant increase in platelet counts along with an increase of IL6 and thrombopoietin levels after Carica papaya leaf extract administration (Sharma et al. Citation2019).
An in vitro study by Chinnappan et al. found that upon direct treatment of Carica papaya leaf extract, a significant reduction in platelet aggregation was observed in the dengue platelet-rich plasma and control platelet-poor plasma. The Carica papaya leaf extract was also found to contain dengue-specific neutralizing effect on dengue infected plasma, suggesting possible protective role on platelets (Chinnappan et al. Citation2016). The expression of envelope, NS1 proteins and intracellular viral load in DENV-infected THP-1 (human monocytic cell line derived from an acute monocytic leukemia patient) cells significantly decreases upon Carica papaya leaf extract treatment (Sharma et al. Citation2019).
Castanospermum australe
Castanospermum australe or commonly known as Blackbean or Moreton Bay Chestnut is indigenous to Australia and also found in Asia (Sajeesh and Parimelazhagan Citation2014). The seeds of Castanospermum australe containing castanospermine, are toxic to horses in particular, and could cause human poisonings but is edible after careful preparations into flour (Sajeesh and Parimelazhagan Citation2014). Castanospermine was found to inhibit virus cell secretion and cell to cell spread of DENV-1, -2, -3, and -4 serotype in vitro and in vivo (Whitby et al. Citation2005). Higher concentration of castanospermine is required to inhibit DENV-2 production in Huh-7 human hepatoma cell (IC50 of 85.7 µM) than BHK-21 cells (IC50 of 1 µM). Castanospermine prevented mortality in DENV infected mouse, with doses of 10, 50, and 250 mg Kg−1 (body weight/per day) and was highly effective in promoting survival (Whitby et al. Citation2005).
Chondrus crispus
Red seaweed Chondrus crispus (Family: Rhodophyta) or Irish Moss, is indigenous to northern Atlantic (Liu et al. Citation2015). Iota-carrageenan, a compound extracted from seaweed, inhibited DENV-2 infection in mosquito C6/36 HT and mammalian Vero cells (Laura B Talarico et al. Citation2011). Iota-carrageenans was less effective in C6/36 HT cells than Vero cells with effective concentration 50% (EC50) values 4.9 to 17.5 fold higher in mosquito cells than mammalian cells (Laura B Talarico et al. Citation2011). In Vero cells, the inhibition was exerted only on the initiation cycle of dengue virion binding while in mosquito cells, DENV-2 adsorption was not affected (Laura B Talarico et al. Citation2011). Inhibition only occurred in mosquito cells if iota-carrageenans was added to cells together with the DENV, after 8-hour post-infection or pre-treatment. The values of EC50 of iota-carrageenan treated DENV-2 infected Vero and C6/36 HT cells was >50 and 1.3 µg ml−1 respectively (Laura B Talarico et al. Citation2011).
Cissampelos pareira
Cissampelos pareira (Family: Menispermaceae) was traditionally used in India as blood purifier and anti-inflammatory remedy (Amresh et al. Citation2007). The roots of Cissampelos pareira was capable of facilitating wound healing besides showing antinociceptive and antiarthritic activity (Amresh et al. Citation2007). Alcoholic extract of Cissampelos pareira inhibited DENV replication in living cells, protected mice against dengue infection and had potent antiviral activity against four DENV-1, -2, -3 and -4 serotypes (Sood et al. Citation2015). The aerial part of Cissampelos pareira showed antiviral activity against DENV-3 with IC50 less than 25 µg ml−1 (Sood et al. Citation2015).
Cladogynos orientalis
Cladogynos orientalis (Family: Euphorbiaceae) or known as Chettaphangki in Thailand, is a plant used in traditional medicine to treat flatulence, stomachache, and as a tonic (Sithisarn et al. Citation2015). A concentration of 12.5 µg ml−1 ethanol extracts of Cladogynos orientalis exhibited inhibitory activity on DENV-2 by 34.85% while a concentration of 100 µg ml−1 ethanol extract of Cladogynos orientals inactivated the viral particle activity by 52.9% (Klawikkan et al. Citation2011). The 50% cytotoxic concentration (CC50) of ethanol extracts of Cladogynos orientalis on Vero cells was 312 µg ml−1 (Klawikkan et al. Citation2011).
Cladosiphon okamuranus
Cladosiphon okamuranus is a type of seaweed consumed in Japan (Shibata et al. Citation2000). Fucoidan derived from Cladosiphon okamuranus had gastric mucosal protection and anti-ulcer properties (Shibata et al. Citation2000). Fucoidans (sulfated polysaccharides) could bind entirely to DENV-2 and interact directly with the envelope glycoprotein (EGP) on DENV-2 (Hidari et al. Citation2008). Dengue virus serotypes -1, -2, -3 and -4 were premixed with fucoidan at various concentrations inoculated onto BHK-21 cells showed IC50 of >1000 (no inhibition), 4.7 (strongest inhibition), 500 and 365 µg ml−1 (Hidari et al. Citation2008).
Coptis chinensis
Coptidis rhizoma is the dried rhizome of Coptis chinensis, also known as Chinese goldthread, wei-lian or huang-lian. Coptis chinensis was used in Traditional Chinese Medicine (TCM) to treat inflammatory diseases and diabetes (Friedemann et al. Citation2014). Bioactive component of Coptis chinensis known as palmatine exhibited inhibitory effects on DENV-2 with EC50 value of 26.4 mM (F. Jia et al. Citation2010).
Cryptocarya chartacea
Cryptocarya chartacea (Family: Lauraceae) contained many secondary metabolites such as alkaloids, α-pyrones, simple and complex flavonoids which have shown biological properties including antiviral, cytotoxic or apoptosis inducer (P. Allard et al. Citation2011). The dialkylated flavanones, chartaceones C−F, showed the most significant inhibition on NS5 RdRp with IC50 ranging from 1.8 to 4.2 µM (P. Allard et al. Citation2011).
Cryptonemia crenulata
The sulfated galactan crude extracts of red seaweed Cryptonemia crenulata (Family: Halymeniaceae) collected from Brazil was found to have antiviral activity against herpes simplex virus type 1 (HSV-1) and 2 (HSV-2) (Laura B. Talarico et al. Citation2004). Cryptonemia crenulata was selective inhibitor of DENV-2 multiplication in Vero cells with IC50 around 1 µg ml−1 but showed lower antiviral effect against DENV-3 (IC50 of 13.9–14.2 µg ml−1) and DENV-4 (IC50 of 29.3 to >50 µg ml−1), inactive against DENV-1 (L.B. Talarico et al. Citation2005).
Curcuma longa
Curcuma longa (Family: Zingiberaceae) is commonly known as turmeric found in Southeast Asian countries, China and India (Karlowicz-Bodalska et al. Citation2017). Curcuma longa has wide range of pharmacological properties including anti-inflammatory, anti-oxidant, anti-diabetic and anti-cancer (Karlowicz-Bodalska et al. Citation2017). Ichsyani et al. tested the antiviral effect of Curcuma longa extract on DENV-2 in vitro and in vivo using Huh7it-1 cell (human cell line derived from liver) and Huh7it-1 infected DENV-2 NGC cells to ddY mice intra-peritoneally (Ichsyani et al. Citation2017). Both in vitro and in vivo study demonstrated that Curcuma longa extract killed the DENV infected cells although not significantly with CC50 of 85.4 µg ml−1 for in vitro where reduction of virus titer in vivo at 6 and 24 h post infection (Ichsyani et al. Citation2017).
Cymbopogon citratus
Cymbopogon citratus (Family: Poaceae) or lemon grass is a plant native to India, Sri Lanka and many Asian countries. Cymbopogon citratus has antifungal, anti-inflammatory and anti-protozoa properties (Brügger et al. Citation2019). Root methanol extract of Cymbopogon citratus inhibited DENV-2 by 98.9% in vitro at a dose of 20 µg ml−1, CC50 and EC50 value of 183.74 and 29.37 µg ml−1 (Rosmalena et al. Citation2019).
Distictella elongata
Distictella elongata (Family: Bignoniaceae) collected from Minas Gerais, Brazil was reported to have antiviral activity against DENV-2 besides also having antioxidant and anticancer properties (Simoes et al. Citation2011). A mixture of pectolinarin and acacetin-7-O-rutinoside was isolated from Distictella elongata ethanol extracts from fruits (FEE) has presented anti-DENV-2 activity with EC50 of 11.1 ± 1.6 µg ml−1 (Simoes et al. Citation2011).
Doratoxylon apetalum
Doratoxylon apetalum (Family: Sapindaceae) is a native medicinal plant to Mascarene Islands, owning protective effects against oxidative stress due to its high polyphenolic content (Marimoutou et al. Citation2015). Doratoxylon apetalum extract has antiviral effect against DENV-1, -2, -3 and -4 with IC50 values of 96.35, 16.75, 25.90 and 23.30 µg ml−1 respectively and DENV-2 was the most susceptible serotype towards Doratoxylon apetalum extract (Haddad et al. Citation2019).
Euphorbia hirta
Euphorbia hirta (Family: Euphorbiaceae) are herbs grown in open grasslands and popular for its folkloric medicinal use in India, Sri Lanka, Malaysia, Java, and Vietnam (Perera et al. Citation2018). Euphorbia hirta has been used to cure dengue fever in the Philippines and tribal groups in America (Mir et al. Citation2012; Perera et al. Citation2018). Euphorbia hirta contained alkenes, triterpenes, phytosterols, tannins, polyphenols and flavonoids which has immunomodulatory effects. Mir et al. found that the intake of Euphorbia hirta herbal water by dengue patients after 24 h was effective in increasing platelet counts, marked recovery in fever and flu-like symptoms (Mir et al. Citation2012). Euphorbia hirta helped in promoting platelet generation, preventing further bleeding which give time for patients to speed up their recovery. Capsules made from Euphorbia hirta were also consumed by the locals in the Philippines to treat dengue (Perera et al. Citation2018).
Flacourtia ramontchi
Flacourtia ramontchi (Family: Salicaceae) or known as Governor’s plum, is an evergreen tree native to southern Asia and Madagascar (Bourjot et al. Citation2012b). Phenolic glycosides of Flacourtia ramontchi showed anti-inflammatory, antimalarial, antiviral activity and could inhibit snake venom phosphodiesterase (Bourjot et al. Citation2012b). Betulinic acid 3β-caffeate isolated from Flacourtia ramontchi showed significant inhibition towards DENV RNA polymerase with IC50 of 0.85 ± 0.1 µM. Flacourtosides A and E as well as scolochinenoside D isolated from Flacourtia ramontchi inhibited DENV RNA polymerase to a lesser extent (Bourjot et al. Citation2012b).
Flagellaria indica
Flagellaria indica (Family: Flagellariaceae) is a climbing plant found in India, Southeast Asia, Polynesia and Australia. The leaves had medicinal value in curing cough, vomiting, wound-healing, and as health tonic (Gnanaraj et al. Citation2015). A concentration of 12.5 µg ml−1 Flagellaria indica ethanol extract was able to inhibit DENV-2 by 45.52% and the CC50 of Flagellaria indica on Vero cells was 312.5 µg ml−1 (Klawikkan et al. Citation2011).
Ficus septica
Ficus septica (Family: Moraceae) commonly known as hauili, is a fig found in Northeast India, South China, Taiwan, Australia and Malesian region (Ragasa et al. Citation2016). The decoction of Ficus septica roots served as toxin neutralizer, diuretic and asthma remedy, while the poultice served for boils. The fruits served as laxative and latex was used against herpes (Ragasa et al. Citation2016). Ficus septica leaf methanol extract inhibited DENV infection in several cell types including A549 human lung epithelial carcinoma cells, HepG2 human hepatocellular carcinoma cells, Huh7.5 human hematoma cells and WS1 human fetal skin normal fibroblasts cells (Huang et al. Citation2017). Interestingly, it was found that Ficus septica methanol extract was disruptive on enveloped virus but not on non-enveloped virus. Ficus septica could disrupt DENV-1 and DENV-2 enveloped viral layer and interfered with DENV contacting cells in vitro (Huang et al. Citation2017). The IC50 values of Ficus septica on DENV infection ranges differently according to type of extracts and part of the plant used: (i) root bark acetone extract with IC50 of 3.05 ± 0.75 µg ml−1, (ii) leaves methanol-ethyl acetate, acetone, chloroform and methanol extract with IC50 of 24.62 ± 4.04, 25.58 ± 9.13, >100, 18.37 ± 10.6 µg ml−1 respectively, (iii) fruit methanol extract with IC50 of 37.46 ± 12.3 µg ml−1, (iv) heartwood methanol extract with IC50 of 24.07 ± 13.18 µg ml−1 and (v) stem methanol extract with IC50 of 35.64 ± 21.2 µg ml−1 (Huang et al. Citation2017).
Garcinia mangostana
Mangosteen or Garcinia mangostana, ‘the queen of fruits’ (Family: Guttiferae) is a tropical evergreen tree grown in India, Sri Lanka, Myanmar, Malaysia, Philippines and Thailand (Cui et al. Citation2010). The pericarp (peel, rind and hull) or fruit of mangosteen was used as treatment for abdominal pain, dysentery, wound infection, ulcer, diarrhoea and suppuration (Cui et al. Citation2010). Bioactive compound purified from pericarp of mangosteen fruit, Garcinia mangostana, known as α-mangostin (α-MG) inhibited both DENV replication in cultured HepG2 and Huh-7 cells. It also reduced HepG2 cells’ cytokine (e.g. IL6, TNFα) or chemokine expression ligand 5 (RANTES), macrophage inflammatory protein 1β (MIP1β), interferon gamma-induced protein 10 (IP10) (Tarasuk et al. Citation2017). The actions were more potent than antiviral agent (ribavirin) and anti-inflammatory drug (dexamethasone) (Tarasuk et al. Citation2017). 20 µM of α-MG treatment to DENV-infected cells significantly gave reduced infection rate by 47-55% of all DENV pan-serotypes (DENV-1 to -4) (Tarasuk et al. Citation2017).
Gastrodia elata
Gastrodia elata (Family: Orchidaceae) was a treasured traditional Chinese herbal medicine recorded in Shen Nong’s Herbal Classic about 2000 years ago. Gastrodia elata served for treatment of headaches, epilepsy, dizziness, rheumatism, neuralgia, cramps and high blood pressure (Zhu et al. Citation2019). WSS45, a sulfated α-D-glucan, from Gastrodia elata strongly inhibited DENV-2 virus infection in vitro by employing baby hamster kidney cells (BHK cells) with EC50 value of 0.68 ± 0.17 µg ml−1, and it also increased the virus detachment from the BHK cells surface (Tong et al. Citation2010).
Glycyrrhiza glabra
Glycyrrhiza glabra (Family: Leguminoseae) or licorice is a plant found in Asia, Europe and Middle East (Zadeh et al. Citation2013). Studies reported that Glycyrrhiza glabra had antibacterial, antioxidant, anti-inflammatory, antihyperglycemic and antiviral activities (Zadeh et al. Citation2013). Prenylated stilbenoids and isoflavonoids from Glycyrrhiza glabra have demonstrated good docking properties to DENV protease (Powers and Setzer Citation2016).
Gymnogongrus griffithsiae
Red seaweed known as Gymnogongrus griffithsiae (Family: Phyllophoraceae) could be found natively occurring in Brazil (Laura B. Talarico et al. Citation2004). The sulfated galactan crude extracts of red seaweed Gymnogongrus griffithsiae has shown antiviral activity against herpes simplex virus type 1 (HSV-1) and 2 (HSV-2) (Laura B. Talarico et al. Citation2004). Gymnogongrus griffithsiae was a selective inhibitor of DENV-2 multiplication in Vero cells with IC50 around 1 µg ml−1 but had lower antiviral effect against DENV-3 (IC50 ranging from 13.9 to 14.2 µg ml−1) and DENV-4 (IC50 ranging from 29.3 to >50 µg ml−1) (L.B. Talarico et al. Citation2005). Gymnogongrus griffithsiae was inactive against DENV-1 but was an active DENV-2 inhibitor only when added together to the virus during early infection (L.B. Talarico et al. Citation2005).
Gymnogongrus torulosus
Gymnogongrus torulosus (Family: Phyllophoraceae) is a red seaweed which could be found in Argentina (Estevez et al. Citation2002). Gymnogongrus torulosus are rich in carrageenans which are sulfated galactans extracts (Estevez et al. Citation2002). The DL-galactan hybrids extracted from red seaweed Gymnogongrus torulosus interfered the binding of viral surface envelope glycoprotein with the cell receptor in vitro (Pujol et al. Citation2002). The DL-galactan hybrids produced IC50 ranging from 0.19 to 1.7 µg ml−1 for DENV-2, as determined in a virus plaque reduction assay in Vero cells (Pujol et al. Citation2002). The DL-galactans did not cause cytotoxic effects on stationary and actively dividing cells, and showed anticoagulant properties (Pujol et al. Citation2002).
Hedyotis auricularia
Hedyotis auricularia originating from Rubiaceae family, a plant locally found in Malaysia, were known for their antiviral effect against herpes simplex type-1 (HSV-1) and vesicular stomatitis (VSV) viruses (Ali et al. Citation1996). Different extracts of Hedyotis auricularia including leaf, stem, root ethanolic and methanolic extract inhibited the DENV-2 NS2B-NS3 protease with IC50 values <100 µg ml−1 (Rothan et al. Citation2014).
Hemigraphis reptans
Hemigraphis reptans also called red flame (Family: Acanthaceae) are green herbs native to Malaysia. Herbs belonging to Hemigraphis genus were traditionally used to treat excessive menstruation, inflammation, diabetes, anaemia, gallstone and wounds (S.M.M. Rahman et al. Citation2019). The leaf ethanolic and methanolic extract of Hemigraphis reptans exhibited inhibitory activities against DENV-2 NS2B-NS3 protease with IC50 values of <100 µg ml−1 (Rothan et al. Citation2014).
Hippophae rhamnoides
Hippophae rhamnoides or Sea buckthorn (Family: Elaeagnaceae) are thorny nitrogen fixing deciduous shrub cultivated in various parts of Asia and Europe for its nutritional and medicinal values as antioxidant, anticancer, anti-inflammatory, antiulcer and as liver tonic (Patel et al. Citation2012). Hippophae rhamnoides leaves alcoholic extract treatment decreased TNF-α but increased IFN-γ production in DENV-2 infected cells (Jain et al. Citation2008). Treatment of infected macrophages with 50 mg ml−1 Hippophae rhamnoides leaves alcoholic extract, decreased the viral titer significantly (p < 0.01) as compared to control cells (Jain et al. Citation2008).
Houttuynia cordata
Houttuynia cordata (Family: Saururaceae) is a perennial herb grown mainly in Eastern Asia which has anti-leukemic, anti-mutagenic, anti-inflammatory and anti-anaphylaxis properties (Yang and Jiang Citation2009). In China, Houttuynia cordata was an edible vegetable and was reported to reduce side-effects of clinically used drugs. It was shown to improve the immunity of severe acute respiratory syndrome (SARS) patients and anti-SARS virus (Yang and Jiang Citation2009). A concentration of 1.56 µg ml−1 ethanol extracts of Houttuynia cordata showed inhibitory activity against DENV-2 by 35.99% while its CC50 value was 312.5 µg ml−1 (Klawikkan et al. Citation2011).
Isatis tinctoria
Isatis tinctoria also known as Isatis indigotica, woad or Ban Lan Gen (Family: Brassicaceae) are herbaceous plants native to Central Asia, South-eastern Russia and South-eastern Europe (Speranza et al. Citation2020). This anti-inflammatory plant is widely utilized in traditional Chinese medicine, as cosmetics raw material production and food in Europe (Speranza et al. Citation2020). Immunomodulatory and antiviral compounds derived from Isatis tinctoria includes indican, isatin, indirubin, and indigotin (Chang et al. Citation2012). 27 compounds namely GB1-27 including alkaloids, flavonoids and phenolic compounds were isolated from the aerial parts of Isatis tinctoria (Gao et al. Citation2018). Among these 27 tested compounds, GB-7 was recommended as anti-DENV candidate (Gao et al. Citation2018). Further in vitro experiments, MTT colorimetric assay, virus load test, and in vivo mouse DENV-2 infection experiments was recommended to validate the roles of GB-7 in treating dengue fever as underlying biological mechanisms are still unclear (Gao et al. Citation2018).
Kaempferia parviflora
Kaempferia parviflora or Krachaidum or Thai ginseng (Family: Zingiberaceae), is a Thai traditional herb which could also be found in tropical areas such as Malaysia, Sumatra and Borneo (Saokaew et al. Citation2017). Kaempferia parviflora had anti-obesity, anti-inflammatory and antiviral effects (Saokaew et al. Citation2017). Main chemical constituents of Kaempferia parviflora are borneol and flavonoids (Sarangi and Padhi Citation2014). Studies have been done on the virucidal activity of leaves and stem extracts of Kaempferia parviflora against DENV-2. It was suggested that some of the bioactive compounds in Kaempferia parviflora inactivated the DENV-2 particles (Kanna and Krishnakumar Citation2019).
Laurentia longiflora
Ethanolic extracts of various parts (leaf, stem and root) of local Malaysian medicinal plant Laurentia longiflora (Family: Campanulaceae) exhibited inhibitory activities against DENV-2 NS2B-NS3pro with all IC50 values <100 µg ml−1 (Rothan et al. Citation2014).
Leucaena leucocephala
Leucaena leucocephala or coffee bush (Family: Fabaceae) is a tropical plant native to Central America and the Yucatan Peninsula of Mexico which require warm temperature for optimal growth (Nehdi et al. Citation2014). Sulfated galactomannans derived from Leucaena leucocephala produced 100-fold decrease in virus titer of DENV-1 at concentration of 37 mg L−1 and at dosage of 49 mg Kg−1, protected mice against death at 96.5% (Ono et al. Citation2003). In vitro experiments of DENV-1 in mosquito C6/36 cell culture assays showed a 100-fold decrease in virus titer of 37 mg L−1 (Ono et al. Citation2003).
Lonicera japonica
Lonicera japonica (Family: Caprifoliaceae) commonly known as Japanese honeysuckle, Jin Yin Hua or Ren Dong, is native to East Asia but also introduced to Western countries (Shang et al. Citation2011). Lonicera japonica and its active compounds had extensive pharmacological actions including antibacterial, anti-inflammatory, antiviral, anti-endotoxin, blood fat reducing, antipyretic properties (Shang et al. Citation2011). Lonicera japonica uptake enhanced innate miRNA let-7a in DENV-2 suppression both in vitro and in vivo (Y.-R. Lee et al. Citation2017). A significant increase of let-7a expression in hepatoma Huh7 cells after Lonicera japonica water extracts treatment for 36 h and 48 h was detected by real-time PCR. Lonicera japonica treatment resulted in NS1 RNA expression reduction by 27%, NS1 protein expression by 52% and reduction of over 30% virus titer in infected mice’s brain tissue (Y.-R. Lee et al. Citation2017). Since Lonicera japonica had a long history in treating flu-like symptoms in China, the possible side effects are negligible (Y.-R. Lee et al. Citation2017). Thus, Lonicera japonica has potential to be developed as antiviral agent.
Mimosa scabrella
Mimosa scabrella (Family: Fabaceae) known as bracatinga, is a fast-growing native leguminous tree, very abundant in Parana, Southern Brazil, where galactomannans extracted from the seeds were used to give high viscosities in dilute aqueous solutions and industrial applications in foods, paints, cosmetics and pharmaceuticals (Ganter et al. Citation1997). Sulfated galactomannan isolated from seeds of Mimosa scabrella produced 100-fold decrease in virus titer of DENV-1 at the concentration of 347 mg L−1 (in vitro) and at dosage of 49 mg Kg−1 (in vivo) protected mice against death at 87.7% (Ono et al. Citation2003).
Momordica charantia
Momordica charantia (Family: Cucurbitaceae) commonly known as bitter gourd, balsam pear, bitter melon, kugua or karela is widely cultivated in Asia, Amazon, Africa, South America, New Zealand and Middle East. Momordica charantia are used as prevention and remedy for diabetes, dysmenorrhea, eczema, jaundice, pneumonia, psoriasis, rheumatism and scabies (S. Jia et al. Citation2017). Methanolic extract of Momordica charantia showed antiviral effects against DENV-1. The antiviral assay was based on cytopathic effects, which was indicated by the degree of inhibition upon treatment of DENV-1 infected Vero E6 cells. The degree of inhibition on DENV-1 infected Vero E6 cells treated with maximum non-toxic dose of Momordica charantia at 0.20 mg/ml was 50% inhibition (Tang et al. Citation2012).
Myristica fatua
Myristica fatua (Family: Myristicaceae) is a scented tree with yellow fruits resembling apricot or peach, normally found in Bangladesh, India and Indonesia, which was used in traditional medicine as antioxidant, analgesics, amenorrheal, aphrodisiacs and digestive agents (Chowdhury et al. Citation2017). DENV-2 infected Huh7it-1 cell lines treated with single dose of 20 µg/ml of Myristica fatua methanol extract showed cell viability (%) in vitro of 122.7% (Rosmalena et al. Citation2019). This showed that the cytotoxic effect on Huh7it-1 cell lines was insignificant. Methanolic extract of Myristica fatua produced an average inhibition of 78.4% of DENV-2 NGC strain in vitro, CC50 and EC50 values of 474.42 and 25.33 µg ml−1 respectively (Rosmalena et al. Citation2019). The viral replication was also reduced as the plant extract concentration increased from 5, 10, 20, 40, 80, to 160 µg ml−1 (Rosmalena et al. Citation2019).
Myrtopsis corymbosa
Myrtopsis corymbosa (Family: Rutaceae) are plants found in New Caledonia which exhibited antiviral activity against DENV-2 RNA dependent RNA polymerase (DENV-NS5 RdRp) (Coulerie et al. Citation2013). The bark extract at 1 µg ml−1 had the strongest inhibition on DENV polymerase at 87% while isolated alkaloids was slightly active against DENV-NS5 (Kanna and Krishnakumar Citation2019). Further analysis of Myrtopsis corymbosa found that the plant contained (i) coumarins: ramosin, myrsellinol, myrsellin and (ii) alkaloids: skimmianine, γ-fagarin and haplopin, but these compounds showed weak anti-DENV activity (Coulerie et al. Citation2013).
Nephelium lappaceum
Native to Southeast Asia, the rambutan or Nephelium lappaceum and also cultivated in South American countries (Hernandez-Hernandez et al. Citation2019), the pulp, seed and peel of Nephelium lappaceum has anti-inflammatory, antioxidant, antiviral and antidiabetic properties (Hernandez-Hernandez et al. Citation2019). Geraniin derived from Nephelium lappaceum rind demonstrated antiviral activity against DENV-2 (Ahmad et al. Citation2017). It was postulated that geraniin inhibited the viral attachment which prevented the initial cell-virus interaction (Ahmad et al. Citation2017). Geraniin was not toxic to Vero cells even at the highest concentration tested and exhibited DENV-2 plaque formation inhibition with an IC50 of 1.75 mM (Ahmad et al. Citation2017).
Norantea brasiliensis
Norantea brasiliensis (Family: Marcgraviaceae) discovered by a Jacques Denis Choisy (1799–1859) is a climbing shrub that grows on the coastal region of Rio de Janeiro, Brazil, popularly known as ‘agarra-pé’ (grab-foot) (Fialho et al. Citation2016). Norantea brasiliensis featured biological activities such as anti-inflammatory, analgesic, and trypanocidal properties (Fialho et al. Citation2016). Crude ethanol extract of Norantea brasiliensis showed antiviral activity against DENV-2 and downregulated TNF-α, IL-6, IL-10 and IFN-α secretion, cellular antigenic viral load and secreted NS1 protein reduction (Fialho et al. Citation2016).
Ocimum sanctum
Ocimum sanctum (Family: Lamiaceae) also known as Ocimum tenuiflorum, Tulsi or ‘holy basil’, native to India and Southeast Asia, is a valuable plant in curing and preventing cough, fever, ulcer and also believed to have hepatoprotective, anti-inflammatory and antiviral properties (Jamshidi and Cohen Citation2017). It was reported that HepG2 cells treated with Ocimum sanctum extract at 1/2 MNTD showed significant plaque inhibitory effects towards DENV-1 of 157.5 ± 67.6 PFU ml−1 (Ling et al. Citation2014).
Pavetta tomentosa
Pavetta tomentosa (Family: Rubiaceae) known as Kattukkaranai and Karanai in Tamil, are shrubs widely distributed in India (Pratheeba et al. Citation2019). The fermented leaves are used as remedy to relieve pain of piles, boils and decoction of bark are administered to children to correct visceral obstructions (Pratheeba et al. Citation2019). DENV infected C6/C36 cells treated with crude extracts of Pavetta tomentosa demonstrated good antiviral activity at 500 µg ml−1 with cell viability of 11.45% (Pratheeba et al. Citation2019).
Piper retrofractum
Piper retrofractum (Family: Piperaceae) is a pepper commonly used as spice, native to India (Salehi et al. Citation2019). Chewing or decoction of the roots of Piper retrofractum can treat colic, dyspepsia and gastralgia. The heated salted and oiled leaves could be used to treat postpartum fevers and chills by applying it to the entire body, while the dried fruits could treat diarrhoea and cough (Salehi et al. Citation2019). 100 µg/ml ethanol extracts of Piper retrofractum inactivated the DENV-2 activity by 84.93% while CC50 value of ethanol and dichloromethane extract was 625 and 156.25 µg ml−1 respectively (Klawikkan et al. Citation2011).
Phyllanthus amarus, Phyllanthus niruri, Phyllanthus urinaria and Phyllanthus watsonii
Phyllanthus species are local Malaysian medicinal plants. Phyllanthus species are used to treat kidney diseases, diabetes, hepatitis, jaundice and cancers. After treatment of aqueous and methanolic extracts cocktail of 4 Phyllanthus species (Phyllanthus amarus, Phyllanthus niruri, Phyllanthus urinaria and Phyllanthus watsonii) on Vero cells infected with DENV-2, all 13 proteins involved in DENV E protein folding and assembly processes were altered (S.H. Lee et al. Citation2013). Synergistic effects of the abundant phytochemicals from 4 Phyllanthus species was anticipated to give more effective impact in challenging the DENV through multiple targets (S.H. Lee et al. Citation2013). The determined proteins consisted of host cell protein and viral protein involved in viral entry and replication, namely Heat shock protein 70 (HSP70), Hepatocyte growth factor receptor (HGFR), Trim 1, RNA binding motif 1 (RBM1), Nonstructural protein NS3, Chain B Dengue virus NS2B/NS3 Protease, DNA topoisomerase 1 (DNA Topo 1), DNA mismatch repair protein Msh2, Histidine triad nucleotide-binding protein, Calreticulin, Glyceraldehyde-3-phosphate dehydrogenase (G3PD), Polysialyltransferase and beta actin (S.H. Lee et al. Citation2013). The MNTD of aqueous and methanolic extract of Phyllanthus on Vero cells were 250.0 and 15.63 µg ml−1 respectively (S.H. Lee et al. Citation2013).
Psidium guajava
Psidium guajava or guava (Family: Myrtaceae) is a tropic tree grown for its fruit in Mexico, Africa, Asia and Central America (Naseer et al. Citation2018). The major ingredients of Psidium guajava are ascorbic acid and citric acid which has anti-mutagenic, antioxidants, anticancer, antiviral and anti-inflammatory properties (Naseer et al. Citation2018). Four out of five phytochemicals from Psidium guajava showed high selective inhibition on DENV-2 replication with Catechin as the most promising phytochemical, showing viral inhibition of more than 90% both in vitro and in vivo (Trujillo-correa et al. Citation2019). The post treatment analysis observed that all phytochemicals except hesperidin inhibited DENV-2 infection significantly, and the highest viral inhibition was found in cultures treated with quercetin, catechin, naringin and gallic acid by 100%, 91.8%, 64.5% and 67.3% respectively (Trujillo-correa et al. Citation2019). The bark ethanolic extracts of Psidium guajava reacted on DENV-2 infected Vero cells resulted in CC50 of 1000.0 µg/ml and EC50 0f 7.8 µg ml−1 (Trujillo-correa et al. Citation2019).
Quercus lusitanica
Quercus lusitanica or Quercus infectoria, is a small tree or shrub mainly present in Greece, Asia Minor, Syria, and Iran (Muliawan et al. Citation2006). The galls of Quercus lusitanica have great medicinal value and was deciphered to be antidiabetic, local anesthetic, antipyretic and anti-inflammatory (Muliawan et al. Citation2006). 180 µg/ml of Quercus lusitanica crude methanol extracts was found to completely inhibit the dengue virus infection at TCID50 of 1–1000 by the absence of cytopathic effect (CPE) (N.A. Rahman et al. Citation2006). Another study reported that low dose of Quercus lusitanica methanol extract at 0.032 mg ml−1 showed 100% inhibition with 10 TCID50 of virus, 50% and 25% inhibition with 100 and 1000 TCID50, respectively (Muliawan et al. Citation2006). The expression of nonstructural NS1 was downregulated as the concentration of Quercus lusitanica increases (Muliawan et al. Citation2006).
Rhizophora apiculata
Rhizophora apiculata (Family: Rhizophoraceae) is a marine mangrove used in traditional medicines in Asia and Africa (Prabhu and Guruvayoorappan Citation2012). The root, leaf, and/or stem extracts of Rhizophora apiculata conveyed inhibitory effect on bacterial, viral and fungal pathogens growth in humans (Prabhu and Guruvayoorappan Citation2012). A concentration of 12.5 µg ml−1 ethanol extract of Rhizophora apiculata exhibited inhibitory activity on DENV-2 by 56.14% and a concentration of 100 µg/ml ethanol extracts Rhizophora apiculata inactivated the viral particle activity by 41.5% (Klawikkan et al. Citation2011). Rhizophora apiculata ethanol extract showed CC50 of 625 µg ml−1 on DENV infected Vero cells (Klawikkan et al. Citation2011).
Schisandra chinensis
Schisandra chinensis (Family: Schisandraceae) is a plant commonly found in northeastern China, whose fruits had been used in traditional Chinese medicine for treatment of disorders of the gastrointestinal (GI) tract, respiratory failure, cardiovascular diseases, body fatigue, excessive sweating, and insomnia besides showing neuro and hepato-protective, anti-inflammatory, antiviral, and anti-cancer activities (Yi et al. Citation2016). Schisandrin A (bioactive lignan from Schisandra chinensis) inhibited DENV-1, -2, -3 and -4 serotypes following concentration and time-dependent manner, and is capable to protect mice against DENV infection (Yu et al. Citation2017). Schisandrin A treated cells resulted in EC50 value of 28.1 ± 0.42 µM against DENV-2 without significant cytotoxicity (Yu et al. Citation2017). The STAT1/2-mediated antiviral interferon response was believed to result in schisandrin A action against DENV replication (Yu et al. Citation2017).
Scutellaria baicalensis
Scutellaria baicalensis (Family: Lamiaceae) is a species of flowering plant indigenous to East Asia, Russia and has been cultivated in many European countries (Zhao et al. Citation2016). Chinese people have used Huang-Qin (dried root of Scutellaria baicalensis) for more than 2000 years as a traditional medicine for treatment of diarrhoea, dysentery, hypertension, hemorrhage, insomnia, inflammation and respiratory infections (Zhao et al. Citation2016). Baicalein extracted from the root of Scutellaria baicalensis inhibited DENV-2 replication in Vero cells and also showed virucidal effects against DENV-2 with IC50 of 1.55 µg ml−1 (Zandi et al. Citation2012).
Senna angustifolia
Senna angustifolia (Family: Caesalpiniaceae) is a local Malaysian plant known for its purpose as herbal laxative (Rothan et al. Citation2014). Ethanolic extract of Senna angustifolia leaves inhibited NS2B-NS3pro with IC50 value of 30.1 µg ml−1. The percentage viral inhibition of Senna angustifolia extract was lower compared to Vernonia cinerea and Tridax procumbens extracts as shown via plaque formation assay and RT-qPCR (Rothan et al. Citation2014).
Tarenna asiatica
Tarenna asiatica also known as Chomelia asiatica (Family: Rubiaceae) is commonly cultivated in India and the leaves, bark and roots of Tarenna asiatica were used in ancient Ayurveda for the treatment of many diseases (Manojj et al. Citation2020). Tarenna asiatica was described to have anti-inflammatory, anticancer and antioxidant properties (Manojj et al. Citation2020). DENV infected C6/C36 cells treated with acetone extracts of Tarenna asiatica recorded CC50 value of 34.35% at concentration of 500 µg ml−1 (Pratheeba et al. Citation2019).
Tephrosia madrensis
Tephrosia plants (Family: Fabaceae) are commonly grown in Mexico (Sánchez et al. Citation2000). The phytoconstituents present in Tephrosia genus demonstrated several biological activities including antidiabetic, antiulcer, antidiarrheal, wound healing, anti-inflammatory, antiviral and anti-fungal properties (Samuel et al. Citation2019). Glabranine and 7-0-methyl-glabranine were isolated from Tephrosia madrensis (Sánchez et al. Citation2000). Intracellular replication of DENV was reduced by 76.9% with 25 µM glabranine whereas 12 µM and 25 µM of 7-O-methyl-glabranine inhibited DENV replication by 75% (Sánchez et al. Citation2000). The inhibitory effects of glabranine and 7-O-methyl-glabranine on DENV-2 infected Rhesus monkey kidney epithelial cells (LLC-MK2) in vitro was in a dose-dependent manner (Sánchez et al. Citation2000).
Tridax procumbens
Tridax procumbens (Family: Asteraceae) commonly known as coatbuttons, is a native tropical plant (Rothan et al. Citation2014). The ethanol extract of Tridax procumbens stems inhibited DENV NS2B-NS3pro with IC50 value of 25.6 µg ml−1. This study recommended that Tridax procumbens was worth further investigation for its anti-DENV-2 activities (Rothan et al. Citation2014).
Trigonostemon cherrieri
Trigonostemon cherrieri (Family: Euphorbiaceae) is an indigenous plant in New Caledonia which possessed insecticidal, acaricidal, cytotoxic and antiviral properties (P.-M. Allard et al. Citation2012). DENV polymerase assay showed that trigocherrin A and trigocherriolides A and B (daphnane diterpenoid orthoesters isolated from Trigonostemon cherrieri) significantly inhibited NS5 RdRp of DENV with IC50 values of 12.7, 3.1 and 16 µM, respectively (P. Allard et al. Citation2012).
Uncaria tomentosa
Uncaria tomentosa (Family: Rubiaceae) are climbing vines native to South and Central America (Keplinger et al. Citation1999). Uncaria tomentosa are used to treat abscess, arthritis, asthma, chemotherapy side-effects, fevers, gastric ulcers, haemorrhages, inflammations, menstrual irregularity, recovery from child birth, rheumatism, wounds and as contraception (Keplinger et al. Citation1999). Anti-DENV-2 and immunomodulating in vitro effects of Uncaria tomentosa pentacyclic oxindole alkaloids reduced infected cell rates and inhibited cytokine activities, reduced TNF-α, IL-10, IFN-α production levels (Reis et al. Citation2008). IL-10 levels in human monocytes infected with DENV-2 was lowered from 572 ± 219 to 244 ± 60 pg ml−1 after Uncaria tomentosa treatment (Reis et al. Citation2008).
Vernonia cinerea
Vernonia cinerea (Family: Asteraceae), a plant native to Malaysia, exhibited inhibitory activities against DENV NS2B-NS3pro (Rothan et al. Citation2014). Among the three medicinal plants (e.g. Senna angustifolia, Tridax procumbens, Vernonia cinerea) studied under the same study, methanolic extract of Vernonia cinerea leaves produced the highest inhibitory effect on DENV NS2B-NS3pro with IC50 of 23.7 µg ml−1. The plaque formation assay and RT-qPCR revealed that percentage viral inhibition of Vernonia cinerea and Tridax procumbens extracts were significantly higher compared to Senna angustifolia extract (Rothan et al. Citation2014). Vernonia cinerea could be potentially developed as DENV-2 inhibitor (Rothan et al. Citation2014).
Zostera marina
Zostera marina (Family: Zosteracea) are eelgrass commonly found in the Sea of Japan (Kolenchenko et al. Citation2005). The pharmacological effects of Zostera marina includes hypocholesteremic, entherosorption, antibacterial, antiviral, antitumor, antiulcer, antioxidant and immunomodulating (Kolenchenko et al. Citation2005). The p-sulfoxy-cinnamic acid, zosteric acid (ZA), derived from Zostera marina, inhibited DENV-1, -2, -3 and -4 serotype and inhibited entry step in the viral life cycle (Rees et al. Citation2008). The anti-DENV activity of ZA are modest with IC50 vales of 2.3 mM (Rees et al. Citation2008).
Essential oils
Considerable evidence from in vitro studies on essential oils derived from plants suggested their potential for the development of antiviral drugs against dengue viral infections. Table summarized the essential oils from medicinal plants that showed pronounced virucidal activity against DENV-2. Essential oils from Lippia alba and Lippia citriodora showed virus plaque reduction and virus inactivation of DENV-1, -2, -3 and -4 serotypes upon treatment before adsorption on cell (Ocazionez et al. Citation2010). Among DENV1-4 serotypes tested, Lippia alba essential oil showed the highest selectivity index of 349 and IC50 value of 0.4 µg ml−1 on DENV-2, suggesting that Lippia alba essential oil is most potent against DENV-2 compared to other serotypes (Ocazionez et al. Citation2010).
Table 2. Essential oils from medicinal plants showing pronounced virucidal activity.
Garcia et al. reported that pronounced virucidal activity was only observed in Artemisia douglasiana and Eupatorium patens which inhibited DENV-2 infectivity with VC50 values (concentration needed to inactivate virus by 50%) of 60 and 150 ppm respectively (C.C. García et al. Citation2003). VC50 values more than 250 ppm shown by Aloysia gratissima, Heterotheca latifolia, Hyptis mutabilis, Lippia junelliana, Lippia turbinate and Tessaria absinthioides were not considered as pronounced virucidal activity (C.C. García et al. Citation2003).
A subsequent study by Garcia et al. reported that essential oils of Cleome aculeate, Eupatorium arnottianum, Eupatorium catarium, Lepechinia floribunda, Lantana camara, Lantana grisebachii, and Trixis divaricata reduced DENV-2 virus titer by 50% (IC50 in (ppm)) as follows; 20.8, 16.9, 21.1, 79.2, 57.3, 38.2 and 56.5 respectively (Cybele C. García et al. Citation2010).
In silico study
(I) DENV envelope protein (E protein)
Hidari et al. studied the amino acid of domain III of envelope glycoprotein among DENV type 1–4 (Hidari et al. Citation2008). The structure-based experiment found that glucuronic acid residues are one of the crucial determinants for fucoidan function and the putative candidate amino acid was within the region of EGP domain III region between the four DENV serotype strains. The substitution of Arg or Lys to Gln at position 323 and the distance from position 310 may cooperatively cause the susceptibility of DENV to fucoidan (Hidari et al. Citation2008). Powers and Setzer carried out molecular docking analysis on 2194 plant-derived secondary compounds with dengue virus protein targets including dengue virus protease (NS2B-NS3pro), helicase (NS3 helicase), methyltransferase (MTase), RNA-dependent RNA polymerase (RdRp) and dengue virus envelope protein (Ep) (Powers and Setzer Citation2016). Compounds from Silybum marianum, Paulownia tomentosa and Cannabis sativa namely 2,3-Dehydrosilybin, 3′,5,7-Trihydroxy-4′-methoxy-5-,6-diprenylisoflavanone, 3′-O-Methyldiplacone, 4′-O-Methyldiplacone, 8β-[4-Hydroxy-5-(5-hydroxytigloyloxy)-tigloyl]santamarin, 8-Prenylmucronulatol, Angusticornin B, Betulafolienetriol, Bonannione A, Caffeoyl-p-coumaroyltartaric acid, Calebin A, Cannflavin A, Chandalone, Cisilandrin, Curcumin I, Diplacone, Furost-20(22)-ene-2,3,26-triol, Hispaglabridin A, Isosilybin A, Isosilybin B, Isosilybin C, Isosilybin D, Kanzonol Y, Paratocarpin L, Phyllanone B, Piperaduncin A, Rhinacanthin K, Silandrin A, Silandrin B, Silybin, Terrestriamide and Tuberine was strongly docked to hydrophobic pore of DENV envelope protein (Powers and Setzer Citation2016). Docking studies showed that geraniin (compound from Nephelium lappaceum) bound to DENV E protein at the DIII region while ELISA assay validated the high affinity interaction between geraniin with recombinant E Domain III (rE-DIII) protein (Ahmad et al. Citation2017). Naringin and hesperidin extracted from Psidium guajava showed better scores than the theoretical threshold of −7.0 kcal/mol with E protein (Trujillo-correa et al. Citation2019).
(II) DENV methyltransferase
Glycyrrhia glabra and Curcuma longa compounds namely 8β–[4-Hydroxy-5-(5-hydroxytigloyloxy)tigloyl] santamarin, Acteol-26-ketone, Chartaceone B4, Curcumin I, Diplacone, Exiguaflavanone A, Flinderole B, Glabraisoflavanone B, Isoborreverine, Neosilyhermin A, Neosilyhermin B, Styracifolin B and Tubulosine showed strong docking energy towards DENV methyltransferase with Edock <−130 kj/mol but not as strong as compared to human RNA methyltransferase (Powers and Setzer Citation2016). These compounds could be considered in the development of DENV methyltransferase inhibitor.
(III) DENV RNA-dependent RNA polymerase (RdRp)
Phytochemicals from Glycyrrhia glabra namely 4,6-Dibenzoyl-2-[phenylhydroxymethyl]-3(2H)-benzofuranone, Dimethylisoborreverine, Drummondin E, Flinderole A, Flinderole B, Kanzonol Y, Neosilyhermin B and Pungiolide A demonstrated strong binding to DENV RNA-dependent RNA polymerase (Powers and Setzer Citation2016). These phytochemicals could be the inhibitors of DENV RNA-dependent RNA polymerase.
(IV) DENV NS2B-NS3 protease
An in silico fragment based study using 4-hydroxyoanduratin and panduratin A from Boesenbergia rotunda and synthetic inhibitor called 246DA against DENV-2 NS2B/NS3 showed that the ligands had good binding affinity towards the active site interacting with His51, Asp75, Ser135 and Gly153 (Frimayanti et al. Citation2012). The fragment-based technique divided the compounds 4-hydroxyoanduratin, panduratin A and 246DA into three different fragments each. New ligands were formed by linking these three fragments and docked into the active site of DENV-2 NS2B/NS3 protease (Frimayanti et al. Citation2012). This study indicated that Boesenbergia rotunda extracts could be potentially developed into competitive DENV inhibitors. Besides, the flavonoid quercetin derived from Carica Papaya showed the highest binding energy against NS2B-NS3 protease (Senthilvel et al. Citation2013). This is evident by the formation of six hydrogen bonds with the amino acid residues at the binding site of the NS2B-NS3 protease receptor (Senthilvel et al. Citation2013).
Qamar et al. screened phytochemicals from several plants (i.e. Gossypium hirsutum, Garcinia mangostana, Garcinia parvifolia, Salvia officinalis, Garcinia vieillardii) against dengue NS2B/NS3 Protease which was known to be responsible of its capability in viral protein cleavage (T. ul Qamar et al. Citation2014). Different classes of phytochemicals such as aromatic, carbohydrates, lignin, saponins, steroids, tannins, terpenoids and xanthones could act as inhibitors against DENV NS2B/NS3 protease (T. ul Qamar et al. Citation2014). The docking results revealed six phytochemicals including (-)-Gossypol, Mangostenone C, Garcidepsidone A, 4-hydroxyacetophenone 4-O-(6′-O-beta-D-apiofuranosyl)-beta-D-glucopyranoside, Demethylcalabaxanthone, Mangostanin showing strong binding affinity inside the active site but Mangostenone C, Garcidepsidone A, Demethylcalabaxanthone and Mangostanin from genus Garcinia showed greater inhibition towards the DENV NS2B/NS3 protease (T. ul Qamar et al. Citation2014). A subsequent study by Qamar et al. investigated another six flavonoids from Desmodium uncinatum, Dalbergia candenatensis, Glycine max, Calophyllum polyanthum, Ficus microcarpa and Glycyrrhiza uralensis namely Uncinanone B, 5-hydroxybowdichione, Prunectin, 5,7,3′,4′-tetrahydroxyisoflavone, Alpunumisoflavone and Glicoisoflavone which showed strong hydrophobic contact with DENV NS2/NS3 binding pocket containing catalytic triad (i.e. His 51, Asp 75, Ser 135) (M.T.U. Qamar et al. Citation2017). It was proposed that any inhibitor against the binding pocket of DENV NS2/NS3 protease could potentially combat all DENV serotypes (M.T.U. Qamar et al. Citation2017).
In addition, compounds from Flemingia stricta, Glycyrrhiza glabra, Macaranga pleiostemona and Populus balsamifera namely 2′,3,4,4′,6,-Pentamethoxychalcone, 3,3′,4,5′-Tetrahydroxy-5-prenylbibenzyl, 3,3′,5′-trihydroxy-4-methoxy-5-prenylbibenzyl, 3,4-Dehydro-5-carboxystrictosidine (aglycone), 3-Acetoxy-4′,5-dihydroxy-3′-prenyldihydrostilbene, 4′-O-Methylglycyrrhisoflavone, 4′-O-Methylneobavaisoflavone, 8-Prenylmucronulatol, Agnuside (aglycone), Balsacone A, Balsacone B, Balsacone C, Chartaceone A2, Euchrestaflavanone A, Flemiflavanone D, Glycyrrhisoflavone, Isosilandrin A, Kanzonol Y, Licobenzo furan, Nishindaside (aglycone), Rhodiolin, Silyhermin, Solophenol D and Umbelliprenin, showed strong docking energy to NS2B/NS3 protease with Edock <−130 kJ/mol but not as strong as compared to mammalian trypsin (Powers and Setzer Citation2016). These compounds could be considered in the development of anti-DENV NS2B/NS3 protease drug.
An in silico study led by Dwivedi et al. found that triterpenoids extracted from Azadirachta indica namely nimbin, desacetylnimbin and desacetylsalannin showed good binding affinity to DENV NS2B-NS3pro with binding energy of −5.56, −5.24 and −3.43 kcal/mol respectively whereas azadirachtin and salannin showed negligible interaction with the target protein (Dwivedi et al. Citation2016). This study suggested that Neem plant could be considered as potential anti-DENV drug. The DENV NS2B/NS3 protease is responsible for genome replication and viral RNA synthesis, thus could be the prime therapeutic target for anti-DENV drug development (Dwivedi et al. Citation2016).
One of the in vitro hits from National Cancer Institute database: D0713 bearing thioguanine scaffold was synthesized into ligands 1–15 and another six compounds (ligand 16–21) subsequently based on their inhibition activity (Hariono et al. Citation2019). The binding energy of ligand 18 (−16.10 kcal/mol) was compared to the control, a known inhibitor called panduratin A (−11.27 kcal/mol) corroborated with the experimental observation (Hariono et al. Citation2019). Ligand 18 and 21 both bound well within the active site of DENV-2 NS2B/NS3pro and they were stabilized by hydrogen bond formation with Asn174 (Hariono et al. Citation2019). Ligand 18 showed the most potent inhibition against DENV protease with IC50 = 0.38 µM and was recommended to be further developed into DENV-2 NS2B/NS3pro inhibitor (Hariono et al. Citation2019).
(V) DENV NS3 helicase
Several cinnamic acid derivatives from black cohosh (Cimiciguga racemosa) and compounds from Curcuma longa including 1,7-Bis(4-hydroxy-3-methoxyphenyl)-1,4,6-heptatrien-3-one, 3′-O-Methyldiplacol, 3′-O-Methyldiplacone, 4,6-Dibenzoyl-2-[phenylhydroxymethylene]-3(2H)-benzofuranone, Angusticornin B, Balsacone A, Balsacone C, Caffeoyl-p-coumaroyltartaric acid, Charteceone B3, Chicoric acid, Cimicifugic acid B, Cimicifugic acid G, Cimiciphenol, Cimiracemate D, Curcumin I, Curcumin II, Curcumin III, Dihydrocurcumin and Mulberrofuran Y showed strong docking energy to ATP binding site of DENV NS3 helicase with Edock <−140 kJ/mol (Powers and Setzer Citation2016). These compounds could be potentially developed into anti-DENV NS3 helicase drug.
(VI) DENV-2 NS5
The constituents from Cymbopogon citratus such as geraniol, geranial and geranyl acetate showed free binding energy of −5.2, −5.3 and −5.5 kcal/mol towards non-structural domain protein (NS5) of DENV-2 while constituents from Acorus calamus (β-asarone, acoric acid, calamusin D) had free binding energy of −4.7, −5.5, −6.1 kcal/mol and constituents from Myristica fatua (artesunic aicd, homoegonol, myristicin) exhibited free binding energy of −7.2, −7.1 and −5.4 kcal/mol (Rosmalena et al. Citation2019). Among the three plants, phytochemical constituents from Myristica fatua showed the highest anti-DENV activities in silico. A negative value indicated the compound binds better and hence, among other constituents from Myristica fatua, artesunic acid was the best inhibitor based on its most negative free energy of binding value at −7.2 kcal/mol (Rosmalena et al. Citation2019). All ligands extracted from Psidium guajava (e.g. quercetin, catechin, naringin, gallic acid, hesperidin) except gallic acid, had binding energy towards NS5 protein above the theoretical threshold of −7.0 kcal/mol (Trujillo-correa et al. Citation2019).
(VII) NS4B
Qaddir et al. performed docking assays on 9 phytochemicals (including Silymarin, Flavobion, Derrisin, Isosilybin, Mundulinol, Silydianin, Isopomiferin, Narlumicine and Oxysanguinarine) derived from various medicinal plants locally present in Pakistan and India including Silybum marianum, Tanacetum parthenium, Fumaria indica, Solanum nigrum, Andrographis paniculata and Melissa officinalis. The findings revealed that they exhibited potential inhibitory properties against DENV with binding affinity ≥−8 kcal/mol against DENV4-NS4B (nonstructural 4B protein from DENV-4 which is highly hydrophobic and associated with lumen side membrane of endoplasmic reticulum) and high reactivity in the binding pocket of DENV4-NS4B (Qaddir et al. Citation2017).
The molecular docking results of phytochemicals from green tea and woody plants including catechin, cianidanol, epicatechin, eupatoretin, glabranin, laurifolin, DL-catehin which interacted with NS4B protein in DENV-1, -2, -3, -4 had shown that plant phytochemicals have anti-dengue properties (Paul et al. Citation2016). NS4B is a membrane protein which is essential for viral life cycle. NS4B interacted with endoplasmic reticulum, and inhibited the interferon-α/β induced signaling cascade. Based on the results, it was deduced that these selected plant phytochemicals with functional groups at different position contributed to excellent NS4B inhibitory binding sites. The (-)-catechin, epicatechin and DL-catechin were suggested as potential candidates to be developed as anti-DENV compounds in combating DENV1-4 serotypes with (-)-catechin showing the most significant anti-DENV activity (Paul et al. Citation2016).
(VIII) NS1, NS3 and NS5
The most recent in silico study by Qamar et al. screened novel plant-derived multi-target drug like molecules namely Canthin-6-one 9-O-beta-glucopyranoside, Kushenol W and Kushenol K against NS1, NS3 and NS5 non-structural proteins (M.T.U. Qamar et al. Citation2019). Natural source of Cathin-6-one 9-O-beta-glucopyranoside is from Asian plant called Eurycoma harmandiana while Kushenol W and Kushenol K are from Sophora flavescens which was a Chinese medicinal herb (M.T.U. Qamar et al. Citation2019). The proposed plant-derived drug molecules showed strong hydrogen bonding and also hydrophobic interaction with crucial active residues of DENV proteins, disrupting their viral replication function and thereby is capable to inhibit DENV infectivity (M.T.U. Qamar et al. Citation2019). From this study, it was proposed that Canthin-6-one 9-O-beta-glucopyranoside, Kushenol W and Kushenol K could function synergistically or additively against pan-serotypes of DENV (M.T.U. Qamar et al. Citation2019).
To sum up, earlier findings of NS2B/NS3pro inhibitor was largely established on non-prime substrates which were identified by profiling dengue virus using tetra-peptides (Frecer and Miertus Citation2010). The earlier inhibitors were not ideal because the NS2B/NS3pro protein had solvent-exposed, shallow active site and substrates selectivity towards substrates containing arginine and lysine at position P1 and P2 (Yotmanee et al. Citation2015). However, there are several peptide or modified peptide molecules which showed good NS2B/NS3pro inhibition activities (Behnam et al. Citation2014; Rothan et al. Citation2014). As mentioned previously, small molecules from plants was also capable of inhibiting NS2B/NS3pro, for instance panduratin (Kiat et al. Citation2006). Nevertheless, based on Table , it could be deduced that NS2B/NS3 protease is the most plausible DENV inhibitor target despite the whole viral particle could be considered as the anti-DENV target.
Table 3. Plant compounds and their respective DENV targets in silico.
Overview of possible action of medicinal plants on DENV and host immune response
During dengue infection, inflammation was the major processes of the host immune response. Cells infected with the DENV produce nitric acid and protective pro-inflammatory cytokines such as IFN-γ, TNF-α, IL-6 and IL-10 to attract and activate leucocytes at the site of infection (Masood et al. Citation2018). IFN-γ, IL-8, -12, -10 and -18 and TNF-α played an important role to exert effective host immune response against DENV infection (Fagundes et al. Citation2011). DENV infected monocytes, dendritic cells and macrophages produces cytokines and TNF-α leading to endothelial activation and nuclear factor-κB (NF-κB), which stimulates platelet-activating factor (PAF) production (Malavige and Ogg Citation2017). Mast cells produces vascular endothelial growth factor (VEGF) which acts on secretory phospholipase A2 (sPLA2) that also contributed to PAF production (Malavige and Ogg Citation2017). Mast cells may also produce sPLA2 leading to synthesis of PAF (Malavige and Ogg Citation2017). DENV may directly infect platelets directly and produces cytokines (Malavige and Ogg Citation2017). These data showed that all factors contribute to ‘cytokine storm’ during DENV infection, causing vascular leakage and increased endothelial permeability, leading to severe dengue haemorrhagic fever or shock (refer Figure a).
Figure 1. (a) DENV is transmitted into the human body via infected Aedes mosquito. DENV attack the immune system leading to ‘cytokine storm’ and vascular leakage. (b) Action of medicinal plants in regulating host immune response during DENV infection. Phytochemicals targeting DENV entry and cytokines producing factors, during DENV infection are responsible to inhibit cytokines production, increase anti-viral IFN-γ and platelet production, thus dampens the cytokine storm and prevent vascular leakage.
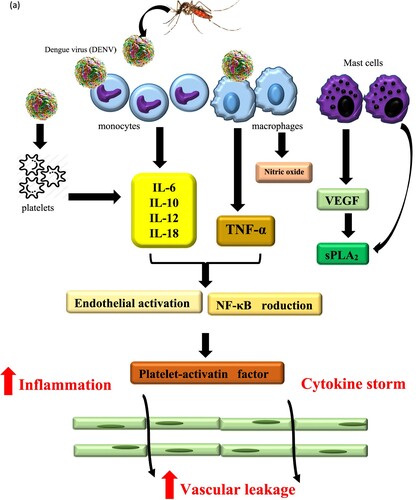
‘Cytokine storm’ which is the correlation between infection severity and high viral titer associated with excessive cytokine generation open ways to antiviral drug combination that could inhibit both DENV (antiviral) and cytokine (anti-inflammatory) production (Tarasuk et al. Citation2017). Many studies have found substantial anti-DENV properties (e.g. inhibit DENV entry to host cells) in medicinal plants compounds including polyphenols, alkaloids, flavonoids, saponins, quinones, terpenes, proanthocyanidins, lignins, tannins, polysaccharides, steroids, thiosulfonates and coumarins (Ali-Seyed and Vijayaraghavan Citation2020). DENV infection causes dysregulated immune response leading to extensive bleeding resulting in platelet reduction and hypovolemia which worsens the situation as the host could not respond to fluid and platelet replacement (Ali-Seyed and Vijayaraghavan Citation2020).
There are several medicinal plants found to potentially contribute to the prevention in vascular leakage during DENV infection. Phytoconstituents extracted from Andrographis paniculata showed anti-platelet properties by modulating PAF through signaling pathways including eNOS-NO/cyclic GMP PLC γ 2-PKC and PI3 kinas/Akt-MAPK (Lu et al. Citation2011). The organosulfur compounds in garlic for instances, namely DAS, DADS and DATS, could reduce oxidative stress response by suppressing nitric oxide production resulting in the inhibition of NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells), reduction of TNF-α and downregulation of nitric oxide synthase (Hall et al. Citation2017). Hippophae Rhamnoides leaf extract was found to decrease TNF-α but in turn increase the anti-viral IFN-γ production in DENV infected cells (Agrawal et al. Citation2016). Hippophae Rhamnoides leaves are rich in flavonoids, terpenoids, polyphenols, isorhamnetin while two of its compounds namely quercetin and fisetin, showed regulatory effects on IFN-γ production (Agrawal et al. Citation2016).
It is plausible to suggest that the anti-DENV drug should be developed focusing on its ability to inhibit the initiation of ‘cytokine storm’ processes and could inhibit vascular leakage by regulating the host immune response. Further studies are required to validate the precise antiviral mechanisms of medicinal plant compounds on DENV infection.
Other than that, the active compounds of Carica papaya including papain, chymopapain, cystatin, tocopherol, ascorbic acid, flavonoids, cyanogenic-glucosides and glucosinolates was reported to inhibit immune facilitated platelet damage, bone marrow suppression by virus and membrane stabilization of DENV infected cells, accelerating the natural course of recovery along with platelet count increase and prevents thrombocytopenia, without any side effects (Gadhwal et al. Citation2016). Euphorbia hirta was widely used in the Philippines to treat dengue and its anti-dengue properties was documented although the exact mechanism warrants further research (Ijaz et al. Citation2017). A study on anti-inflammatory and anxiolytic effects of Euphorbia hirta extracts using rat model found that treatment of Euphorbia hirta reduced the total leukocytes, eosinophils, IL-6, TNF-α, lipid peroxidation besides an increase in antioxidant levels. Therefore, medicinal plants capable to regulate host immune response by inhibiting the virus entry, factors which stimulates the synthesis of cytokines and PAF eventually leading to ‘cytokine storm’ would be one of the best anti-DENV candidate. The overview of the possible action of medicinal plants in regulating the host immune response in inhibiting cytokine storm and vascular leakage is shown in Figure b.
Possible anti-DENV protein targets
In addition, based on the data reviewed above, anti-DENV drug development should also target on the viral proteins, in which the inhibitor drug could bind to the active or on the surface viral proteins to stop viral replication or its attachment to the host cells. More efforts should be made to design inhibitors against DENV using dengue virus NS2B/NS3 protease as a molecular target. Although numerous studies reported on anti-DENV medicinal plants, most of the studies had no further follow up investigation. Nevertheless, studies on using Carica papaya as anti-DENV agent for instance have been active for years but its mechanism of action has yet to be discovered and animal model studies were limited. Besides, many of the small molecules derived from medicinal plants were not officially approved to be used in humans despite its positive outcome against DENV as reported in several in vitro and in silico studies.
The NS2B protein is located upstream of NS3 protease domain and functions as a cofactor in promoting NS3 conformational changes. NS2B/NS3 complex is responsible for the proteolytic processing of DENV polyprotein at the NS2A/NS2B, NS2B/NS3, NS3/NS4A and NS4B/NS5 junction. NS3 contains an ATPase/helicase and RNA tri-phosphatase domain in its C-terminal region, which is vital for viral RNA replication and capping. NS3 is an important target for screening drug candidates and efficacy evaluation because of its ability to cleave different parts of the polyprotein precursor and its participation in viral replication (Silva et al. Citation2019). The mechanism by which NS2B contributes to high activation of NS3pro remains poorly understood. The overview of the viral structure and the NS2B/NS3 protease as anti-DENV plant targets is shown in (Figure ).
Figure 2. Overview of the DENV structural and non-structural proteins and the proposed potential target sites (e.g. NS2b/NS3) for DENV inhibition by phytochemicals.
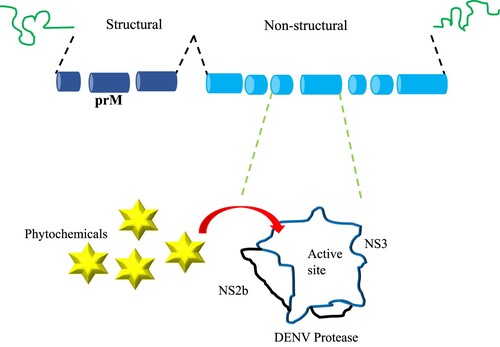
Conclusion
Herbal medicine targets and newly discovered potent plant derivatives have shown to be anti-DENV. However, the targets or mechanisms are yet to be validated. The constituents in medicinal plants are complex and may interact with targets either in synergistic or additive manner. Whether a single or several phytochemical(s) contributed to the antiviral action of medicinal plants is a critical concern to be revealed. Based on this literature, there is a ray of hope for the potential development of novel herbal anti-DENV drug. The DENV inhibitors is acclaimed to inhibit (i) viral entry; (ii) the viral protein NS4B; (iii) the viral capsid; (iv) the viral protease; (v) the viral helicase; (vi) the viral methyl-transferase; (vii) the viral polymerase; and (viii) the host target. It will be much effective in developing anti-DENV drugs by determining which viral factors are targeted by the diverse plant phytochemicals. This review therefore warrants to explore the possible mechanisms of action and provide platform for future investigations. Nevertheless, the observed plant anti-DENV effect requires further validation via in vitro, in silico and in vivo with larger study cohorts as small cohort analysis was not power-driven to recognize significant anti-DENV variances.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- Agrawal MJ, Chanda S, Rao EM, Ganju L. 2016. Effect of hippophae rhamnoides leaf extract against dengue virus infection in virology & mycology. Virol Mycol. 5(2):1–8.
- Ahmad SAA, Palanisamy UD, Tejo BA, Chew MF, Tham HW, Hassan SS. 2017. Geraniin extracted from the rind of nephelium lappaceum binds to dengue virus type-2 envelope protein and inhibits early stage of virus replication. Virol J. 14(229):1–13.
- Ali-Seyed M, Vijayaraghavan K. 2020. Dengue virus infections and anti-dengue virus activities of andrographis paniculata. Asian Pac J Trop Med. 13(2):49–55.
- Ali AM, Mackeen MM, El-Sharkawy SH, Hamid JA, Ismail NH, Ahmad FBH, Lajis NH. 1996. Antiviral and cytotoxic activities of some plants used in Malaysian indigenous medicine. Pertanika J Trop Agric Sci. 19(2/3):129–136. Available from: https://www.researchgate.net/publication/236960016.
- Allard P, Dau ETH, Eydoux C, Guillemot J-C, Dumontet V, Poullain C, Canard B, Gueritte F, Litaudon M. 2011. Alkylated flavanones from the bark of cryptocarya chartacea as dengue virus NS5 polymerase inhibitors. J Nat Prod. 74:2446–2453.
- Allard P, Leyssen P, Martin M, Bourjot M, Dumontet V, Eydoux C, Guillemot J, et al. 2012. Antiviral chlorinated daphnane diterpenoid orthoesters from the bark and wood of trigonostemon cherrieri. Phytochemistry. 84:160–168. doi:10.1016/j.phytochem.2012.07.023.
- Allard P-M, Martin M-T, Dau M-ETH, Leyssen P, Gueritte F, Litaudon M. 2012. Trigocherrin A, the first natural chlorinated daphnane diterpene orthoester from trigonostemon cherrieri. Org Lett. 14(1):342–345.
- Alzohairy MA. 2016. Therapeutics role of azadirachta indica (neem) and their active constituents in diseases prevention and treatment. Evidence-Based Complementary Altern Med. 2016(Article ID 7382506):1–11.
- Amresh G, Singh PN, Rao CV. 2007. Antinociceptive and antiarthritic activity of cissampelos pareira roots. J Ethnopharmacol. 111:531–536.
- Assir MZK, Nasir NU, Mansoor H, Waseem T, Ahmed HI, Riaz F, Bukhari SK, Akram J. 2012. Effect of carica papaya leaf extract on platelet count in dengue fever: a randomized controlled trial (plead trial). Int J Infect Dis. 16S(June):e317–e473.
- Behnam MAM, Nitsche C, Vechi M, Klein CD. 2014. C-Terminal residue optimization and fragment merging: discovery of a potent peptide-hybrid inhibitor of dengue protease. ACS Med Chem Lett. 5:1037–1042.
- Ben-Shabat S, Yarmolinsky L, Porat D, Dahan A. 2020. Antiviral effect of phytochemicals from medicinal plants: applications and drug delivery strategies. Drug Deliv Transl Res. 10:354–367.
- Bharati P, Sinha R. 2012. Study the effect of tinospora cardifolia (wild) miers and boerhaavia diffusia linn on dengue. Int J Ayur Herbal Med. 2(3):574–577.
- Bourjot M, Leyssen P, Eydoux C, Guillemot J, Canard B, Rasoanaivo P, Guéritte F, Litaudon M. 2012a. Chemical constituents of anacolosa pervilleana and their antiviral activities. Fitoterapia. 83:1076–1080. doi:10.1016/j.fitote.2012.05.004.
- Bourjot M, Leyssen P, Eydoux C, Guillemot JC, Canard B, Rasoanaivo P, Guéritte F, Litaudon M. 2012b. Flacourtosides A-F, phenolic glycosides isolated from flacourtia ramontchi. J Nat Prod. 75(4):752–758.
- Brandão GC, Kroon EG, Souza DER, Filho JDS, Oliveira AB. 2013. Chemistry and antiviral activity of arrabidaea pulchra (bignoniaceae). Molec. 18:9919–9932.
- Brügger BP, Martínez LC, Plata-Rueda A, de Castro e Castro BM, Soares MA, Wilcken CF, Carvalho AG, Serrão JE, Zanuncio JC. 2019. Bioactivity of the cymbopogon citratus (poaceae) essential oil and its terpenoid constituents on the predatory bug, podisus nigrispinus (heteroptera: pentatomidae). Sci Rep. 9(8358):1–8.
- Cao-Lormeau VM. 2009. Dengue viruses binding proteins from aedes aegypti and aedes polynesiensis salivary glands. Virol J. 6(35):1–4.
- Chambers TJ, Hahn CS, Galler R, Rice CM. 1990. Flavivirus genome organization, expression, and replication. Annu Rev Microbiol. 44:649–688.
- Chandra D, Prasad K. 2017. Phytochemicals of acorus calamus (sweet flag). J Med Plant Stu. 5(5):277–281.
- Chang SJ, Chang YC, Lu KZ, Tsou YY, Lin CW. 2012. Antiviral activity of isatis indigotica extract and its derived indirubin against Japanese encephalitis virus. Evidence-Based Complementary Altern Med. 2012:925830
- Chinnappan S, Ramachandrappa S, Tamilarasu K, Krishnan UM, Pillai AKB, Rajendiran S. 2016. Inhibition of platelet aggregation by the leaf extract of carica papaya during dengue infection: an in vitro study. Viral Immunol. 29(3):1–5.
- Chowdhury MAR, Manirujjaman, Haq MM. 2017. Phytochemical and pharmacological activity of myristica fragrans houtt (myristicaceae). Int J Toxicol Pharmacol Res. 9(1):56–63.
- Coulerie P, Maciuk A, Lebouvier N, Hnawia E, Guillemot JC, Canard B, Figadère B, Nour M. 2013. Phytochemical study of myrtopsis corymbosa, perspectives for anti-dengue natural compound research. Rec Nat Prod. 7(3):250–253.
- Cruz-Oliveira C, Freire JM, Conceição TM, Higa LM, Castanho MARB, Da Poian AT. 2015. Receptors and routes of dengue virus entry into the host cells. FEMS Microbiol Rev. 39(2):155–170.
- Cui J, Hu W, Cai Z, Liu Y, Li S, Tao W, Xiang H. 2010. New medicinal properties of mangostins: analgesic activity and pharmacological characterization of active ingredients from the fruit hull of garcinia mangostana L. Pharmacol Biochem Behav. 95:166–172. doi:10.1016/j.pbb.2009.12.021.
- Dwivedi VD, Tripathi IP, Mishra SK. 2016. In silico evaluation of inhibitory potential of triterpenoids from azadirachta indica against therapeutic target of dengue virus, NS2B-NS3 protease. J Vec Borne Disease. 53(June 2016):156–161.
- Eng-Chong T, Yean-Kee L, Chin-Fei C, Choon-Han H, Sher-Ming W, Li-Ping CT, Gen-Teck F, et al. 2012. Boesenbergia rotunda: from ethnomedicine to drug discovery. Evidence-Based Complementary Altern Med. 2012(Article ID 473637):1–25.
- Estevez JM, Ciancia M, Cerezo AS. 2002. Carrageenans biosynthesized by carposporophytes of red seaweeds gigartina skottsbergii (gigartinaceae) and gymnogongrus torulosus (phyllophoraceae) [Carrageenans biosynthesized by gametophytic and tetrasporic plants of seaweeds belonging to the gigartinace]. J Phycol. 38:344–350. Available from: https://www.ijabbr.com.
- Fagundes CT, Costa VV, Cisalpino D, Amaral FA, Souza PRS, Souza RS, Ryffel B, et al. 2011. IFN-γ Production depends on IL-12 and IL-18 combined action and mediates host resistance to dengue virus infection in a nitric oxide-dependent manner. PLoS Negl Trop Dis. 5(12):e1449.
- Ferreira-De-Lima VH, Lima-Camara TN. 2018. Natural vertical transmission of dengue virus in aedes aegypti and aedes albopictus: a systematic review. Parasites and Vectors. 11(77):1–8.
- Fialho LG, da Silva VP, Regina S, Reis NI, Azeredo EL, Kaplan MAC, Figueiredo MR, Kubelka CF. 2016. Antiviral and immunomodulatory effects of norantea brasiliensis choisy on dengue virus-2. Intervirology. 59:217–227.
- Frecer V, Miertus S. 2010. Design, structure-based focusing and in silico screening of combinatorial library of peptidomimetic inhibitors of dengue virus NS2B-NS3 protease. J Comput-Aided Mol Des. 24:195–212.
- Friedemann T, Otto B, Klätschke K, Schumacher U, Tao Y, Leung AKM, Efferth T, Schröder S. 2014. Coptis chinensis franch. Exhibits neuroprotective properties against oxidative stress in human neuroblastoma cells. J Ethnopharmacol. 155:607–615. doi:10.1016/j.jep.2014.06.004.
- Frimayanti N, Zain SM, Lee VS, Wahab HA, Yusof R, Rahman NA. 2012. Fragment-based molecular design of new competitive dengue Den2 Ns2b/Ns3 inhibitors from the components of fingerroot (boesenbergia rotunda). In Silico Biol. 11:29–37.
- Gadhwal AK, Ankit B, Chahar C, Tantia P, Sirohi P, Agrawal R. 2016. Effect of carica papaya leaf extract capsule on platelet count in patients of dengue fever with thrombocytopenia. J Asso Phys India. 64(June):22–26.
- Ganter JLMS, Cardoso ATM, Kaminski M, Reicher F. 1997. Galactomannan from the seeds of mimosa scabrella: a scale-up process. Int J Biol Macromol. 21:137–140.
- Gao B, Zhang J, Xie L. 2018. Structure analysis of effective chemical compounds against dengue viruses isolated from isatis tinctoria. Canadian J Infect Disease Med Microbiol. 2018(Article ID 3217473):1–11.
- García CC, Acosta EG, Carro AC, Belmonte MCF, Bomben R, Duschatzky CB, Perotti M, Schuff C, Damonte EB. 2010. Virucidal activity and chemical composition of essential oils from aromatic plants of central west Argentina. Nat Prod Commun. 5(8):1307–1310.
- García CC, Talarico L, Almeida N, Colombres S, Duschatzky C, Damonte EB. 2003. Virucidal activity of essential oils from aromatic plants of San Luis, Argentina. Phytother Res. 17:1073–1075.
- Gnanaraj C, Shah MD, Haque ATME, Iqbal M. 2015. Phytochemical screening, antioxidant properties in various extracts from the leaves of Flagellaria indica L. from Sabah, Malaysia. Int J Pharm Pharmac Sci. 7(9):510–512.
- Gubler DJ. 1998. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 11(3):480–496.
- Haddad JG, Koishi AC, Gaudry A, dos Santos CND, Viranaicken W, Desprès P, El Kalamouni C. 2019. Doratoxylon apetalum, an indigenous medicinal plant from mascarene islands, is a potent inhibitor of zika and dengue virus infection in human cells. Int J Mol Sci. 20(2382):1–13.
- Hall A, Troupin A, Londono-Renteria B, Colpitts TM. 2017. Garlic organosulfur compounds reduce inflammation and oxidative stress during dengue virus infection. Viruses. 9(159):1–10.
- Hariono M, Choi SB, Roslim RF, Nawi MS, Tan ML, Kamarulzaman EE, Mohamed N, et al. 2019. Thioguanine-based DENV-2 NS2B/NS3 protease inhibitors: virtual screening, synthesis, biological evaluation and molecular modelling. PLoS ONE. 14(1):e0210869. doi:10.1371/journal.pone.0210869.
- Hernandez-Hernandez C, Aguilar CN, Rodriguez-Herrera R, Flores-Gallegos AC, Morlett-Chavez J, Govea-Salas M, Ascacio-Valdes JA. 2019. Rambutan (nephelium lappaceum L.): nutritional and functional properties. Trends Food Sci Technol. 85:201–210. doi:10.1016/j.tifs.2019.01.018.
- Herrmann F, Romero MR, Blazquez AG, Kaufmann D, Ashour ML, Kahl S, Marin JJG, Efferth T, Wink M. 2011. Diversity of pharmacological properties in Chinese and european medicinal plants: cytotoxicity, antiviral and antitrypanosomal screening of 82 herbal drugs. Diversity. 3:547–580.
- Hidari KIPJ, Takahashi N, Arihara M, Nagaoka M, Morita K, Suzuki T. 2008. Structure and anti-dengue virus activity of sulfated polysaccharide from a marine alga. Biochem Biophys Res Commun. 376:91–95.
- Huang N-C, Hung W-T, Tsai W-L, Lai F-Y, Lin Y-S, Huang M-S, Chen J-J, Lin W-Y, Weng J-R, Chang T-H. 2017. Ficus septica plant extracts for treating dengue virus in vitro. PeerJ. 2017(5):e3448.
- Ichsyani M, Ridhanya A, Risanti M, Desti H, Ceria R, Putri DH, Sudiro TM, Dewi BE. 2017. Antiviral effects of Curcuma longa L. against dengue virus in vitro and in vivo. IOP Conf Series: Earth and Environ Sci Paper. 101:1–10. Available from: https://iopscience.iop.org/article/10.1088/1755-1315/140/1/012115/meta.
- Ijaz B, Ayub MA, Ben Ghnia J, Nisar S, Jilani MI. 2017. Potential use of tawa tawa: a schematic review of literature. Int J Chem Biochem Sci. 12:107–112. Available from: www.iscientific.org/Journal.html.
- Jain M, Ganju L, Katiyal A, Padwad Y, Mishra KP, Chanda S, Karan D, Yogendra KMS, Sawhney RC. 2008. Effect of hippophae rhamnoides leaf extract against dengue virus infection in human blood-derived macrophages. Phytomedicine. 15:793–799.
- Jamshidi N, Cohen MM. 2017. The clinical efficacy and safety of tulsi in humans: a systematic review of the literature. Evidence-Based Complementary Altern Med. 2017(Article ID 9217567):1–13.
- Jayakumar T, Hsieh C, Lee J, Sheu J. 2013. Experimental and clinical pharmacology of andrographis paniculata and its major bioactive phytoconstituent andrographolide. Evidence-Based Complementary Altern Med. 2013(Article ID 846740):1–16.
- Jia S, Shen M, Zhang F, Xie J. 2017. Recent advances in momordica charantia: functional components and biological activities. Int J Mol Sci. 18(2555):1–25.
- Jia F, Zou G, Fan J, Yuan Z. 2010. Identification of palmatine as an inhibitor of west Nile virus. Arch Virol. 155:1325–1329.
- Jiang W, Luo X, Kuang S. 2005. Effects of alternanthera philoxeroides griseb against dengue virus in vitro. J First Military Med Univ. 25(4):454–456.
- Jitoboam K, Phaonakrop N, Libsittikul S, Thepparit C, Roytrakul S, Smith DR. 2016. Actin interacts with dengue virus 2 and 4 envelope proteins. PLoS ONE. 11(3):1–18.
- Kadir SLA, Yaakob H, Zulkifli RM. 2013. Potential anti-dengue medicinal plants: a review. J Nat Med. 67:677–689.
- Kala CP. 2012. Leaf juice of carica papaya L.: a remedy of dengue fever. Med Aromat Plants (Los Angel). 1(6):1–2.
- Kanna SU, Krishnakumar N. 2019. Anti-Dengue medicinal plants: a mini review. J Pharmacogn Phytochem. 8(3):4245–4249.
- Kao Y-T, Lai MMC, Yu C-Y. 2018. How dengue virus circumvents innate immunity. Front Immunol. 9(Article 2860):1–8.
- Karlowicz-Bodalska K, Han S, Freier J, Smolenski M, Bodalska A. 2017. Curcuma longa as medicinal herb in the treatment of diabetic complications. Acta Pol Pharm. 74(2):605–610.
- Kaur M, Talniya NC, Sahrawat S, Kumar A, Stashenko EE. 2019. Ethnomedicinal uses, phytochemistry and pharmacology of carica papaya plant: a compendious review. Mini-Rev Org Chem. 16:1–18.
- Keplinger K, Laus G, Wurm M, Dierich MP, Teppner H. 1999. Uncaria tomentosa (willd.) DC. – ethnomedicinal use and new pharmacological, toxicological and botanical results. J Ethnopharmacol. 64:23–34.
- Khetarpal N, Khanna I. 2016. Dengue fever: causes, complications, and vaccine strategies. J Immunol Res. 2016(Article ID 6803098):1–14.
- Kiat TS, Pippen R, Yusof R, Ibrahim H, Khalid N, Rahman NA. 2006. Inhibitory activity of cyclohexenyl chalcone derivatives and flavonoids of fingerroot, boesenbergia rotunda (L.), towards dengue-2 virus NS3 protease. Bioorg Med Chem Lett. 16(12):3337–3340.
- Klawikkan N, Nukoolkarn V, Jirakanjanakit N, Yoksan S, Wiwat C, Thirapanmethee K. 2011. Effect of Thai medicinal plant extracts against dengue virus in vitro. Original Article Mahidol Univ J Pharmac Sci. 38(1–2):13–18.
- Kolenchenko EA, Sonina LN, Khotimchenko YS. 2005. Comparative in vitro assessment of antioxidant activities of low-etherified pectin from the eelgrass zostera marina and antioxidative medicines. Russian J Marine Biol. 31(5):331–334.
- Kusyati E, Nyoman N. 2017. Garlic extract to increase platelet levels in dengue hemorrhagic fever patients. Health Notions. 1(2):83–85.
- Lee SH, Tang YQ, Rathkrishnan A, Wang SM, Ong KC, Manikam R, Payne BJ, Jaganath IB, Sekaran SD. 2013. Effects of cocktail of four local Malaysian medicinal plants (Phyllanthus Spp.) against dengue virus 2. BMC Complement Altern Med. 13(192):1–13.
- Lee Y-R, Yeh S-F, Ruan X-M, Zhang H, Hsu S-D, Huang H-D, Hsieh C-C, et al. 2017. Honeysuckle aqueous extract and induced let-7a suppress dengue virus type 2 replication and pathogenesis. J Ethnopharmacol. 198:109–121. doi:10.1016/j.jep.2016.12.049.
- Ling APK, Khoo BF, Seah CH, Foo KY, Cheah RK, Chye SM, Koh RY. 2014. Inhibitory activities of methanol extracts of andrographis paniculata and ocimum sanctum against dengue – 1 virus. International conference on biological, environment and food engineering, August 4–5, p. 47–52.
- Liu J, Banskota AH, Critchley AT, Hafting J, Prithiviraj B. 2015. Neuroprotective effects of the cultivated chondrus crispus in a C. Elegans model of Parkinson’s disease. Mar Drugs. 13:2250–2266.
- Lu WJ, Lee JJ, Chou DS, Jayakumar T, Fong TH, Hsiao G, Sheu JR. 2011. A novel role of andrographolide, an NF-kappa B inhibitor, on inhibition of platelet activation: the pivotal mechanisms of endothelial nitric oxide synthase/cyclic GMP. J Mol Med. 89:1261–1273.
- Madhavan V, Yoganarasimhan S, Prasad R, Gurudeva M, Deveswaran R. 2013. Pharmacognostical studies on the leaves of basilicum polystachyon moench. J Tradi Med. 8(1):118–126.
- Malavige GN, Ogg GS. 2017. Pathogenesis of vascular leak in dengue virus infection. J Cells Molecul Sys Technol. 151:261–269.
- Manojj D, Yasasve M, Kanmani K, Sai Ramesh A. 2020. In vitro cytotoxicity study and anti-brucella activity of tarenna asiatica (L). S Afr J Bot. 128:54–61. doi:10.1016/j.sajb.2019.09.021.
- Marimoutou M, Le Sage F, Smadja J, D’Hellencourt CL, Gonthier MP, Da Silva CR. 2015. Antioxidant polyphenol-rich extracts from the medicinal plants antirhea borbonica, doratoxylon apetalum and gouania mauritiana protect 3T3-L1 preadipocytes against H2O2, TNFα and LPS inflammatory mediators by regulating the expression of superoxide dismut. J Inflamm. 12(10):1–15.
- Masood KI, Jamil B, Rahim M, Islam M, Farhan M, Hasan Z. 2018. Role of TNF α, IL-6 and CXCL10 in dengue disease severity. Iran J Microbiol. 10(3):202–207.
- Mir M, Khurshid R, Aftab R. 2012. Management of thrombocytopenia and flu-like symptoms in dengue patients with herbal water of euphorbia hirta. J Ayub Med College, Abbottabad: JAMC. 24(3–4):6–9.
- Mishra S, Aeri V, Gaur PK, Jachak SM. 2014. Ethnopharmacological overview for a traditionally important herb: boerhavia diffusa linn. BioMed Res Int. 2014(Article ID 808302):1–19.
- Mohd Abd Razak MR, Norahmad NA, Md Jelas NH, Jusoh B, Muhammad A, Mohmad Misnan N, Zainol M, Thayan R, Syed Mohamed AF. 2019. Preliminary study on the expression of endothelial cell biology related genes in the liver of dengue virus infected mice treated with carica papaya leaf juice. BMC Res Notes. 12(206):1–6. doi:10.1186/s13104-019-4242-z.
- Mukhopadhyay S, Kuhn RJ, Rossmann MG. 2005. A structural perspective of the flavivirus life cycle. Nat Rev Microbiol. 3(1):13–22.
- Muliawan SY, Kit LS, Devi S, Hashim O, Yusof R. 2006. Inhibitory potential of quercus lusitanica extract on dengue virus type 2 replication. Southeast Asian J Trop Med Public Health. 37:132–135.
- Murphy BR, Whitehead SS. 2011. Immune response to dengue virus and prospects for a vaccine. Annu Rev Immunol. 29:587–619.
- Naseer S, Hussain S, Naeem N, Pervaiz M, Rahman M. 2018. The phytochemistry and medicinal value of psidium guajava (guava). Clinical Phytoscience. 4(32):1–8.
- Navarro-Sánchez E, Desprès P, Cedillo-Barrón L. 2005. Innate immune responses to dengue virus. Arch Med Res. 36:425–435.
- Nehdi IA, Sbihi H, Tan CP, Al-Resayes SI. 2014. Leucaena leucocephala (Lam.) de Wit seed oil: characterization and uses. Ind Crops Prod. 52:582–587. doi:10.1016/j.indcrop.2013.11.021.
- Norahmad NA, Mohd Abd Razak MR, Mohmad Misnan N, Md Jelas NH, Sastu UR, Muhammad A, Ho TCD, et al. 2019. Effect of freeze-dried carica papaya leaf juice on inflammatory cytokines production during dengue virus infection in AG129 mice. BMC Complement Altern Med. 19(1):1–10.
- Nuri THM, Ming LC. 2016. Papaya leaves juice as an alternative treatment for dengue fever. J Appl Pharmac Sci. 6(3):172–173.
- Ocazionez RE, Meneses R, Torres FÁ, Stashenko E. 2010. Virucidal activity of Colombian lippia essential oils on dengue virus replication in vitro. Memórias Do Instituto Oswaldo Cruz. 105(3):304–309.
- Ogbole OO, Akinleye TE, Segun PA, Faleye TC, Adeniji AJ. 2018. In vitro antiviral activity of twenty-seven medicinal plant extracts from southwest Nigeria against three serotypes of echoviruses. Virol J. 15(110):1–8.
- Oliveira AFCdS, Teixeira RR, de Oliveira AS, de Souza APM, da Silva ML, de Paula SO. 2017. Potential antivirals: natural products targeting replication enzymes of dengue and chikungunya viruses. Molecules. 22(505):1–20.
- Ono L, Wollinger W, Rocco IM, Coimbra TLM, Gorin PAJ, Sierakowski M. 2003. In vitro and in vivo antiviral properties of sulfated galactomannans against yellow fever virus (BeH111 strain) and dengue 1 virus (hawaii strain). Antiviral Res. 60:201–208.
- Paemanee A, Hitakarun A, Wintachai P, Roytrakul S, Smith DR. 2019. A proteomic analysis of the anti-dengue virus activity of andrographolide. Biomed Pharmacother. 109:322–332.
- Panraksa P, Ramphan S, Khongwichit S, Smith DR. 2017. Activity of andrographolide against dengue virus. Antiviral Res. 139:69–78.
- Panya A, Yongpitakwattana P, Budchart P, Sawasdee N, Krobthong S, Paemanee A, Roytrakul S, Rattanabunyong S, Choowongkomon K, Yenchitsomanus P. 2019. Novel bioactive peptides demonstrating anti-dengue virus activity isolated from the Asian medicinal plant Acacia catechu. Chem Biol Drug Des. 93(May 2018):100–109.
- Parida MM, Upadhyay C, Pandya G, Jana AM. 2002. Inhibitory potential of neem (azadirachta indica juss) leaves on dengue virus type-2 replication. J Ethnopharmacol. 79:273–278.
- Patel CA, Divakar K, Santani D, Solanki HK, Thakkar JH. 2012. Remedial prospective of hippophae rhamnoides linn (sea buckthorn). ISRN Pharmacol. 2012(Article ID 436857):1–6.
- Paul A, Vibhuti A, Raj S. 2016. Molecular docking NS4B of DENV 1-4 with known bioactive phyto-chemicals. Bioinformation. 12(3):140–148.
- Pena J, Harris E. 2011. Dengue virus modulates the unfolded protein response in a time-dependent manner. J Biol Chem. 286(16):14226–14236.
- Perera SD, Jayawardena UA, Jayasinghe CD. 2018. Potential Use of euphorbia hirta for dengue: a systematic review of scientific evidence. J Trop Med. 2018(Article ID 2048530):1–7.
- Powers CN, Setzer WN. 2016. An In-silico investigation of phytochemicals as antiviral agents against dengue fever. Comb Chem High Throughput Screening. 19(7):516–536.
- Prabhu VV, Guruvayoorappan C. 2012. Anti-inflammatory and anti-tumor activity of the marine mangrove rhizophora apiculata. J Immunotoxicol. 9(4):341–352.
- Pratheeba T, Taranath V, Sai Gopal DVR, Natarajan D. 2019. Antidengue potential of leaf extracts of pavetta tomentosa and tarenna asiatica (rubiaceae) against dengue virus and its vector aedes aegypti (diptera: culicidae). Heliyon. 5:e02732. doi:10.1016/j.heliyon.2019.e02732.
- Pujol CA, Estevez JM, Carlucci MJ, Ciancia M, Cerezo AS, Damonte EB. 2002. Novel DL-galactan hybrids from the red seaweed gymnogongrus torulosus are potent inhibitors of herpes simplex virus and dengue virus. Antiviral Chem Chemother. 13:83–89.
- Pulipati S, P SB, Devi BS, Devi GR, Bhanuja M. 2015. Pharmacognostic studies of alternanthera philoxeroides (mart.) griseb. J Pharmacogn Phytochem. 4(2):202–204.
- Qaddir I, Rasool N, Hussain W, Mahmood S. 2017. Computer-aided analysis of phytochemicals as potential dengue virus inhibitors based on molecular docking, ADMET and DFT studies. J Vector Borne Dis. 54:255–262.
- Qamar MTU, Ashfaq UA, Tusleem K, Mumtaz A, Tariq Q, Goheer A, Ahmed B. 2017. In-silico identification and evaluation of plant flavonoids as dengue NS2B/NS3 protease inhibitors using molecular docking and simulation approach. Pak J Pharm Sci. 30(6):2119–2137.
- Qamar MTU, Maryam A, Muneer I, Xing F, Ashfaq UA, Khan FA, Anwar F, et al. 2019. Computational screening of medicinal plant phytochemicals to discover potent pan-serotype inhibitors against dengue virus. Sci Rep. 9(1433):1–16.
- Qamar Tu, Mumtaz A, Ashfaq UA, Azhar S, Fatima T, Hassan M, Hussain SS, Akram W, Idrees S. 2014. Computer aided screening of phytochemicals from garcinia against the dengue NS2B/NS3 protease. Bioinformation. 10(3):115–118.
- Ragasa CY, Macuha MR, De Los Reyes MM, Mandia EH, Van Altena IA. 2016. Chemical constituents of ficus septica burm, F. Int J Pharmac Clinical Res. 8(11):1464–1469.
- Rahman SMM, Atikullah M, Islam MN, Mohaimenul M, Ahammad F, Islam MS, Saha B, Rahman MH. 2019. Anti-inflammatory, antinociceptive and antidiarrhoeal activities of methanol and ethyl acetate extract of hemigraphis alternata leaves in mice. Clinical Phytosci. 5(16):1–13.
- Rahman NA, Hadinur SM, Rashid NN, Muhamad M, Yusof R. 2006. Studies on quercus iusitanica extracts on DENV-2 replication. Dengue Bulletin. 30:260–269.
- Raut R, Beesetti H, Tyagi P, Khanna I, Jain SK, Jeankumar VU, Yogeeswari P, Sriram D, Swaminathan S. 2015. A small molecule inhibitor of dengue virus type 2 protease inhibits the replication of all four dengue virus serotypes in cell culture. Virol J. 12(16):1–7.
- Rees CR, Costin JM, Fink RC, Mcmichael M, Fontaine KA, Isern S, Michael SF. 2008. In vitro inhibition of dengue virus entry by p-sulfoxy-cinnamic acid and structurally related combinatorial chemistries. Antiviral Res. 80:135–142.
- Reis SRIN, Valente LMM, Sampaio AL, Siani AC, Gandini M, Azeredo EL, D’Avila LA, Mazzei JL, Henriques MdGM, Kubelka CF. 2008. Immunomodulating and antiviral activities of uncaria tomentosa on human monocytes infected with dengue virus-2. Int Immunopharmacol. 8:468–476.
- Rosmalena R, Elya B, Dewi BE, Fithriyah F, Desti H, Angelina M, Hanafi M, Lotulung PD, Prasasty VD, Seto D. 2019. The antiviral effect of Indonesian medicinal plant extracts against dengue virus In vitro and In silico. Pathogens. 8(85):1–11.
- Rothan H, Zulqarnain M, Ammar Y, Tan E, Rahman N, Yusof R. 2014. Screening of antiviral activities in medicinal plants extracts against dengue virus using dengue NS2B-NS3 protease assay. Trop Biomed. 31(2):286–296.
- Sajeesh T, Parimelazhagan T. 2014. Analgesic, anti-inflammatory, and GC-MS studies on castanospermum australe A. Cunn. & C. Fraser Ex hook. Scientific World J. 2014(Article ID 587807):1–9.
- Salami D, Capinha C, Martins MdRO, Sousa CA. 2020. Dengue importation into Europe: a network connectivity-based approach. Plos One. 15(3):e0230274.
- Salehi B, Zakaria ZA, Gyawali R, Ibrahim SA, Rajkovic J, Shinwari ZK, Khan T, et al. 2019. Piper species: a comprehensive review on their phytochemistry, biological activities and applications.
- Samuel VJ, Mahesh AR, Murugan V. 2019. Phytochemical and pharmacological aspects of tephrosia genus: a brief review. J Appl Pharmac Sci. 9(3):117–125.
- Sánchez I, Gómez-Garibay F, Taboada J, Ruiz BH. 2000. Antiviral effect of flavonoids on the dengue virus. Phytother Res. 14:89–92.
- Saokaew S, Wilairat P, Raktanyakan P, Dilokthornsakul P, Dhippayom T, Kongkaew C, Sruamsiri R, Chuthaputti A, Chaiyakunapruk N. 2017. Clinical effects of krachaidum (kaempferia parviflora): a systematic review. J Eviden-Based Complement Alter Med. 22(3):413–428.
- Sarala N, Paknikar S. 2014. Papaya extract to treat dengue: a novel therapeutic option? Ann Med Health Sci Res. 4(3):320–324.
- Sarangi MK, Padhi S. 2014. Dengue and its phytotherapy: a review. Int J Pharmac Phytopharmacol Res. 4(1):37–46.
- Sathyapalan DT, Padmanabhan A, Moni M, P-prabhu B, Prasanna P, Balachandran S, Trikkur SP, et al. 2020. Efficacy & safety of carica papaya leaf extract µl) in adult dengue – results of a pilot study. PLoS ONE. 15(2):e0228699.
- Senthilvel P, Lavanya P, Kumar KM, Swetha R, Anitha P, Bag S, Sarveswari S, Vijayakumar V, Ramaiah S, Anbarasu A. 2013. Flavonoid from carica papaya inhibits NS2B-NS3 protease and prevents dengue 2 viral assembly. Bioinformation. 9(18):889–895.
- Shang X, Pan H, Li M, Miao X, Ding H. 2011. Lonicera japonica thunb.: ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J Ethnopharmacol. 138:1–21. doi:10.1016/j.jep.2011.08.016.
- Sharma N, Mishra KP, Chanda S, Bhardwaj V, Tanwar H, Ganju L, Kumar B, Singh SB. 2019. Evaluation of anti-dengue activity of carica papaya aqueous leaf extract and its role in platelet augmentation. Arch Virol. 164:1095–1110. doi:10.1007/s00705-019-04179-z.
- Shibata H, Kimura-Takagi I, Nagaoka M, Hashimoto S, Aiyama R, Iha M, Ueyama S, Yokokura T. 2000. Properties of fucoidan from cladosiphon okamuranus tokida in gastric mucosal protection. BioFactors. 11:235–245.
- Silva EM, Conde JN, Allonso D, Ventura GT, Coelho DR, Carneiro PH, Silva ML, et al. 2019. Dengue virus nonstructural 3 protein interacts directly with human glyceraldehyde- 3-phosphate dehydrogenase (GAPDH) and reduces its glycolytic activity. Sci Rep. 9(2651):1–19.
- Simoes L, Maciel G, Brandao G, Kroon E, Castilho R, Oliveira A. 2011. Antiviral activity of distictella elongata (vahl) urb. (bignoniaceae), a potentially useful source of anti-dengue drugs from the state of minas gerais, Brazil. Lett Appl Microbiol. 53:602–607.
- Sithisarn P, Rojsanga P, Sithisarn P, Kongkiatpaiboon S. 2015. Antioxidant activity and antibacterial effects on clinical isolated streptococcus suis and staphylococcus intermedius of extracts from several parts of cladogynos orientalis and their phytochemical screenings. Evidence-Based Complementary Altern Med. 2015(Article ID 908242):1–8.
- Soo KM, Khalid B, Ching S-M, Chee H-Y. 2016. Meta-analysis of dengue severity during infection by different dengue virus serotypes in primary and secondary infections. PLoS ONE. 11(5):1–16.
- Sood R, Raut R, Tyagi P, Kumar P. 2015. Cissampelos pareira linn: natural source of potent antiviral activity against all four dengue virus serotypes. PLoS Negl Trop Dis. 9(12):e0004255.
- Speranza J, Miceli N, Taviano MF, Ragusa S, Kwiecień I, Szopa A, Ekiert H. 2020. Isatis tinctoria L. (woad): a review of its botany, ethnobotanical uses, phytochemistry. Biolog Activ Biotechnol Stud Plant. 9(298):1–40.
- Talarico LB, Noseda MD, Ducatti DRB, Duarte MER, Damonte EB. 2011. Differential inhibition of dengue virus infection in mammalian and mosquito cells by iota-carrageenan. J Gen Virol. 92:1332–1342.
- Talarico LB, Pujol CA, Zibetti RGM, Faria PCS, Noseda MD, Duarte MER, Damonte EB. 2005. The antiviral activity of sulfated polysaccharides against dengue virus Is dependent on virus serotype and host cell. Antiviral Res. 66:103–110.
- Talarico LB, Zibetti RGM, Faria PCS, Scolaro LA, Duarte MER, Noseda MD, Pujol CA, Damonte EB. 2004. Anti-herpes simplex virus activity of sulfated galactans from the red seaweeds gymnogongrus griffithsiae and cryptonemia crenulata. Int J Biol Macromol. 34:63–71.
- Tan YP, Houston SD, Modhiran N, Savchenko AI, Boyle GM, Young PR, Watterson D, Williams CM. 2019. Stachyonic acid: a dengue virus inhibitor from basilicum polystachyon. Chem European J. 25:5664–5667.
- Tang LIC, Ling APK, Koh RY, Chye SM, Voon KGL. 2012. Screening of anti-dengue activity in methanolic extracts of medicinal plants. BMC Complement Altern Med. 12(3):1–10. Available from: https://www.biomedcentral.com/1472-6882/12/3.
- Tarasuk M, Songprakhon P, Chimma P, Sratongno P, Na-Bangchang K, Yenchitsomanus P. 2017. Alpha-Mangostin inhibits both dengue virus production and cytokine/chemokine expression. Virus Res. 240:180–189.
- Tong XK, Qiu H, Zhang X, Shi LP, Wang GF, Ji FH, Ding HY, Tang W, Ding K, Zuo JP. 2010. WSS45, a sulfated α-D-glucan, strongly interferes with dengue 2 virus infection in vitro. Acta Pharmacol Sin. 31:585–592.
- Trujillo-correa AI, Quintero-gil DC, Diaz-castillo F, Quiñones W, Robledo SM, Martinez-gutierrez M. 2019. In vitro and in silico anti-dengue activity of compounds obtained from psidium guajava through bioprospecting. BMC Complement Altern Med. 19(298):1–16.
- Whitby K, Pierson TC, Geiss B, Lane K, Engle M, Zhou Y, Doms RW, Diamond MS. 2005. Castanospermine, a potent inhibitor of dengue virus infection in vitro and in vivo. J Virol. 79(14):8698–8706.
- WHO. 2016. Dengue vaccine: WHO position paper – July 2016, 16, no. July 2016.
- Yang L, Jiang J. 2009. Bioactive components and functional properties of hottuynia cordata and its applications. Pharm Biol. 47(12):1154–1161.
- Yao X, Ling Y, Guo S, Wu W, He S, Zhang Q, Zou M, Nandakumar KS, Chen X, Liu S. 2018. Tatanan A from the acorus calamus L. Root inhibited dengue virus proliferation and infections. Phytomedicine. 42:258–267.
- Yi H, Chen Y, Liu J, Zhang J, Guo W, Xiao W, Yao Y. 2016. Extraction and separation of active ingredients in schisandra chinensis (turcz.) baill and the study of their antifungal effects. PLoS ONE. 11(5):e0154731.
- Yotmanee P, Rungrotmongkol T, Wichapong K, Choi SB, Wahab HA, Kungwan N, Hannongbua S. 2015. Binding specificity of polypeptide substrates in NS2B/NS3pro serine protease of dengue virus type 2: a molecular dynamics study. J Mol Graphics Modell. 60:24–33. doi:10.1016/j.jmgm.2015.05.008.
- Yu J, Wu Y, Tseng C, Lin C, Hsu Y, Chen Y-H, Lee J-C. 2017. Schisandrin A inhibits dengue viral replication via upregulating antiviral interferon responses through STAT signaling pathway. Sci Rep. 7(45171):1–12. doi:10.1038/srep45171.
- Yunita F, Hanani E, Kristianto J. 2012. The effect of carica papaya L. Leaves extract capsules on platelets count and hematocrit level in dengue fever patient. Int J Med Aromatic Plants. 2(4):573–578.
- Zadeh JB, Kor ZM, Goftar MK. 2013. Licorice (glycyrrhiza glabra linn) as a valuable medicinal plant. Int J Advan Biolog Biomed Res. 1(10):1281–1288. Available from: https://www.ijabbr.com.
- Zandi K, Teoh B, Sam S, Wong P, Mustafa MR, Abubakar S. 2012. Novel antiviral activity of baicalein against dengue virus. BMC Complement Altern Med. 12(214):1–9. BMC Complementary and Alternative Medicine.
- Zhao Q, Chen XY, Martin C. 2016. Scutellaria baicalensis, the golden herb from the garden of Chinese medicinal plants. Sci Bull. 61(18):1391–1398.
- Zhu H, Liu C, Hou J, Long H, Wang B, Guo D, Lei M, Wu W. 2019. Gastrodia elata blume polysaccharides: a review of their acquisition, analysis, modification, and pharmacological activities. Molecules. 24(2436):1–18.