Abstract
Paclitaxel-induced peripheral neuropathic pain (PIPNP) is a major therapy- and dose-limiting side effect that is extremely laborious to treat. Resveratrol (Res), with a structure name of 3,5,40-trihydroxystilbene, is a kind of natural polyphenolic chemical with plenty of useful characteristics such as anti-inflammatory, and anti-oxidative stress. However, the impact of Res on PIPNP remains unclear. Therefore, the current study intends to examine the analgesic effect of Res on PIPNP. In the present study, the results revealed that Res could effectively prevent the development of paclitaxel-induced PIPNP in a dose-dependent manner when repeated doses of Res were given during PIPNP induction. This prophylactic effect of Res was involved in inhibited paclitaxel-induced up-regulation of TRPV1 instead of TRPA1 in the dorsal root ganglia. Furthermore, the inhibition of TRPV1 up-regulation by Res may be involved in a phosphorylation of ERK mechanism. In summary, we conclude that Res plays an intriguing role in the prevention of PIPNP and thus may work as a therapeutic compound for treating and preventing PIPNP.
KEYWORDS:
Introduction
Anti-cancer drugs including taxanes, platinum analogues, proteasome inhibitors, and vinca alkaloids frequently possess several side effects and chemotherapy-induced peripheral neuropathic pain (CIPNP) is the most severe and common one (Argyriou et al. Citation2012; Park et al. Citation2013). CIPNP reduces the patient’s quality of life and leads to the decreased use or discontinuation of life-saving therapeutic drugs. Currently, no effective therapeutic drugs were successfully prepared for the prevention and treatment of CIPNP. Paclitaxel is a widely utilized drug for cancer chemotherapy that usually results in serious peripheral neuropathic pain in numerous treated patients (Postma et al. Citation1995). This kind of side effect deeply does harm on the patient’s life quality, limits or results in discontinuation of paclitaxel utilization. Since the present analgesics possess only mild effects and exhibit unacceptable side effects, clinical treatments of paclitaxel-induced peripheral neuropathic pain (PIPNP) remain formidable (Farquhar-Smith Citation2011). Until now, no effective therapy has been successfully developed in PIPNP prevention as well as treatment (Brewer et al. Citation2016).
Members of the transient receptor potential family of ion channels (TRP channels) were determined as contributors to the induction of PIPNP by previous literatures (Chen et al. Citation2011; Hara et al. Citation2013). TRPV4 and TRPM8 displayed sensitivities in DRG neurons in PIPNP while TRPV1 was regarded as a mediator of cold and mechanical allodynia (Anand et al. Citation2010; Descoeur et al. Citation2011; Nassini et al. Citation2011; Chen et al. Citation2015). PIPNP in rodents will be reduced, which resulted from selective inhibition by TRPV4 or TRPV1 (Chen et al. Citation2011). Furthermore, paclitaxel leads to an enhancement of expression levels of two critical neurotransmitters, consisting of substance P and Calcitonin Gene-Related Peptide (CGRP), engaged in the neuropathic pain (Chiba et al. Citation2016; Darby et al. Citation2017).
In the therapy of chronic comorbidities such as cancer, diabetes, and pain, plant natural extracts are considered as traditional Chinese medicine (Yuan et al. Citation2015). The pain-relieving effect of antioxidant is known and well described, a direct relationship between the antioxidant and pain-relieving effect was demonstrated, probably by the decrease in ROS but also by a neuroprotective effect (Di Cesare Mannelli et al. Citation2012). Resveratrol (Res), with a structure name of 3,5,40-trihydroxystilbene, is a kind of natural polyphenolic chemical with plenty of useful characteristics such as anti-tumorigenic activities, anti-inflammatory, and anti-oxidative stress (Baur and Sinclair Citation2006). Res possesses neuroprotective effects as well as improves the behavioral and pathological outcomes of diverse types of nerve injury, including traumatic brain injury, peripheral nerve injury, stroke, and spinal cord injury (Ates et al. Citation2006; Singleton et al. Citation2010; Girbovan et al. Citation2012; Xu et al. Citation2018). Res could also reduce peripheral sensitization by inhibiting inflammatory responses in nerves or local tissues (Singh and Vinayak Citation2016). Moreover, previous studies revealed that in the spinal cord, the anti-inflammatory effect of Res was associated with the suppression of glial activation (Yang et al. Citation2016). These profiles give prominence to the capacity of Res in the therapy of pains arising from the peripheral or central nervous system.
As an adjuvant during chemotherapy, few investigations so far have been performed to study the influence and mechanism of Res. In the former publication, TRPV1 receptor was reported to be associated with the endometriosis pain by ERK signaling pathway (Liu et al. Citation2018). Considering this, we assume that whether some kind of drugs could block TRPV1 up-regulation and ERK phosphorylation in PIPNP rats. In the present study, therefore, the effects of the repeated dose of Res were examined on the prevention of PIPNP in rats. Besides, the reversal of paclitaxel-induced alterations of TRPA1 and TRPV1 channel in the DRG by Res was studied in order to further clarify the deep mechanism.
Materials and methods
Animals
Male Sprague–Dawley rats (200–220 g) were purchased from the Laboratory Animals Center of Sun Yat-sen University (Guangzhou, China) and a 12 h/12 h light/dark cycle were maintained. Water and food were obtained ad libitum. All procedures in the present study were conducted according to the Guide for the Care and Use of Laboratory Animals and approved by the Animal Care and Use Committee of Sun Yat-sen University. The approval number is GD-2019007.
Drug and design of the experiments
Paclitaxel (>99% degree) was bought from Dalian Meilun Biology Technology Co. Ltd (China). It was dissolved in DMSO and consecutively diluted using saline buffer, followed by intraperitoneally injection every other day. According to previous literature, a final cumulative doses of 8 mg/kg were acquired on days 1, 3, 5, and 7 in order for peripheral neuropathic pain induction (Ba et al. Citation2018).
Res purchased from Sigma-Aldrich (USA) was dissolved in 2% DMSO and saline buffer, followed by once-daily injection (i.p. 100 or 200 mg/kg) into rats for 21 days, which begins on the day surgery started. Based on previous studies concerning the i.p. treatment research of resveratrol on spinal pathology, we chose this dose. The same volume of 2% DMSO in saline were administered and set as the control group. All the solutions were performed 0.22 μm membrane filtration and sterilization steps. Concerning on behavioral experiments, rats (n = 8) were randomly divided into the following four groups: saline + vehicle group, saline + paclitaxel group, Res + paclitaxel (100 mg/kg) group, and Res + paclitaxel (200 mg/kg) group. Res was conducted intraperitoneal (i.p.) injection every 24 h in rats, while the same volume of DMSO in saline was conducted the same operation for vehicle and paclitaxel-treated animals groups. After the final behavioral tests, tissue samples were collected within 24 h. The operators who were blinded to the drug treatments carried out all the behavioral tests.
Mechanical allodynia
The Von Frey test was performed for the assessment of mechanical allodynia. A clear plastic box with a size of 20*17*13 cm and with a wire mesh floor was placed 40 cm above the bench and utilized for the rats placements. Before testing, rats were of 15 min to habituate to these environments. An electronic Von Frey hair unit obtained from IITC (USA) was utilized: with a 0.2 g accuracy, the withdrawal threshold was assessed by employing forces from the range 0–50 g. Withdrawal threshold would automatically exhibit on the screen when punctuate stimulus was transformed to the mid-plantar area of each hind paw from below the mesh floor through a plastic tip. The minimum force required to extract a robust and immediate withdrawal reflex of the paw is defined as the paw sensitivity threshold. It would not be regarded as a withdrawal response when voluntary movements were related with locomotion. Every 5 min intervals, each hind paw was stimulated. Every measurement was performed three times and the mean value of five measurements was determined as the final value (Sun et al. Citation2018). At the starting and on days 7, 14, and 21 after injection of paclitaxel (i.p. 2.0 mg/kg), the paw withdrawal threshold in the von Frey test was evaluated and determined.
Thermal hyperalgesia
A hot-plate analgesia meter (BIO-CHP, Bioseb, France) was utilized for thermal hyperalgesia assessment. A hot plate kept at a temperature of 53 ± 1°C was utilized at a time for animals placement. An electronic timer was applied for recording the latency of response either by hind-paw-lick or jump. A cut-off time of 30 s was employed in order to protect tissue from damage. With a 10 min interval, each rat was performed triplicate measurement and calculated the mean value. It excluded the lifting for normal locomotion and operators who are blinded to the treatment conditions carried out the behavioral tests. At the starting and on days 7, 14, and 21 after injection of paclitaxel (i.p. 2.0 mg/kg), the paw licking latency in the hot-plate test was evaluated and determined.
Coordination between body weight and motor
Weighing was performed every week on days 0, 7, 14, and 21 for all animals. A rotarod apparatus (Shanghai Mobile Datum, Shanghai, China) was utilized for the assessment of motor coordination. Precisely, rats firstly got familiar and trained on the rotarod apparatus for 10 min for 3 consecutive days at the speed of 10, 20, and 30 rpm and then stopped until they maintained unfalling for 60 s. A variable speed of 4–40 rpm was applied for rats running on the testing day until reaching the cut-off time of 5 min or falling and recorded the falling latency.
Tissue collection
Rats were deeply anesthetized with i.p. 50 mg/kg sodium pentobarbital (Sigma-Aldrich, USA) upon behavioral testing ending and performed perfusion intracardially with saline. L4–L6 DRG in the left and right lumbar were removed followed by flash frozen in liquid nitrogen. Storing the resulting samples at −80°C for further experiments.
Quantitative real-time RT–PCR
Place the resulting samples into the tubes with Trizol reagent (Sigma-Aldrich, USA). Chloroform extraction as well as isopropanol precipitation steps were performed for RNA isolation. The iScript™ cDNA Synthesis kit (Biorad, USA) was utilized for cDNA synthesis. Quantitative real-time RT–PCR (qRT–PCR) method were carried out for detecting each gene expression level. For the convenience of comparison, the results were normalized to the mRNA level of β-actin in each sample. The primers utilized in the present study were consistent with the previous literatures (Obata et al. Citation2005; Malin et al. Citation2011). The mRNA expressions of Trpa1, Trpv1, Trpv4, and Trpm8 in DRG of rats at starting and on days 7, 14 ,and 21 after injection of paclitaxel were performed.
Protein preparation and western blot
The samples were firstly performed homogenization step followed by centrifugation in lysis buffer (pH 7.4, 50 mM Tris–HCl, 150 mM NaCl, 1% Triton-100, 0.1% SDS, 1% sodium deoxycholate) in order for proteins extraction. The resulting preparations were then stored at −80°C. The same amounts of protein were firstly performed 10% SDS–PAGE and subsequently transferred onto PVDF membranes. Then the membranes were blocked for 1 h at room temperature in 10% skimmed milk and subsequently performed overnight incubation with primary antibodys (Abcam, MA, USA) including 1:1000 anti-TRPV1, 1:200 ERK, 1:500 pERK, and 1:5000 GAPDH. Then secondary antibody 1:2000 goat anti-rabbit IgG (Abcam, Massachusetts, USA) were utilized for incubation at room temperature for 30 min. Chemiluminescent substrate was utilized for the development of immunoblots and image J Software were utilized for quantification.
Statistical analysis
All the experiments data were analyzed by using one-way ANOVA or mixed factorial designs as appropriate, followed by simple-effects ANOVA or Fisher’s least significant difference (LSD) tests.
Results
Mechanical allodynia and thermal hyperalgesia induced by paclitaxel
The model of PIPNP model rats by a single injection of paclitaxel (i.p. 2.0 mg/kg) was firstly established. As shown in Figure (a,b), at the starting and on days 7, 14, and 21 after injection of paclitaxel, we evaluated and determined the paw licking latency in the hot-plate test and paw withdrawal threshold in the von Frey test. After the paclitaxel injection and throughout the whole experiment, paclitaxel-treated rats evolved reliable mechanical allodynia (Figure (a); P < .01) and thermal hyperalgesic (Figure (b); P < .01) from day 7 when compared with the vehicle-treated rats. Moreover, the body weight of vehicle-treated rats gained throughout the experiment, while the body weight of paclitaxel-treated rats failed to gain enough as much as vehicle-treated rats (Figure (c); P < .01). The motor function of paclitaxel-treated rats was considerably impaired (Figure (d); P < .01). These findings suggest that treatment of paclitaxel could considerably result in mechanical allodynia as well as thermal hyperalgesia. In addition, the impairment of body weight gain and motor function also suggest that the PIPNP model was successfully established. We also investigated the mRNA expression levels of Trpa1, Trpv1, Trpv4, and Trpm8 in DRG. These findings demonstrate that treatment of paclitaxel significantly induced the up-regulation of Trpv1 and Trpa1 instead of Trpv4 and Trpm8 (Figure (e); P < .01).
Figure 1. Establishment of PIPNP. Mechanical (a) and thermal tests (b) in PIPNP rats. Body weight (c) and motor coordination (d) were also monitored on days 7, 14, and 21 post the injection of paclitaxel. Mean ± SEM, n = 8 each group; **P < .01 vs. Vehicle group; ## P < .01 vs. paclitaxel group; repeated-measures ANOVA followed by LSD post hoc test. (e) The mRNA expression levels of Trpv1, Trpv4, Trpm8, and Trpa1 in DRG. Mean ± SEM, n = 8 each group; **P < .01 vs. Vehicle group. Unpaired Student’s t-test.
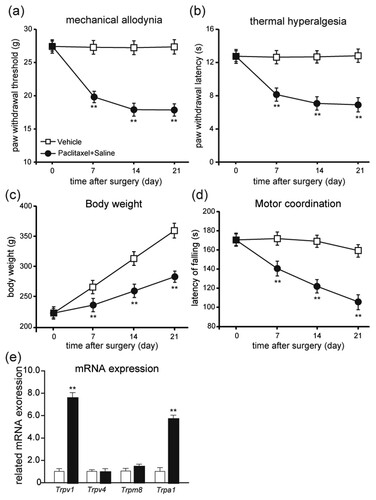
Effects of repeated doses of Res on mechanical allodynia and thermal hyperalgesia induced by paclitaxel
As shown in Figure , the effects of repeated given doses of Res were evaluated during the induction of PIPNP. After the paclitaxel injection and throughout the whole experiment, Res (100 and 200 mg/kg) treated rats evolved reliable mechanical allodynia and thermal hyperalgesic from days 7 to 21 when compared with the vehicle-treated rats. The results demonstrated that when Res was repeatedly injected during the induction of PIPNP, it could prevent the establishment of PIPNP in a dose-dependent manner. During the whole testing days, the Res + paclitaxel-treated rats displayed only partial development of mechanical allodynic behavior, which is considerably less than that of paclitaxel-treated rats (Figure (a); P < .01). In the hot-plate tests, paw licking latency of the Res + paclitaxel-treated rats displayed no considerable discrepancy compared with the vehicle-treated rats (Figure (b); P > .05). The body weight of rats treated by Res + paclitaxel did not gain, which was similar to paclitaxel-treated rats (Figure (c); P < .01). When compared with paclitaxel-treated rats, the motor function of the Res + paclitaxel-treated rats considerably improved (Figure (d); P < .01). The present results suggest that Res dose-dependently prevented the development of PIPNP in rats without affecting motor function.
Figure 2. Amelioration of paclitaxel-induced mechanical allodynia and thermal hyperalgesia by consecutive injection of Res. Prevention of paclitaxel-induced mechanical allodynia (a) and thermal hyperalgesia (b) by repeated injection of Res. Res 100 or 200 mg/kg was injected (i.p.) during the induction of PIPNP. Paw withdrawal threshold in the von Frey test, number of responses in cold acetone test and paw licking latency in the hot-plate test were measured before and on days 7, 14 and 21 post the injection of oxaliplatin. Body weight (c) and motor coordination (d) were also monitored on days 7, 14 and 21 post the injection of paclitaxel. Mean ± SEM, n = 8 each group; **P < .01 vs. Vehicle group; ##P < .01 vs. paclitaxel + saline group; repeated-measures ANOVA followed by LSD post hoc test (n = 8–10 each group).
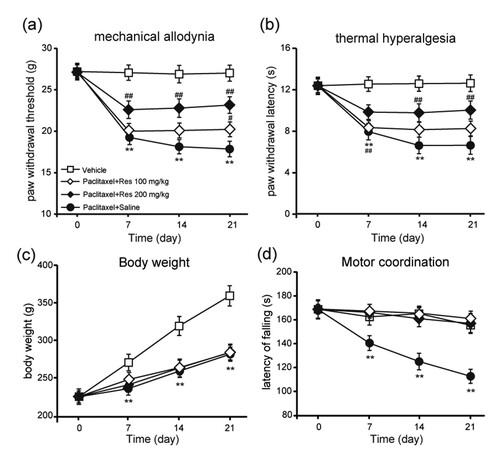
Reversed up-regulation of paclitaxel-induced TRPV1 by repeated injection of Res
We further determined the mRNA expression of Trpa1 and Trpv1 in DRG of rats at starting and on days 7, 14, and 21 after injection of paclitaxel in order to investigate the mechanism of Res on PIPNP. The results demonstrate that paclitaxel could considerably induce the up-regulation of Trpa1 and Trpv1 mRNA after injection on days 14 and 21 (Figure (a,b); P < .01). Furthermore, that 200 mg/kg of Res was repeatedly injected during the induction of PIPNP considerably inhibited the up-regulation of Trpv1 instead of Trpa1 mRNA in DRG of rats treated with paclitaxel (Figure (a,b); P < .01).
Figure 3. Effect of repeated Res injection on the mRNA expression of Trpv1 and Trpa1 in DRG. Effect of repeated Res (200 mg/kg) injection on the mRNA expression of TRPV1 (a) and TRPV4 (b) in DRG. Mean ± SEM, n = 8; **P < .01 vs. day 0; ##P < .01 vs. paclitaxel + saline; one-way analysis of variance followed by Fisher’s least significant difference post hoc test.
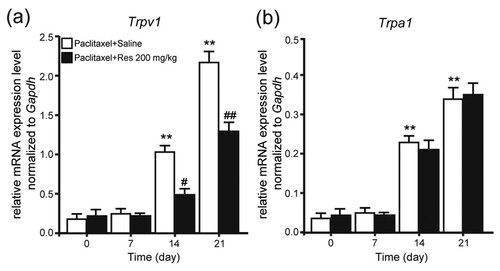
Inhibited up-regulation of TRPV1 by Res is associated with ERK phosphorylation
Protein expression of TRPV1 was further investigated in DRG of rats. Western blot results revealed that after injection on day 21, the protein expression of TRPV1 was considerably enhanced in DRG of rats. Moreover, treatment of repeated 200 mg/kg Res could protect TRPV1 in DRG from up-regulation (Figure (a,b); P < .01). Signaling pathway concerned with the analgesic effects of Res was then studied. Western blot analysis revealed that the phosphorylation of ERK was considerably enhanced in DRG of rats after injection day 21, while this kind of enhancement failed to occur in the DRG of rats by repeated treatment with 200 mg/kg Res (Figure (a,c); P < .01). The resulting finding demonstrated that neuropathic pain could be effectively alleviated with Res by blocking TRPV1 up-regulation and ERK phosphorylation in DRG of PIPNP rats.
Figure 4. Protein expression of TRPV1 and pERK in DRG of PIPNP rats after consecutively Res treatment (a) Representative images of western blot result of TRPV1, phosphorylation of ERK (pERK), ERK, and GAPDH from DRG of PIPNP rats consecutively treated with Res (200 mg/kg). Comparison of TRPV1 (b) and pERK (c) protein levels in DRG of PIPNP rats consecutively treated with Res (i.p.). Mean ± SEM, n = 8, ** P < .01 vs. day 0, ##P < .01 vs. paclitaxel + saline group. One-way analysis of variance followed by Fisher’s least significant difference post hoc test.
Discussion
Up to 80% of patients who obtained cytostatic pharmacotherapy were observed CIPNP (Sisignano et al. Citation2014). Treating this annoying side effect turns to be urgent because of the numbers of cancer survivors are increasing. Few agents display adequate advantages in clinical application, although several drugs have been developed for their efficacy in CIPNP prevention or treatment (Majithia et al. Citation2016; Hao et al. Citation2019). In the present study, we found that given repeated injections of Res during the induction of PIPNP could prevent the occurrence of paclitaxel-induced PIPNP, which was related to the inhibited up-regulation of TRPV1 and phosphoralytion of ERK in DRG. The resulting findings suggest that Res may exert a potential analgesic role by blocking TRPV1 up-regulation in PIPNP.
The given Res during the induction of PIPNP protected paclitaxel-induced PIPNPI from occurrence in behavioral study. Although preventing CIPNP is the ideal treating strategy, no drugs so far have displayed beneficial efficacy in clinical application. The underlying mechanisms of paclitaxel-induced peripheral neuropathy involved in ion channel dysregulation, neuronal damage, neuronal inflammation and oxidative stress (Sisignano et al. Citation2014). Previous study suggests that TRP channels could be served as chemical, thermal, and mechanical sensors (Julius Citation2013). An increasing number of evidences demonstrate that these TRP channels are associated with chemotherapy-induced neuropathy. During the PIPNP induction, the repeated given Res considerably inhibited the up-regulation of paclitaxel-induced TRPV1, which suggested that TRPV1 is concerned with the effect of Res on PIPNP.
TRPV1, both in central nervous and peripheral systems, is a well-known channel that is reported to be concerned with thermosensation and nociception (Tominaga et al. Citation1998). Under pathological conditions, the expression of TRPV1 in nociceptors changed in different models of neuropathy (Biggs et al. Citation2007; Kim et al. Citation2008). Previous study also revealed the up-regulation of the TRPV1 expression in DRG in bone cancer pain (Schwartz et al. Citation2013). In the present study, we discovered that the expression level of TRPV1 is significantly enhanced in DRG of PIPNP animals, and Res dramatically inhibited this up-regulation, indicating that Res may alleviate PIPNP by TRPV1 (Figures and ).
Currently, the underlying analgesic mechanism of Res in PIPNP remains unestablished. However, several literatures may provide some evidences that could lay a foundation on the future investigation. Large quantity of evidences put emphasis on the importance of TRPV1 up-regulation in CIPNP (Ba et al. Citation2018), which leads to a sense of serious pain by selectively activating sensory neurons that deliver information regarding noxious stimuli to the central nervous system (Caterina et al. Citation1997). Our results demonstrated that Res injection dramatically prevented TRPV1 up-regulation in PIPNP. Mitogen-activated protein kinases (MAPK) are the major protein phospho-regulating effectors that regulate nociceptive sensitization (Edelmayer et al. Citation2014). A large number of TRPV1 receptors are distributed on DRG neurons, and the receptor channel is a non-selective cation channel. ERK is involved in regulating the proliferation and differentiation of nerve cells and plays an important role in the sensitization process of TRPV1. Previous literature reported that TRPV1 receptor is involved in endometriosis pain by ERK signaling pathway (Liu et al. Citation2018). Coincidentaly, the present results showed that Res blocks TRPV1 up-regulation and ERK phosphorylation in DRG of PIPNP rats. Our investigations suggest that TRPV1 may exert an effective analgesic role in signal transduction of neuropathic pain, which may be regulated by ERK signaling pathway. Moreover, it has been reported that Res modulates the expression of PKC (Shou et al. Citation2017; Shi et al. Citation2018), which is the upstream of TRPV1 (Tominaga Citation2010; Mandadi et al. Citation2011; Ozdemir et al. Citation2016; Simoes et al. Citation2016). Therefore, we could not exclude the other possible mechanisms which Res may be involved in paclitaxel-induced PIPNP.
Conclusion
The present investigation exhibits the analgesic effect of Res on paclitaxel-induced CIPNP. What’s more, Res prevents the establishment of paclitaxel-induced CIPNP by inhibiting the up-regulation of TRPV1 in DRG. However, the present study has some limitations due to the fact that further investigations including the expression of TRPV1 and TRPA1 before and after the treatment with resveratrol in the spinal cord have not been performed, but in the future, we will perform these experiments in depth. To sum up, the present results demonstrate that Res harbors effective analgesic efficacy for CIPNP and further confirmation of its effectiveness in clinically related CIPNP is deserved.
Acknowledgements
This work was supported by grants from National Natural Science Foundation of Sun Yat-sen University [Grant number 201901245].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data are not available.
Additional information
Funding
References
- Anand U, Otto WR, Anand P. 2010. Sensitization of capsaicin and icilin responses in oxaliplatin treated adult rat DRG neurons. Mol Pain. 6:82. doi:10.1186/1744-8069-6-82.
- Argyriou AA, Bruna J, Marmiroli P, Cavaletti G. 2012. Chemotherapy-induced peripheral neurotoxicity (CIPN): an update. Crit Rev Oncol Hematol. 82:51–77.
- Ates O, Cayli S, Altinoz E, Gurses I, Yucel N, Kocak A, Yologlu S, Turkoz Y. 2006. Effects of resveratrol and methylprednisolone on biochemical, neurobehavioral and histopathological recovery after experimental spinal cord injury. Acta Pharmacol Sin. 27:1317–1325.
- Ba X, Wang J, Zhou S, Luo X, Peng Y, Yang S, Hao Y, Jin G. 2018. Cinobufacini protects against paclitaxel-induced peripheral neuropathic pain and suppresses TRPV1 up-regulation and spinal astrocyte activation in rats. Biomed Pharmacother. 108:76–84.
- Baur JA, Sinclair DA. 2006. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 5:493–506.
- Biggs JE, Yates JM, Loescher AR, Clayton NM, Boissonade FM, Robinson PP. 2007. Changes in vanilloid receptor 1 (TRPV1) expression following lingual nerve injury. Eur J Pain. 11:192–201.
- Brewer JR, Morrison G, Dolan ME, Fleming GF. 2016. Chemotherapy-induced peripheral neuropathy: current status and progress. Gynecol Oncol. 140:176–183.
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. 1997. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 389:816–824.
- Chen Y, Yang C, Wang ZJ. 2011. Proteinase-activated receptor 2 sensitizes transient receptor potential vanilloid 1, transient receptor potential vanilloid 4, and transient receptor potential ankyrin 1 in paclitaxel-induced neuropathic pain. Neuroscience. 193:440–451.
- Chen K, Zhang ZF, Liao MF, Yao WL, Wang J, Wang XR. 2015. Blocking PAR2 attenuates oxaliplatin-induced neuropathic pain via TRPV1 and releases of substance P and CGRP in superficial dorsal horn of spinal cord. J Neurol Sci. 352:62–67.
- Chiba T, Oka Y, Kambe T, Koizumi N, Abe K, Kawakami K, Utsunomiya I, Taguchi K. 2016. Paclitaxel-induced peripheral neuropathy increases substance P release in rat spinal cord. Eur J Pharmacol. 770:46–51.
- Darby LM, Meng H, Fehrenbacher JC. 2017. Paclitaxel inhibits the activity and membrane localization of PKCα and PKCβI/II to elicit a decrease in stimulated calcitonin gene-related peptide release from cultured sensory neurons. Mol Cell Neurosci. 82:105–117.
- Descoeur J, Pereira V, Pizzoccaro A, Francois A, Bourinet E. 2011. Oxaliplatin-induced cold hypersensitivity is due to remodelling of ion channel expression in nociceptors. EMBO Mol Med. 3:266–278.
- Di Cesare Mannelli L, Zanardelli M, Failli P, Ghelardini C. 2012. Oxaliplatin-induced neuropathy: oxidative stress as pathological mechanism. Protective effect of silibinin. J Pain. 13:276–284.
- Edelmayer RM, Brederson JD, Jarvis MF, Bitner RS. 2014. Biochemical and pharmacological assessment of MAP-kinase signaling along pain pathways in experimental rodent models: a potential tool for the discovery of novel antinociceptive therapeutics. Biochem Pharmacol. 87:390–398.
- Farquhar-Smith P. 2011. Chemotherapy-induced neuropathic pain. Curr Opin Support Palliat Care. 5:1–7.
- Girbovan C, Morin L, Plamondon H. 2012. Repeated resveratrol administration confers lasting protection against neuronal damage but induces dose-related alterations of behavioral impairments after global ischemia. Behav Pharmacol. 23:1–13.
- Hao Y, Luo XX, Ba XY, Wang JL, Zhou SY, Yang SM, Fang CJ, Jiang CY, Sun WP. 2019. Huachansu suppresses TRPV1 up-regulation and spinal astrocyte activation to prevent oxaliplatin-induced peripheral neuropathic pain in rats. Gene. 680:43–50.
- Hara T, Chiba T, Abe K, Makabe A, Ikeno S, Kawakami K, Utsunomiya I, Hama T, Taguchi K. 2013. Effect of paclitaxel on transient receptor potential vanilloid 1 in rat dorsal root ganglion. Pain. 154:882–889.
- Julius D. 2013. TRP channels and pain. Annu Rev Cell Dev Biol. 29(29):355–384.
- Kim HY, Park CK, Cho IH, Jung SJ, Kim JS, Oh SB. 2008. Differential changes in TRPV1 expression after trigeminal sensory nerve injury. J Pain: Off J Am Pain Soc. 9:280–288.
- Liu YT, Liu W, Wang XQ, Wan ZH, Liu YQ, Leng YF. 2018. Dexmedetomidine relieves acute inflammatory visceral pain in rats through the ERK pathway, toll-like receptor signaling, and TRPV1 channel. J Mol Neurosci. 66:279–290.
- Majithia N, Loprinzi CL, Smith TJ. 2016. New practical approaches to chemotherapy-induced neuropathic pain: prevention, assessment, and treatment. Oncology-Ny. 30:1020–1029.
- Malin S, Molliver D, Christianson JA, Schwartz ES, Cornuet P, Albers KM, Davis BM. 2011. TRPV1 and TRPA1 function and modulation are target tissue dependent. J NeurosciOff J Soc Neurosci. 31:10516–10528.
- Mandadi S, Armati PJ, Roufogalis BD. 2011. Protein kinase C modulation of thermo-sensitive transient receptor potential channels: implications for pain signaling. J Nat Sci Biol Med. 2:13–25.
- Nassini R, Gees M, Harrison S, De Siena G, Materazzi S, Moretto N, Failli P, Preti D, Marchetti N, Cavazzini A, et al. 2011. Oxaliplatin elicits mechanical and cold allodynia in rodents via TRPA1 receptor stimulation. Pain. 152:1621–1631.
- Obata K, Katsura H, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Tokunaga A, Tominaga M, Noguchi K. 2005. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J Clin Invest. 115:2393–2401.
- Ozdemir US, Naziroglu M, Senol N, Ghazizadeh V. 2016. Hypericum perforatum attenuates spinal cord injury-induced oxidative stress and apoptosis in the dorsal root ganglion of rats: involvement of TRPM2 and TRPV1 channels. Mol Neurobiol. 53:3540–3551.
- Park SB, Goldstein D, Krishnan AV, Lin CS, Friedlander ML, Cassidy J, Koltzenburg M, Kiernan MC. 2013. Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin. 63:419–437.
- Postma TJ, Vermorken JB, Liefting AJ, Pinedo HM, Heimans JJ. 1995. Paclitaxel-induced neuropathy. Ann Oncol. 6:489–494.
- Schwartz ES, La JH, Scheff NN, Davis BM, Albers KM, Gebhart GF. 2013. TRPV1 and TRPA1 antagonists prevent the transition of acute to chronic inflammation and pain in chronic pancreatitis. J Neurosci. 33:5603–5611.
- Shi L, Hao ZX, Zhang SB, Wei MJ, Lu B, Wang ZT, Ji LL. 2018. Baicalein and baicalin alleviate Acetaminophen-induced liver injury by activating Nrf2 antioxidative pathway: The involvement of ERK1/2 and PKC. Biochem Pharmacol. 150:9–23.
- Shou X, Wang B, Zhou R, Wang L, Ren A, Xin S, Zhu L. 2017. Baicalin suppresses hypoxia-reoxygenation-induced arterial endothelial cell apoptosis via suppressing PKCdelta/p53 signaling. Med Sci Monit Int Med J Exp Clin Res. 23:6057–6063.
- Simoes RR, Dos Santos Coelho I, Do Espirito Santo CC, Morel AF, Zanchet EM, Santos AR. 2016. Oral treatment with methanolic extract of the root bark of condalia buxifolia Reissek alleviates acute pain and inflammation in mice: potential interactions with PGE2, TRPV1/ASIC and PKA signaling pathways. J Ethnopharmacol. 185:319–326.
- Singh AK, Vinayak M. 2016. Anti-nociceptive effect of resveratrol during inflammatory hyperalgesia via differential regulation of pro-inflammatory mediators. Phytother Res. 30:1164–1171.
- Singleton RH, Yan HQ, Fellows-Mayle W, Dixon CE. 2010. Resveratrol attenuates behavioral impairments and reduces cortical and hippocampal loss in a rat controlled cortical impact model of traumatic brain injury. J Neurotrauma. 27:1091–1099.
- Sisignano M, Baron R, Scholich K, Geisslinger G. 2014. Mechanism-based treatment for chemotherapy-induced peripheral neuropathic pain. Nat Rev Neurol. 10:694–707.
- Sun WP, et al. 2018. Oxytocin relieves neuropathic pain through GABA release and presynaptic TRPV1 inhibition in spinal cord. Front Mol Neurosci. 11:248. doi:10.3389/fnmol.2018.00248.
- Tominaga M. 2010. Activation and regulation of nociceptive transient receptor potential (TRP) channels, TRPV1 and TRPA1. Yakugaku Zasshi: J Pharm Soc Jpn. 130:289–294.
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. 1998. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 21:531–543.
- Xu M, Cheng Z, Ding Z, Wang Y, Guo Q, Huang C. 2018. Resveratrol enhances IL-4 receptor-mediated anti-inflammatory effects in spinal cord and attenuates neuropathic pain following sciatic nerve injury. Mol Pain. 14:1744806918767549.
- Yang YJ, Hu L, Xia YP, Jiang CY, Miao C, Yang CQ, Yuan M, Wang L. 2016. Resveratrol suppresses glial activation and alleviates trigeminal neuralgia via activation of AMPK. J Neuroinflammation. 13(1):84. doi:10.1186/s12974-016-0550-6.
- Yuan QL, Guo TM, Liu L, Sun F, Zhang YG. 2015. Traditional Chinese medicine for neck pain and low back pain: a systematic review and meta-analysis. PloS One. 10:e0117146.
