 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Radiotherapy (RT) is one of the key treatments for thyroid cancer (TC). Our previous results showed that RT enhances autophagy and blocks RT-induced P53-dependent TC cell apoptosis. However, how RT induces autophagy in TC remains poorly understood. Human TC cell lines, SW579 cells (primary squamous cell carcinoma cells) and FTC-133 (follicular thyroid carcinoma cells) and were used to expose to X-ray irradiation. The relationships between reactive oxygen species (ROS), apoptosis, and autophagy were analyzed by FACS in TC cells. In X-ray-treated TC cells, the proportion of 2’, 7’–dichlorofluorescein (DCF)-positive cells indicating intracellular ROS levels were significantly higher. X-ray irradiation reduced superoxide dismutase (SOD) activity in SW579 cells. The ROS scavengers N-acetyl-L-cysteine (NAC) and SOD enhanced X-ray-induced apoptosis via blocking PI3K-Akt-dependent autophagy in TC cells. ROS mediate the induction of PI3K–Akt-dependent protective autophagy by RT in TC cells.
Introduction
Thyroid cancer (TC) is the top fifth common cancer in women in USA and typically has papillary, follicular, or medullary histology (Teppo and Hakulinen Citation1998; Sherman Citation2003). Primary squamous cell carcinoma (SCC) of the thyroid is a rare sporadic malignant cancer. Radiotherapy (RT) is a widely used approach to improve the survival time and life quality of TC patients. However, the effectiveness and application of RT are limited by incomplete responses to treatment and toxicity. A clinical study showed that 67% of differentiated TC patients have an incomplete structural response to RT, which is associated with significantly worse clinical outcome (Vaisman et al. Citation2011). A recent study by our group indicated that RT-induced autophagy exerts protective effects in TC, especially SCC, contributing to the incomplete response to RT (Gao et al. Citation2019). In the present study, we investigated the mechanisms by which RT induces autophagy and inhibits apoptosis in TC cells.
Reactive oxygen species (ROS) have been reported to play a key role in regulating various pathways associated with both cell survival and apoptosis. Many stimulations, including nutrient starvation, mitochondrial toxins, hypoxia, and oxidative stress, induce ROS-dependent autophagy (Azad et al. Citation2009). RT has been reported to cause ROS-dependent as well as ROS-independent cell damage and autophagy in a context-dependent manner in different cells (Palumbo and Comincini Citation2013; Chaurasia et al. Citation2016). However, little is known about the role of ROS in RT-induced autophagy and apoptosis in TC.
Moreover, the phosphoinositide 3-kinase (PI3 K)-Akt pathway plays a central role in cancer metastasis and RT, and it plays a key role in regulating cell growth, death, adhesion, and migration, particularly during cancer progression and metastasis and in radioresistance (Vo et al. Citation2013; Chang et al. Citation2014). Moreover, the PI3K-Akt pathway has been widely reported to regulate autophagy in various diseases, including cancer, diabetes, and neurodegenerative diseases (Heras-Sandoval et al. Citation2014; Wang et al. Citation2018a). In the present study, the role of ROS in PI3K-Akt-dependent autophagy was investigated to clarify the role of ROS in incomplete responses of TC to RT.
Therefore, SW579 cells, an SCC cell line, and FTC-133, a human follicular thyroid carcinoma were treated with X-ray irradiation, as in our previous report (Gao et al. Citation2019). Intracellular ROS levels were measured by FACS, and cells were treated with ROS scavengers to investigate the role of ROS in X-ray-induced autophagy and apoptosis in TC cells.
Materials and methods
Cell culture
SW579 cells (HTB-107), FTC-133 and HeLa were obtained from American Type Culture Collection (Manassas), and cultured in Leibovitz’s L-15 medium (Gibco) or DMEM (Gibco) with 10% FBS (Gibco), glutamine (2 mM, Gibco), penicillin (100 U/ml) and streptomycin (100 μg/ml), and cultured in a humidified atmosphere with 5% CO2 at 37°C. Cells were passaged every 3–4 days when cells were grown until around 80% confluent.
X-ray irradiation
3 × 105 cells per well were seeded in 6-well plates or 2 × 104 cells per well were seeded in 96-well plate. After 24 h culture, N-acetyl-L-cysteine (NAC, Sigma), Superoxide dismutase (SOD, Sigma) and wortmannin were pretreated 2 h prior to X-ray irradiation. Then the cell culture medium was removed, and the cells were irradiated by X-ray linear accelerator (160 kV, 25 mA; RadSource, Suwanee, GA, USA). The focus was set at 40 cm far from the focus skin, and 280 × 180 mm was irradiated with a dose uniformity of > 95%. A fixed dose rate of 0.1 Gy/s was used to irradiated 10-60s to produce 1–6 Gy X-ray at room temperature. Control group was sham-irradiated cells. Then culture mediums with or without NAC, SOD or wortmannin were refilled, and the cells were further cultured for 24 h until analysis. All the experiments were repeated 3 times.
Growth inhibition assay
After X-ray and NAC, SOD or wortmannin treated as indicated in 96-well plate, PBS was used to wash the cells for 3 times and inculcated in 100 µL of 0.5 mg/mL 5-diphenyl tetrazolium bromide (MTT, Sigma) solution at 37°C for 2 h. Viable cells could be labeled by MTT since NADPH-dependent cellular oxidoreductase enzymes in viable cells could reduce MTT to insoluble formazan, which has a purple color in DMSO. A microplate reader (Thermo Scientific) was used to measure the optical density at 490 nm wavelength in 150 µL of DMSO. Growth inhibition was calculated using the following equation:
MDC staining
Monodansylcadaverine (MDC, Sigma) was used to label autophagic vesicles since it is preferentially accumulated in autophagic vesicles via interactions with vesicle membrane lipids to emit a blue fluorescence (Murugan and Amaravadi Citation2016). The cells were staining at 37°C for 40 min by 0.05 mM MDC buffer containing 100 mM Tris, pH 7.4, 150 mM NaCl, 1 mM CaCl2, 0.5 mM MgCl2, 0.1% Nonidet P-40. After washing with PBS, the fluorescent changes were observed by Olympus IX70 reverse fluorescence microscopy (Olympus).
Fluorescence-activated cell sorting (FACS) analysis
Flow cytometer (Becton Dickinson) was used to measure the cells population at descripted gates. Cells were staining with MDC, 10 mM 2’,7’-Dichlorofluorescin diacetate (DCF-DA, Sigma) and FITC Annexin V Apoptosis Detection Kit with PI (Biolegend) for 30 min to label autophagy, ROS and apoptosis, respectively.
Western blot analysis
After treatment, SW579 cells were harvested and washed twice with PBS, and lysed in SDS lysis buffer. Equal amounts supernatants were collected for western blot analysis, and run in SDS-PAGE and trans-bolting to PVDF membrane. After blocking with 3% skim milk-TBST buffer, primary antibodies against Beclin-1 (sc-48341, SCBT), caspase 3 (sc-7272, SCBT), caspase 8 (sc-56070, SCBT), P53 (sc-126, SCBT), PI3 K (sc-1637, SCBT), p-PI3 K (#4228, CST), Akt1 (sc-5298, SCBT), p-Akt (#9271, CST), β-actin (sc-47778, SCBT) were used and incubated overnight at 4°C. Then horseradish perosidase (HRP)-conjugated secondary antibody (SCBT) was incubated for 2 h at room temperature, and ECL (Thermo), the HRP substrate, was used, and the specific protein bands were visualized in Tanon 5500 chemiluminometer (Tanon).
Superoxide dismutase (SOD) activity assay
The intracellular SOD activity was quantified according the manuscript by Superoxide Dismutase (SOD) assay kit (A001-3-2, Nanjing Jiancheng). Non-treated samples were used as a control for normalization. The relative level was showm.
Statistical analysis
All data are expressed as mean ± SD. Three independent experiments were performed. Statistical comparisons were made using Student’s t-test by Prism8 (ver. 8.3.0). P < 0.05 was identified as significantly difference.
Results
X-ray irradiation increases ROS levels in SW579 cells
To understand the role of ROS in RT-treated TC cells, SW579 cells (a human thyroid SCC cell line) were treated with X-ray at various dosages. DCF-DA, which is oxidized by cellular ROS into the fluorescent compound DCF, was used to measure the intracellular ROS levels after X-ray irradiation by FACS. X-ray irradiation (6 Gy) increased the proportion of DCF-positive cells from 2.61% to 16.95% at 24 h post-irradiation (Figure (A)); this increase was dose-dependent (Figure (B)), indicating that X-ray irradiation increased ROS levels in TC cells.
Figure 1. X-ray irradiation induces ROS accumulation in TC cells. X-ray irradiation increased the percentage of DCF-positive SW579 cells (A and B), FTC-113 cells (D) and HeLa cells (E). All the cells were exposed to X-ray at the indicated dosages. Intracellular ROS levels were measured by FACS with DCF-DA staining at 24 h post-irradiation. (C) X-ray irradiation reduced SOD activity in SW579 cells, as indicated by quantification using the kit described in the Methods section. Relative activities are shown as mean ± SD (n = 3). *P < 0.05, **P < 0.01.
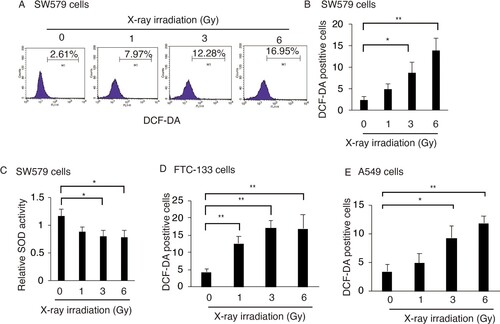
Superoxide dismutase (SOD) is an antioxidant enzyme that catalyzes the conversion of the superoxide (O2−) radical into either molecular oxygen (O2) or hydrogen peroxide (H2O2). Cellular SOD activity was quantified in irradiated and non-irradiated SW579 cells. After X-ray irradiation (6 Gy), SOD activity was significantly reduced, suggesting that X-ray irradiation increased ROS levels, especially superoxide, in TC cells via repressing SOD activity.
Then FTC-133 cells (a human thyroid SCC cell line) and HeLa cells, non-TC cells were used to confirm the generation of ROS in response to X-ray irradiation. X-ray irradiation could significantly increase the ROS generation in both FTC-113 and HeLa cells in dose-dependent manner (Figure (D and E)). This indicated that X-ray-induced ROS generation may occurre in multiply tumor cells lines including TC cells.
X-ray-induced ROS exert protective effects in SW579 cells
To understand the role of ROS in X-ray-induced TC cell death, SW579 cells were treated with the ROS scavengers N-acetyl-L-cysteine (NAC) and SOD, and after X-ray irradiation, cell viability and apoptosis were analyzed by MTT assay and FACS with Annexin V and PI staining, respectively. The addition of NAC and SOD further enhanced the X-ray-induced upregulation of apoptosis (Figure (A)). When either NAC or SOD was added to the cells, the percentage of apoptotic cells was significantly increased by around 13%. Then NAC was added to X-ray-treated FTC-113 cells to confirm the role of ROS in X-ray-induced cell death by MTT assay. X-ray-induced cell death was significantly increased after NAC addition in FTC-113 cells (Figure (C)).
Figure 2. ROS exert protective effects in X-ray-irradiated TC cells. NAC and SOD reduced viability of X-ray-treated cells (A) and enhanced X-ray-induced apoptosis (B) in SW579 cells. (C) NAC reduced viability of X-ray-treated FTC-113 cells. SW579 or FTC-113cells were pretreated with NAC (5 mM) or SOD (500 U/mL) for 2 h and subsequently treated with 6 Gy X-ray. (A and C) Cell viability was analyzed at 24 h post-irradiation by MTT assay. (B) The percentage of apoptotic cells was measured by FACS with Annexin V and PI staining at 24 h post-irradiation. Annexin V-positive cells were considered as apoptotic. Results are expressed as mean ± SD (n = 3). *P < 0.05. (D) The levels of apoptosis-related proteins and P53 in SW579 cells after X-ray irradiation and NAC treatment were measured by western blot.
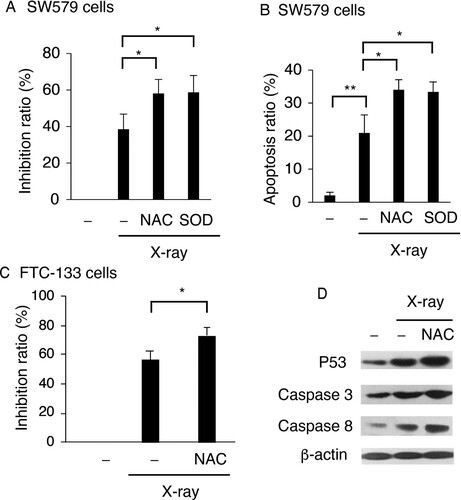
Moreover, the expression of apoptosis-associated proteins in X-ray-treated SW579 cells was analyzed by western blots. Our previous data indicated that X-ray could induce cell death via P53 in SW579 cells (Gao et al. Citation2019). Under the same conditions, after the addition of NAC to the cells, the X-ray-induced increased in the levels of P53, caspase 3, and caspase 8 in SW579 cells were further enhanced, indicating that X-ray-induced ROS, mainly superoxide, exerted protective effects in TC cells.
X-ray irradiation induces autophagy in SW579 cells via ROS
Our previous report showed that X-ray irradiation induces protective autophagy in SW579 cells (Gao et al. Citation2019). In the present study, SW579 and FTC-113 cells were treated with NAC and SOD, and MDC positive cell population was measured by FACS indicating the level of autophagy. NAC significantly reduced the percentage of MDC-positive SW579 and FTC-113 cells (Figure (A and B)). SOD repressed MDC-positive SW579 after X-ray irradiation which indicates that superoxide plays as an autophagy inducer role in SW579 cells (Figure (A)).
Figure 3. ROS promotes X-ray-induced autophagy in TC cells. NAC or SOD reduced X-ray-induced autophagy in SW579 cells (A) and FTC-113 cells (B), as measured by FACS with MDC staining. Results are expressed as mean ± SD (n = 3). *P < 0.05, ***P < 0.001. (C) NAC reduced X-ray-induced Beclin-1 expression in SW579 cells, as analyzed by western blot. (D) Autophagic vacuoles were visualized by MDC staining in SW579 cells at 24 h post-irradiation. Representative photos are shown.
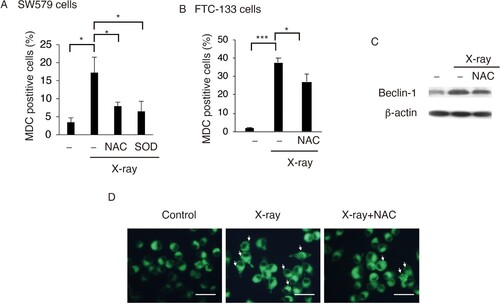
NAC decreased the expression levels of the autophagic protein Beclin-1 (Figure (C)). MDC staining indicated NAC reduced the number of autophagic vacuoles in X-ray-treated cells (Figure (C)). These results indicated that X-ray induced protective autophagy via ROS in SW579 cells.
X-ray-induced ROS promotes protective autophagy via the PI3K-Akt pathway in SW579 cells
PI3 K and Akt are well-known regulators of autophagy, and ROS has been reported to regulate PI3 K and Akt signaling (Kim et al. Citation2018). In the present study, we investigated the relationship between X-ray-induced ROS and PI3K-Akt-mediated autophagy in SW579 cells. First, Wortmannin, a PI3 K inhibitor, was used to investigate the relationship between the PI3K-Akt pathway and autophagy in X-ray-treated SW579 cells. Wortmannin significantly repressed the increase in the percentage of MDC-positive cells in X-ray-treated cells (Figure (A)) and (ii) significantly increased cell death in X-ray-treated cells (Figure (B)). Consistently, in X-ray-treated SW579 cells, more phosphorylated PI3 K and Akt were observed (Figure (C), suggesting that the protective effects of X-ray irradiation are mediated by the PI3K-Akt pathway.
Figure 4. X-ray irradiation induces protective autophagy in SW579 cells via the PI3K-Akt pathway. X-ray induced (A) increases in autophagy and (B) decreases in cell viability via PI3 K in SW579 cells. SW579 cells were pretreated with 10 mM Wortmannin, a PI3 K inhibitor. Autophagy was measured by FACS, and cell viability was quantified by MTT assay at 24 h post-irradiation, and the cell inhibition ratio was calculated. Results are expressed as mean ± SD (n = 3). *P < 0.05. (C) Expression levels of proteins in the PI3K–Akt pathway in SW579 cells were analyzed by western blot. (D) X-ray induced protective autophagy via ROS accumulation and PI3K-Akt pathway activation in SW579 cells.
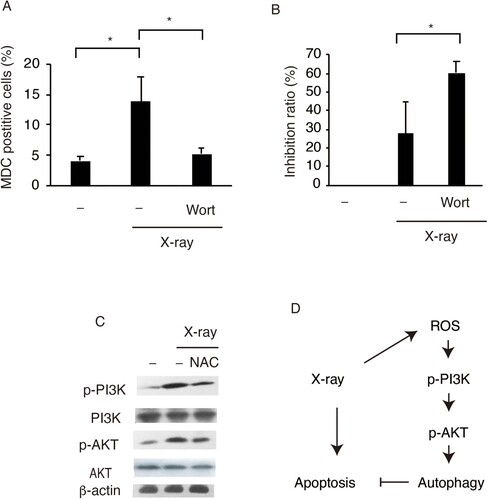
The role of ROS in the activation of PI3 K and Akt in X-ray-treated SW579 cells was investigated by western blots. NAC clearly decreased the phosphorylation status of PI3 K and Akt in SW579 cells after X-ray treatment (Figure (C)). These results suggested that X-ray-induced ROS accumulation promoted protective autophagy via the PI3K-Akt pathway in SW579 cells.
Discussion
In this work, we found ROS levels were increased after X-ray stimulation in a human TC cell line. ROS, including superoxide, hydroxyl radical (·OH), and hydrogen peroxide, are mainly generated in mammalian mitochondria (Zorov et al. Citation2014). Ion radiation induced ROS generation was found in cancer cells. Bioinformatics analysis further indicated that these upregulated mitochondrial respiratory chain proteins enhance ATP production and, simultaneously, ROS release (Fan et al. Citation2019). In addition, radiation has been reported to induce ROS generation prior to oxidative DNA damage in human peripheral T cells (Ogawa et al. Citation2003), indicating that RT-induced mitochondrial ROS generation might occur as an early event. Moreover, SOD activity was found to be reduced in response to X-ray irradiation in SW579 cells. Consistently, exogenous SOD in hepatocellular carcinoma cells could repress radiation-induced ROS accumulation (Motoori et al. Citation2001), indicating that RT-induced ROS generation is dependent on mitochondrial SOD in TC cells.
RT-induced SOD-regulated ROS exert protective effects in TC cells. However, the relationship between ROS levels and cell survival is complex and controversial in different cancer cells. Increased intracellular ROS levels can induce cancer cell cycle arrest, senescence, and apoptosis. Mitochondrial ROS can active the caspases and cell apoptosis by releasing cytochrome C (Cadenas Citation2004). However, ROS have also been reported to contribute to cell proliferation. Decreased SOD activity favors proliferation, due to increased superoxide and decreased hydrogen peroxide levels, while increased SOD activity induces cell cycle arrest (Wang et al. Citation2005). In breast cancer cells, ROS contributes to estrogen-induced tumor proliferation (Parkash et al. Citation2006). In a previous study, a reduction in ROS generation in X-ray-treated cancer cells did not increase cell death (Wang and Zhang Citation2019). Moreover, γ-ray irradiation can induce cancer cell invasion by increasing ROS generation and decreasing SOD activity (Jung et al. Citation2019). These observations indicate that the context-dependent functions of ROS in cell survival and death might be associated with the different species and levels of intracellular ROS (Kuwabara et al. Citation2008). Superoxide, which is dismutated by SOD, may be a signaling molecule that regulates multiply pathways involved in cell survival and migration.
Autophagy, a catabolic process, was aimed to recycle cellular components and stresses-induced cellular damaged organelles (Glick et al. Citation2010). In the present study, X-ray-induced ROS-dependent autophagy was observed in SW579 cells. Starvation has been reported to induce ROS-dependent autophagy in TC cells via a highly conserved nuclear protein, high mobility group box 1 (Chai et al. Citation2019). Furthermore, the increased superoxide levels, which, at least partially, resulted from decreased SOD activity, were found to induce autophagy in X-ray-treated SW579 cells. Consistently, superoxide has been reported to be a major reactive oxygen species regulating autophagy; starvation-induced autophagy was blocked by overexpression of SOD and a concomitant decrease in superoxide levels (Chen et al. Citation2009). These observations indicate that RT-induced protective autophagy is mainly dependent on superoxide in TC cells.
As a member of lipid kinases which phosphorylate the 3′-hydroxyl group of phosphoinositides, PI3 K activity is negatively regulated by dephosphorylation by the tumor suppressor PTEN (Cantley Citation2002). Excessive ROS can activate PI3K-Akt signaling via inhibiting the activity of PTEN (Koundouros and Poulogiannis Citation2018). In the present study, ROS were found to activate the PI3K-Akt pathway, regulating autophagy in X-ray-treated SW579 cells. Consistently, radiation has been found to induce ROS-dependent autophagy via the PI3K-Akt pathway in intestinal epithelial cells (Wang et al. Citation2018b). Akt-mediated protective autophagy has been observed in TC cells that were treated with an inhibitor of vascular endothelial growth factor receptor-2 (Feng et al. Citation2018). Thus, PI3K-Akt-mediated autophagy in RT-treated TC cells might be regulated by ROS.
Conclusion
This work clarified the role of ROS in RT-induced changes in autophagy and apoptosis. RT might induce the accumulation of ROS, which further activated the PI3K-Akt pathway and induced protective autophagy (Figure (D)). Thus, the incomplete response to RT of TC cells might be associated with increased ROS-dependent autophagy.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data is not available.
Reference
- Azad MB, Chen Y, Gibson SB. 2009. Regulation of autophagy by reactive oxygen species (ROS): implications for cancer progression and treatment. Antioxid Redox Signal. 11:777–790.
- Cadenas E. 2004. Mitochondrial free radical production and cell signaling. Mol Aspects Med. 25:17–26.
- Cantley LC. 2002. The phosphoinositide 3-kinase pathway. Science. 296:1655–1657.
- Chai W, Ye F, Zeng L, Li Y, Yang L. 2019. HMGB1-mediated autophagy regulates sodium/iodide symporter protein degradation in thyroid cancer cells. J Exp Clin Cancer Res. 38:325.
- Chang L, Graham PH, Hao J, Ni J, Bucci J, Cozzi PJ, Kearsley JH, Li Y. 2014. PI3K/Akt/mTOR pathway inhibitors enhance radiosensitivity in radioresistant prostate cancer cells through inducing apoptosis, reducing autophagy, suppressing NHEJ and HR repair pathways. Cell Death Dis. 5:e1437.
- Chaurasia M, Bhatt AN, Das A, Dwarakanath BS, Sharma K. 2016. Radiation-induced autophagy: mechanisms and consequences. Free Radic Res. 50:273–290.
- Chen Y, Azad MB, Gibson SB. 2009. Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ. 16:1040–1052.
- Fan PC, Zhang Y, Wang Y, Wei W, Zhou YX, Xie Y, Wang X, Qi YZ, Chang L, Jia ZP, et al. 2019. Quantitative proteomics reveals mitochondrial respiratory chain as a dominant target for carbon ion radiation: delayed reactive oxygen species generation caused DNA damage. Free Radic Biol Med. 130:436–445.
- Feng H, Cheng X, Kuang J, Chen L, Yuen S, Shi M, Liang J, Shen B, Jin Z, Yan J, Qiu W. 2018. Apatinib-induced protective autophagy and apoptosis through the AKT–mTOR pathway in anaplastic thyroid cancer. Cell Death Dis. 9:1030.
- Gao P, Hao F, Dong X, Qiu Y. 2019. The role of autophagy and Beclin-1 in radiotherapy-induced apoptosis in thyroid carcinoma cells. Int J Clin Exp Pathol. 12:885–892.
- Glick D, Barth S, Macleod KF. 2010. Autophagy: cellular and molecular mechanisms. J Pathol. 221(1):3–12.
- Heras-Sandoval D, Pérez-Rojas JM, Hernández-Damián J, Pedraza-Chaverri J. 2014. The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell Signal. 26:2694–2701.
- Jung CH, Kim EM, Song JY, Park JK, Um HD. 2019. Mitochondrial superoxide dismutase 2 mediates γ-irradiation-induced cancer cell invasion. Exp Mol Med. 51:14.
- Kim JH, Choi TG, Park S, Yun HR, Nguyen NNY, Jo YH, Jang M, Kim J, Kim J, Kang I, et al. 2018. Mitochondrial ROS-derived PTEN oxidation activates PI3K pathway for mTOR-induced myogenic autophagy. Cell Death Differ. 25:1921–1937.
- Koundouros N, Poulogiannis G. 2018. Phosphoinositide 3-kinase/Akt signaling and redox metabolism in cancer. Front Oncol. 8:160.
- Kuwabara M, Asanuma T, Niwa K, Inanami O. 2008. Regulation of cell survival and death signals induced by oxidative stress. J Clin Biochem Nutr. 43(2):51–57.
- Motoori S, Majima HJ, Ebara M, Kato H, Hirai F, Kakinuma S, Yamaguchi C, Ozawa T, Nagano T, Tsujii H, Saisho H. 2001. Overexpression of mitochondrial manganese superoxide dismutase protects against radiation-induced cell death in the human hepatocellular carcinoma cell line HLE. Cancer Res. 61:5382–5388.
- Murugan S, Amaravadi RK. 2016. Methods for studying autophagy within the tumor microenvironment. Adv Exp Med Biol. 899:145–166.
- Ogawa Y, Kobayashi T, Nishioka A, Kariya S, Hamasato S, Seguchi H, Yoshida S. 2003. Radiation-induced reactive oxygen species formation prior to oxidative DNA damage in human peripheral T cells. Int J Mol Med. 11:149–152.
- Palumbo S, Comincini S. 2013. Autophagy and ionizing radiation in tumors: the “survive or not survive” dilemma. J Cell Physiol. 228:1–8.
- Parkash J, Felty Q, Roy D. 2006. Estrogen exerts a spatial and temporal influence on reactive oxygen species generation that precedes calcium uptake in high-capacity mitochondria: implications for rapid nongenomic signaling of cell growth. Biochemistry. 45:2872–2881.
- Sherman SI. 2003. Thyroid carcinoma. Lancet. 361:501–511.
- Teppo L, Hakulinen T. 1998. Variation in survival of adult patients with thyroid cancer in Europe. EUROCARE Working group. Eur J Cancer. 34:2248–2252.
- Vaisman F, Tala H, Grewal R, Tuttle RM. 2011. In differentiated thyroid cancer, an incomplete structural response to therapy is associated with significantly worse clinical outcomes than only an incomplete thyroglobulin response. Thyroid. 21:1317–1322.
- Vo BT, Morton D Jr., Komaragiri S, Millena AC, Leath C, Khan SA. 2013. TGF-β effects on prostate cancer cell migration and invasion are mediated by PGE2 through activation of PI3K/AKT/mTOR pathway. Endocrinology. 154:1768–1779.
- Wang M, Kirk JS, Venkataraman S, Domann FE, Zhang HJ, Schafer FQ, Flanagan SW, Weydert CJ, Spitz DR, Buettner GR, Oberley LW. 2005. Manganese superoxide dismutase suppresses hypoxic induction of hypoxia-inducible factor-1alpha and vascular endothelial growth factor. Oncogene. 24:8154–8166.
- Wang JT, Xie WQ, Liu FQ, Bi Y, Zhu XJ, Wang QE, Zheng YF. 2018b. NADH protect against radiation enteritis by enhancing autophagy and inhibiting inflammation through PI3K/AKT pathway. Am J Transl Res. 10:1713–1721.
- Wang H, Zhang X. 2019. ROS reduction does not decrease the anticancer efficacy of X-ray in two breast cancer cell lines. Oxid Med Cell Longev. 2019:3782074.
- Wang B, Zhong Y, Li Q, Cui L, Huang G. 2018a. Autophagy of macrophages is regulated by PI3k/Akt/mTOR signalling in the development of diabetic encephalopathy. Aging. 10:2772–2782.
- Zorov DB, Juhaszova M, Sollott SJ. 2014. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 94:909–950.
