?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Gastrointestinal stromal tumor (GIST) is one of the most common mesenchymal tumors of the gastrointestinal tract by activating mutations in the related receptor tyrosine kinases KIT and PDGFRA. Few reliable biomarkers have been established despite illustration of the molecular mechanisms of GISTs development. Accumulating evidence has demonstrated that long non-coding RNA (lncRNAs) plasmacytoma variant translocation 1 (lncRNA PVT1) is a tumor regulator in many cancers. The goal of our research was to investigate the level and biological function of PVT1 in GIST-882 and GIST-T1 cells. The relative expression of PVT1 was higher in the GISTs cells. Down-regulation of PVT1 has shown that suppressed the proliferation and migration abilities of GISTs cells, and significantly stimulated cell apoptosis. Furthermore, western blot assay confirmed that knockdown of PVT1 obviously reduced the expression of CCND1, CCNB1, CDK6, PIK3CA, p-AKT, Rictor, mSIN1 and mLST8. The results implicated that PTV1 may regulate the progression of GISTs cells through PI3K/AKT/mTOR pathway. In conclusion, our study provides the evidence that PVT1 plays a vital oncogenic role in GISTs cells, and is a potential prognostic biomarker of recurrence after surgical procedures of GISTs. This highlights the possible of PVT1 serve as a therapeutic target for GISTs treatment.
Background
Gastrointestinal stromal tumors (GISTs) are the most common sarcoma in the gastrointestinal tract, accounting for 10–15 cases per million population every year (Soreide et al. Citation2016). GISTs occur at every segment of the alimentary tract, but most commonly in the stomach and small intestine, accounting for 60–70% and 20–30% of cases, respectively (Connolly et al. Citation2003). GISTs drive from the interstitial cells of Cajal lineage or its precursors by activating mutations in receptor tyrosine kinase (KIT) and platelet-derived growth factor receptor alpha (PDGFRA) genes (Hirota et al. Citation1998, Citation2003). Surgical resection and drug are the principal of treatment for patients with primary GISTs (Milhem and Deutsch Citation2015; Casali et al. Citation2018; von Mehren et al. Citation2018). But in fact, approximately 50% of patients’ recurrence within 5 years after surgery and the wide use of the drug in clinical cases along with the emergence of the drug resistance (DeMatteo et al. Citation2000; Li, Huynh et al. Citation2015). Recently, several clinical indices including tumor size and location, tumor rupture and mitotic index have been established to predict the risk of GIST recurrence, but precious little reliable prognostic indictors of molecular biomarkers have been used for GISTs (Fletcher et al. Citation2002). Thus, further research into the potential mechanisms responsible for GISTs and search the possible of novel therapeutic targets to overcome the unfavorable prognostic are urgently required.
In the mammalian genome, only approximately 1.2% of the transcripts are protein-coding genes, and the vast majority of non-protein-coding transcripts, such as long non-coding RNAs (lncRNAs) with diversity and complexity of sequences, which are longer than 200 nt (Wilusz et al. Citation2009; Consortium Citation2012). In previous studies, lncRNAs have been shown to play an integral role in regulating of cellular functions, including cell growth, proliferation, cell cycle, division, apoptosis and migration (Cheetham et al. Citation2013; Pickard et al. Citation2013; Lian et al. Citation2018; Fan et al. Citation2019). Furthermore, aberrant expression of lncRNA may contribute to the progression of various diseases, particularly tumors (Bo et al. Citation2019; Wei et al. Citation2019). LncRNA plasmacytoma variant translocation 1 (PVT1) was first identified as an activator of MYC in murine lymphomas (Zeidler et al. Citation1994). Studies have been demonstrated that high expression of PVT1 in various human cancer including hepatocellular carcinoma, gastric cancer, ovarian cancer, breast cancer, bladder cancer and pancreatic cancer (Cho et al. Citation2018; Ding et al. Citation2018; He et al. Citation2018; Huang et al. Citation2018; Zhao et al. Citation2018). Recently, studies have showed that PVT1 can promote the proliferation, migratory and invasive characteristics of cancer cell. In addition, up-regulation of PVT1 is considered as an important oncogene as shown by the previous study and can promote cell proliferation and inhibit cell apoptosis in bladder cancer (Liu and Zhang Citation2017). Thus, PVT1 may also act as a possible predictor of cancer progression and patient’s prognosis (Ding et al. Citation2018; Huang et al. Citation2018).
The aim of our study was to demonstrate the biological function of PVT1 in Gastrointestinal stromal tumors. We observed that PVT1 was upper expressed both in GIST-882 and GIST-T1 cells. Moreover, it was identified that silencing PVT1 can promote the apoptosis, and suppress the migration and proliferation of GIST-882 and GIST-T1 cells. Based on the above-mentioned information, we detected the differentially expressed genes when PVT1 was suppressive. Finally, we authorized that the functions of PVT1 in the regulation of GIST-882 and GIST-T1 cells may involve in PI3K/AKT/mTOR pathway.
Materials and methods
GIST cells culture and the lentivirus infection
GIST-882 cell line and GIST-T1 cell line were purchased from BNBIO (Beijing, China). All cells were grown in Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum, and in a humidified 37°C incubator with 95% air and 5% CO2. Firstly, three short hairpin RNAs (shRNA) against the PVT1 were purchased from Genechem (Shanghai, China), and the sequence is as follows:
The concentration of LV-shCtrl and LV-shPVT1 was both 1 × 108 TU/mL, and as described previously that 2 × 105 cells were seeded and infected with LV-shCtrl or LV-shPVT1 at the Multiplicity of infection (MOI) of 10. 72 h after infected, the infection efficiency was evaluated by fluorescence microscope (Olympus).
RNA isolation and the RT-qPCR assays
Total RNA was isolated from GIST cells with TRIzol (Invitrogen, USA). The quality and concentration of each sample were detected by a NanoDrop spectrophotometer (Thermo, USA). The mRNA reverse transcription and qPCR were conducted as described previously with the PrimeScript RT Master Mix Kit (Takara, Japan) and ChamQ Universal SYBR qPCR Master Mix (Takara, Japan), respectively. The primers were purchased from Realgene (Shanghai Shenggong), and the specific primer sequences are given as follows:
CCK8 assay
CCK8 assay was performed to measure the effect PVT1 on the proliferation of GIST cells. 2000 cells were plated per well in a 96-well plate and incubated in incubator. For a desired period (e.g. after infected 1, 2, 3, 4, and 5 days), add 10 µL/well of CCK8 Solution to each well 4–6 h before the end. The absorbance increases were measured under 450 nm, and all experiments were repeated three times.
Cell apoptosis and cell cycle assay
As described previously, the cell apoptosis and cell cycle were analyzed using flow cytometric methods. First, the cells were washed three times with pre-cold phosphate-buffered saline (PBS). Next, cells were collected and centrifuged at 1000 rpm for 8 min. Finally, protecting from light and following the manufacturer’s instructions, apoptotic cells were stained with Annexin V-FITC and propidium iodide (PI; BD, USA) and subjected to flow cytometry (FACSCalibur, BD, USA). For analyzing cell cycle, the cells were stained with PI and subjected to FACS.
Transwell assay
Transwell assay was used to measure the ability of GIST cells to migration after PVT1 knockdown. Cells were seeded on the upper transwell chamber which were placed in 24-well plates. Cells were monitored under the BDS200 Inverted Biological Microscope (Optec, Chongqing, China) and pictures were taken at 24 h later. And migrated cells were stained with Methylrosanilinium chloride solution and counted the number. All experiments were repeated three times.
Western blot assay
GISTs cells were washed in PBS and harvested in 200 µL RIPA lysis buffer, which contains protease and phosphatase inhibitor (KeyGen, Nanjing). As described previously, proteins were quantified with the BCA assay kit (Beyotime, Shanghai). After electrophoresis, the proteins were transferred onto polyvinylidene fluoride membranes. The membranes were blocked in 5% bovine serum albumin solution and probed with the primary antibodies against with AKT (4685, Cell Signaling Technology, USA), p-AKT(Ser473) (AF887-sp, R&D, USA), p-AKT(Thr308) (13,038, Cell Signaling Technology, USA), CCND1 (2978, Cell Signaling Technology, USA), CDK6 (ab151247, Abcam, USA), PIK3CA (ab40776, Abcam, USA), Rictor (MAB4598-SP, R&D, USA), DEPTOR (AF7255-SP, R&D, USA), mLST8 (also known as GβL) (AF4004-SP, R&D, USA), mSIN1 (also known as MAPKAP1) (MAB8168-SP, R&D, USA) and GAPDH (AP0063, Bioworld, USA) overnight at 4°C. The following day, membranes were incubated with the specific secondary antibody for 1 h at room temperature. The Horse radish peroxidase (HRP) signal was detected using the enhanced chemiluminescence western blot detection kit (Millipore) and the western blot images were obtained by AI600 (GE).
Statistical analysis
All data analyses were conducted using the SPSS statistical software (version 22.0; IBM Corp.), and a P < .05 was considered statistically significant.
Results
lncRNA PVT1 is highly expressed in GIST cells
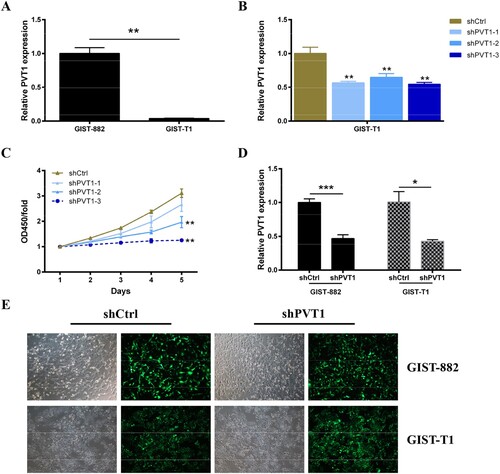
GIST-882 and GIST-T1 cell lines have been used as a substitute to study biological function in GISTs (Hemming et al. Citation2019). First, the expression level of PVT1 in GIST-882 cells and GIST-T1 cells was confirmed by qRT-PCR analyze. The results showed that the PVT1 was expressed in both of GIST-882 cells and GIST-T1 cells and was higher in GIST-882 cells (Figure (A)). To ascertain the effect of PVT1 on biological function in GISTs cells, the expression of PVT1 was knockdown by interference technology. As shown in Figure (B), GIST-T1 cells were harvested after transfection 48 h for RNA extraction. The silencing efficiency was evaluated by qRT-PCR, which revealed that the expression level of PVT1 was reduced in three groups (shPVT1-1, shPVT1-2, and shPVT1-3) and the inhibition efficiency was highest in the shPVT1-3 group compared with shCtrl (P < .01). Meanwhile, we verified the effect of PTV1 knockdown on GIST-T1 cell proliferation. The results of CCK8 assays were showed that the proliferation of GIST-T1 cells was suppressed after transient transfected with sh-PVT1-2(P < 0.01) and sh-PVT1-3 (P < .01) compared with shCtrl, and the inhibition efficiency was highest in the shPVT1-3 group (Figure (C)). Therefore, we chose the adequate sh-PVT1-3 for the following study.
Figure 1. The chosen of shPVT1. (A) The expression level of PVT1 was detected in GIST-882 and GIST-T1 cells via RT-qPCR. GAPDH was used as the internal control. (B) The expression level of PVT1 was detected in PVT1 knockdown GIST-T1 cells via RT-qPCR. GAPDH was used as the internal control. (C) The viability of GIST-T1 cells transfected with shCtrl and shPVT1 were examined by CCK8 assays. (D) The expression level of PVT1 was detected in PVT1 knockdown GIST-882 and GIST-T1 cells via RT-qPCR. GAPDH was used as the internal control. (E) The efficiency of shCtrl and shPVT1 transfected were identified by expression of GFP. *P < .05, **P < .01, and ***P < .001.
Down-regulation of lncRNA PVT1 inhibits cell viability and increase apoptosis of GIST cells
To tested the effect of PVT1 knockdown on the proliferation of GIST-882 and GIST-T1 cells. First, qRT-PCR analysis was performed to confirm the expression of PVT1 in GIST-882 (Figure (D), P < .001) and GIST-T1 (Figure (D), P < .05) cells following sh-PVT1-3 transfection (Figure (E)), and the results showed that the relative PVT1 expression was down-regulated compared with shCtrl group. Next, CCK8 assays were performed to investigate the proliferation viability of GIST-882 and GIST-T1 cells following sh-PVT1-3 transfection. As shown in Figure (A,B), the GIST-882 and GIST-T1 cells growth were inhibited after transfected with sh-PVT1-3 compared with shCtrl control (P < .01).
Figure 2. The apoptosis of GIST cells was increased after PVT1 knockdown. (A) The viability of GIST-882 and GIST-T1 cells were reduced after shPVT1 transfected. (B) The effect of PVT1 expression alteration on cell apoptosis was detected by FACS analysis. **P < .01 and ***P < .001.
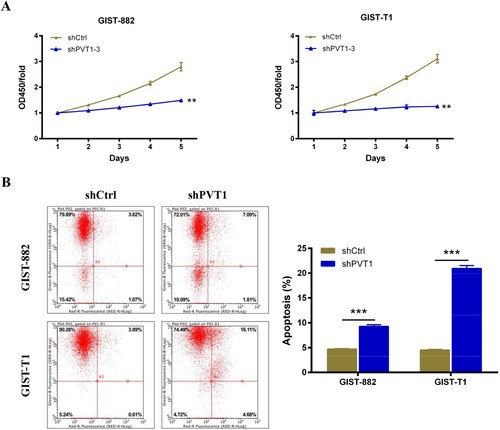
To determine the effect of PVT1 on GIST-882 and GIST-T1 cells apoptosis, flowcytometry assay was performed to detect the apoptosis rate of GIST-882 and GIST-T1 cells after 96 h of sh-PVT1-3 transfection. As shown in Figure (B), the apoptosis rate of GIST-882 and GIST-T1cells transfected with sh-PVT1-3 was 9.28% (P < .001) and 20.97% (P < .001) compared with negative control, respectively. These revealed that the PVT1 silencing enhances GIST-882 and GIST-T1 cells apoptosis.
Down-regulation of lncRNA PVT1 distributes cell cycle of GIST cells
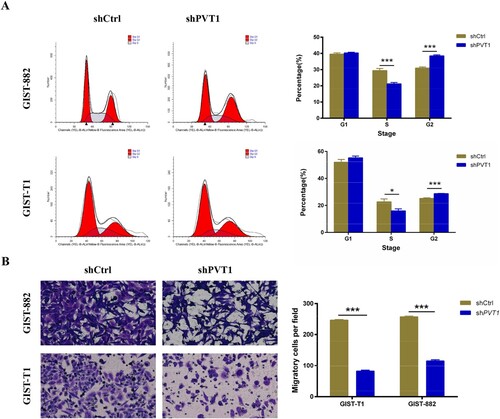
To investigate the mechanism of PVT1 knockdown-induced cell proliferation inhibition, cell-cycle analysis was used to confirm the effects of PTV1 on cell cycle distribution. Cell populations in the G1, S, and G2 phases were 39.58%, 29.46%, and 30.96%, respectively, in the shCtrl group, and 40.32%, 21.21% and 38.47%, respectively, in the shPTV1 GIST-882 group. Cell populations in the G1, S, and G2 phases were 52.08%, 22.70%, and 25.21%, respectively, in the shCtrl group, and 55.27%, 15.98%, and 28.75%, respectively, in the shPTV1 GIST-T1 group. The population of the S-phase cells significantly decreased in the shPTV1 GIST-882 group (P < .001) and shPTV1 GIST-T1 group (P < .05), while the populations of shPTV1 GIST-882 group (P < .001) and shPTV1 GIST-T1 group (P < .001) in G2 phases whole increased, indicating the S phase arrest (Figure (A)).
Figure 3. The analyses of cell cycle and migration in PVT1 knockdown GIST-882 and GIST-T1 cells. (A) The cell cycle of GIST-882 and GIST-T1 cells were analyzed by FACS after shPVT1 transfected. (B) The number of migrating cells was reduced in GIST-882 and GIST-T1 cells while PVT1 knockdown. ***P < .001.
Down-regulation of lncRNA PVT1 inhibits GIST-882 and GIST-T1 cells migration
We next measured migration of GIST-882 and GIST-T1 cells upon PVT1-KD using Transwells chamber and found that the ability of migration was reduced in both GIST-882 and GIST-T1 cells by silenced PTV1 when compared with the shCtrl group (P < .001 and P < .001) (Figure (B)). These data showed that PTV1 may present a positive relation with the GISTs cells progression, which may facilitate GISTs cells to sustain in a malignant state.
lncRNA PVT1 promotes GIST cell growth through the PI3K/AKT/mTOR pathway
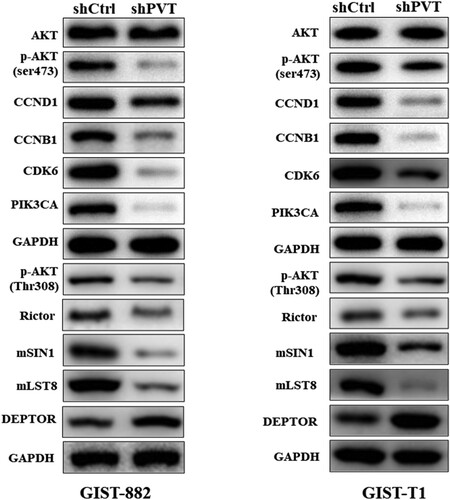
To further illustrate the mechanism by which PVT1 regulates the function of GIST-882 and GIST-T1 cells, we performed western blot analysis of shPVT1 and shCtrl in GIST-882 and GIST-T1 cells. We found that knockdown of PVT1 significantly reduced the expression of CCND1, CCNB1, CDK6, PIK3CA, and p-AKT enriched in PI3K/AKT pathway (Figure ). The results showed that PTV1 interacted with PI3K/AKT pathways were used in these cells to achieve their proliferative and migratory processes. Due to the phosphorylation of AKT has been identified as a mammalian target of rapamycin (mTOR) in an insensitive complex of mSIN1-Rictor–mTOR (Jacinto et al. Citation2006), we found that the protein expression of Rictor, mSIN1 and mLST8 were suppressed by shPVT1 but DEPTOR was upregulated (Figure ). In a word, down-regulation of PVT1 regulates the proliferation, apoptosis, cell cycle, and migration in GISTs cells, and PVT1 affected GIST-882 and GIST-T1 cells functions may via PI3K/AKT/mTOR pathway.
Discussion
GISTs are the most common mesenchymal sarcoma of the gastrointestinal tract with poor prognosis and high recurrence (DeMatteo et al. Citation2000). miRNAs and lncRNAs have been confirmed to have the potential biological function in the induction and progression of tumors (Qi et al. Citation2017; Yang et al. Citation2017; Liu et al. Citation2018). LncRNAs are related to several tumor signaling pathways including Notch, mTOR, NF-κb, and Wnt (Li, Yu, et al. Citation2015). They regulate cell proliferation, migration, apoptosis, invasion, tumorigenicity, cell cycle, and metastasis. Besides, accumulated evidence show that the abnormal expressions of lncRNAs have clinical significances in gastric cancer diagnosis (Shao et al. Citation2014; Zhu et al. Citation2015). These gastric cancer-associated lncRNAs may be used as potential biomarkers for indicating metastasis of gastric cancer (Endo et al. Citation2013; Hu et al. Citation2014).
Recently studies have demonstrated that high expression of PVT1 in various human cancer, such as hepatocellular carcinoma, gastric cancer, ovarian cancer, breast cancer, bladder cancer, and pancreatic cancer (Cho et al. Citation2018; Ding et al. Citation2018; He et al. Citation2018; Huang et al. Citation2018; Zhao et al. Citation2018). Studies on the relation between PVT1 and cancer have shown that PVT1 is a potential oncogene in various cancers (Guo et al. Citation2017). It can promote the occurrence and development of cancers by affecting cell proliferation, migration, invasion, and apoptosis. In our study, we identified that PVT1 can promote the migration and proliferation of GIST-882 and GIST-T1 cells and suppress apoptosis. Furthermore, down expression of PVT1 shows the opposite results. In a prior study, PVT1 promotes the development of cisplatin resistance in colorectal cancer; thus, silencing PVT1 inhibits tumorigenesis and cisplatin resistance in colorectal cancer (Ping et al. Citation2018). Down regulate of PVT1 to a certain extent enhances the radiosensitivity of non-small cell lung cancer cells through inhibiting cell proliferation and promoting apoptosis (Wu et al. Citation2017). In addition, over-expression of PVT1 in gastric cancer can significantly promote the proliferation and invasion of gastric cancer cells, which is associated with poor prognosis (Jia et al. Citation2017; Huang et al. Citation2017).
Currently, the molecular mechanisms of PVT1 in progression of cancer are not completely clear. In this research, we confirmed that knockdown of PVT1 obviously reduced the expression of CCND1, CCNB1, CDK6, PIK3CA, and P-AKT. Thus, we speculated that the functions of PVT1 in the regulation of GISTs cells may involve in PI3K/AKT pathway. PI3K/Akt signaling pathway plays a crucial role in cell proliferation, migration, and invasion (Li, Han et al. Citation2015). AKT can further stimulate different downstream factors, which play a pivotal role in angiogenesis and migration (Fukumura et al. Citation2001). Furthermore, studies showed that the activation of PI3K/AKT pathway was used to achieve their proliferative, migratory, and invasive processes by cancer cells (Ferretti et al. Citation2006). Similarly, lncRNA PVT1 interacts with downstream signaling pathway of c-Myc to synergistically promote tumorigenesis (Liang et al. Citation2019). Moreover, the study also revealed that silencing lncRNA PVT1 up-regulated miR-145 and inhibited the viability, migration, invasion capacity, and induced apoptosis of esophageal carcinoma cells via inhibition of FSCN1(Shen et al. Citation2019). The molecular mechanisms for the function of lncRNAs are very complex, which can act on any biological processes, such as epigenetic modification of DNA, DNA transcription and protein translation, protein mRNA stability (Sang et al. Citation2019). Interestingly, the Rictor-mTOR complex directly phosphorylated Akt/PKB on Ser473 in vitro and facilitated Thr308 phosphorylation by PDK1 (Sarbassov et al. Citation2005). High DEPTOR expression is necessary to maintain PI3 K and Akt activation and a reduction in DEPTOR levels leads to apoptosis (Peterson et al. Citation2009). Furthermore, mLST8 is necessary to maintain the Rictor–mTOR interaction, and both mLST8 and Rictor are required for the hydrophobic motif phosphorylation of Akt/PKB (Guertin et al. Citation2006). Here, when the phosphorylation level of AKT was inhibited by the knockdown of PVT1, the components of TORC1 and TORC2 followed in turn dramatically, which are following that Rictor, mSIN1, and mLST8 were down-regulated but DEPTOR was u-pregulated response to shPVT1. It is well-known that mTOR controls cell growth and proliferation via the raptor-mTOR (TORC1) and Rictor-mTOR (TORC2) protein complexes (Jacinto et al. Citation2006). Therefore, PVT1 may regulate the malignant progressions in GISTs cells via PI3K/AKT/mTOR pathway. Hence, further studies are urgently needed to acquire more insight into the regulatory events between PVT1 and PI3 K/AKT/mTOR pathway.
Our study has several limitations. First, the molecular mechanism of PTV1 and PI3 K/AKT/mTOR pathway in GISTs remains unclear. Furthermore, studies are needed to reveal the detailed mechanism of PTV1 regulation in GISTs. Second, the number of experiments enrolled in our study was relatively small, and we will increase the in vivo and molecular experiments in the future study. In summary, our study supplied the evidence of a functional link between PVT1 and GISTs cells, which added novel insights into the molecular mechanism of GISTs development.to identify the mechanism of PTV1 regulation in GISTs. Despite have some limitations, our results have several clinical implications. PTV1 may as potential biomarkers for indicating prognosis of GISTs.
Acknowledgements
The subject design was completed by Yang Ling. The operation of the experiment was carried out by Yanzhi Bi and Dongxiang Zeng. Data analysis by Wei Ye and Min Xiao. Article written by Yanzhi Bi and Quanliang Yang. The final data review and article review by Yang Ling.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Due to some confidential reasons, participants of this study did not agree for their data to be shared publicly and all authors do not wish to share the data with the database online before publication, so supporting data is not available. However, the data used to support the findings of this study are available from the corresponding author upon reasonable request.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Bo H, Fan L, Gong Z, Liu Z, Shi L, Guo C, Li X, Liao Q, Zhang W, Zhou M, Xiang B. 2019. Upregulation and hypomethylation of lncRNA AFAP1AS1 predicts a poor prognosis and promotes the migration and invasion of cervical cancer. Oncol Rep. 41(4):2431–2439. Available from: https://www.ncbi.nlm.nih.gov/pubmed/30816545. doi:10.3892/or.2019.7027.
- Casali PG, Abecassis N, Bauer S, Biagini R, Bielack S, Bonvalot S, Boukovinas I, Bovee JVMG, Brodowicz T, Broto JM, et al. 2018. Gastrointestinal stromal tumours: ESMO-EURACAN clinical Practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 29(Suppl 4):iv68–iv78. Available from: https://www.ncbi.nlm.nih.gov/pubmed/29846513. doi:10.1093/annonc/mdy095.
- Cheetham SW, Gruhl F, Mattick JS, Dinger ME. 2013. Long noncoding RNAs and the genetics of cancer. Br J Cancer. 108(12):2419–2425. Available from: https://www.ncbi.nlm.nih.gov/pubmed/23660942. doi:10.1038/bjc.2013.233.
- Cho SW, Xu J, Sun R, Mumbach MR, Carter AC, Chen YG, Yost KE, Kim J, He J, Nevins SA, et al. 2018. Promoter of lncRNA Gene PVT1 is a tumor-suppressor DNA boundary element. Cell. 173(6):1398–1412.e22. e1322. Available from: https://www.ncbi.nlm.nih.gov/pubmed/29731168. doi:10.1016/j.cell.2018.03.068.
- Connolly EM, Gaffney E, Reynolds JV. 2003. Gastrointestinal stromal tumours. Br J Surg. 90(10):1178–1186. Available from: https://www.ncbi.nlm.nih.gov/pubmed/14515284. doi:10.1002/bjs.4352.
- Consortium EP. 2012. An integrated encyclopedia of DNA elements in the human genome. Nature. 489(7414):57–74. Available from: https://www.ncbi.nlm.nih.gov/pubmed/22955616. doi:10.1038/nature11247.
- DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. 2000. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 231(1):51–58. Available from: https://www.ncbi.nlm.nih.gov/pubmed/10636102. doi:10.1097/00000658-200001000-00008.
- Ding H, Liu J, Liu B, Zeng Y, Chen P, Su Y. 2018. Long noncoding RNA PVT1 inhibits interferon-α mediated therapy for hepatocellular carcinoma cells by interacting with signal transducer and activator of transcription 1. Biochem Biophys Res Commun. 500(4):973–980. Available from: https://www.ncbi.nlm.nih.gov/pubmed/29715456. doi:10.1016/j.bbrc.2018.04.219.
- Endo H, Shiroki T, Nakagawa T, Yokoyama M, Tamai K, Yamanami H, Fujiya T, Sato I, Yamaguchi K, Tanaka N, et al. 2013. Enhanced expression of long Non-coding RNA HOTAIR Is associated with the development of gastric cancer. PLoS One. 8(10):e77070. Available from: <Go to ISI>://WOS:000325814200054. doi:ARTN e7707010.1371/journal.pone.0077070.
- Fan C, Tang Y, Wang J, Wang Y, Xiong F, Zhang S, Li X, Xiang B, Wu X, Guo C, et al. 2019. Long non-coding RNA LOC284454 promotes migration and invasion of nasopharyngeal carcinoma via modulating the Rho/Rac signaling pathway. Carcinogenesis. 40(2):380–391. Available from: https://www.ncbi.nlm.nih.gov/pubmed/30380023. doi:10.1093/carcin/bgy143.
- Ferretti C, Bruni L, Dangles-Marie V, Pecking AP, Bellet D. 2006. Molecular circuits shared by placental and cancer cells, and their implications in the proliferative, invasive and migratory capacities of trophoblasts. Hum Reprod Update. 13(2):121–141. Available from: https://www.ncbi.nlm.nih.gov/pubmed/17068222. doi:10.1093/humupd/dml048.
- Fletcher CDM, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP, et al. 2002. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Int J Surg Pathol. 10(2):81–89. Available from: https://www.ncbi.nlm.nih.gov/pubmed/12075401. doi:10.1177/106689690201000201.
- Fukumura D, Gohongi T, Kadambi A, Izumi Y, Ang J, Yun C-O, Buerk DG, Huang PL, Jain RK. 2001. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci USA. 98(5):2604–2609. Available from: https://www.ncbi.nlm.nih.gov/pubmed/11226286. doi:10.1073/pnas.041359198.
- Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM. 2006. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCα, but Not S6K1. Dev Cell. 11(6):859–871. doi:10.1016/j.devcel.2006.10.007.
- Guo K, Yao J, Yu Q, Li Z, Huang H, Cheng J, Wang Z, Zhu Y. 2017. The expression pattern of long non-coding RNA PVT1 in tumor tissues and in extracellular vesicles of colorectal cancer correlates with cancer progression. Tumor Biol. 39(4). Available from: <Go to ISI>://WOS:000400268800073. doi:Artn 699122 10.1177/1010428317699122.
- He Y, Jing Y, Wei F, Tang Y, Yang L, Luo J, Yang P, Ni Q, Pang J, Liao Q, et al. 2018. Long non-coding RNA PVT1 predicts poor prognosis and induces radioresistance by regulating DNA repair and cell apoptosis in nasopharyngeal carcinoma. Cell Death Dis. 9(2):235. Available from: https://www.ncbi.nlm.nih.gov/pubmed/29445147. doi:10.1038/s41419-018-0265-y.
- Hemming ML, Lawlor MA, Andersen JL, Hagan T, Chipashvili O, Scott TG, Raut CP, Sicinska E, Armstrong SA, Demetri GD, Bradner JE. 2019. Enhancer Domains in gastrointestinal stromal tumor regulate KIT expression and Are Targetable by BET Bromodomain inhibition. Cancer Res. 79(5):994–1009. Available from: https://www.ncbi.nlm.nih.gov/pubmed/30630822. doi:10.1158/0008-5472.CAN-18-1888.
- Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Tunio GM. 1998. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 279(5350):577–580. Available from: https://www.ncbi.nlm.nih.gov/pubmed/9438854. doi:10.1126/science.279.5350.577.
- Hirota S, Ohashi A, Nishida T, Isozaki K, Kinoshita K, Shinomura Y, Kitamura Y. 2003. Gain-of-function mutations of platelet-derived growth factor receptor α gene in gastrointestinal stromal tumors. Gastroenterology. 125(3):660–667. Available from: https://www.ncbi.nlm.nih.gov/pubmed/12949711. doi:10.1016/s0016-5085(03)01046-1.
- Hu Y, Wang J, Qian J, Kong X, Tang J, Wang Y, Chen H, Hong J, Zou W, Chen Y, et al. 2014. Long noncoding RNA GAPLINC regulates CD44-dependent cell invasiveness and associates with poor prognosis of gastric cancer. Cancer Res. 74(23):6890–6902. Available from: https://www.ncbi.nlm.nih.gov/pubmed/25277524. doi:10.1158/0008-5472.CAN-14-0686.
- Huang F, Chen W, Peng J, Li Y, Zhuang Y, Zhu Z, Shao C, Yang W, Yao H, Zhang S. 2018. LncRNA PVT1 triggers Cyto-protective autophagy and promotes pancreatic ductal adenocarcinoma development via the miR-20a-5p/ULK1 Axis. Mol Cancer. 17(1):98. Available from: https://www.ncbi.nlm.nih.gov/pubmed/30001707. doi:10.1186/s12943-018-0845-6.
- Huang T, Liu HW, Chen JQ, Wang SH, Hao LQ, Liu M, Wang B. 2017. The long noncoding RNA PVT1 functions as a competing endogenous RNA by sponging miR-186 in gastric cancer. Biomed Pharmacother. 88:302–308. Available from: <Go to ISI>://WOS:000395528000035. doi:10.1016/j.biopha.2017.01.049.
- Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B. 2006. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 127(1):125–137. doi:10.1016/j.cell.2006.08.033.
- Jia X, Chen J, Megger DA, Zhang X, Kozlowski M, Zhang L, Fang Z, Li J, Chu Q, Wu M, et al. 2017. Label-free proteomic analysis of exosomes derived from inducible hepatitis B virus-replicating HepAD38 cell line. Mol Cell Proteomics. 16(4 suppl 1):S144. Available from: http://www.mcponline.org/content/16/4_suppl_1/S144.abstract. doi:10.1074/mcp.M116.063503.
- Li H, Han L, Yang Z, Huang W, Zhang X, Gu Y, Li Y, Liu X, Zhou L, Hu J, et al. 2015. Differential Proteomic analysis of syncytiotrophoblast extracellular vesicles from early-onset severe preeclampsia, using 8-Plex iTRAQ labeling coupled with 2D Nano LC-MS/MS. Cell Physiol Biochem. 36(3):1116–1130. Available from: https://www.ncbi.nlm.nih.gov/pubmed/26113202. doi:10.1159/000430283.
- Li F, Huynh H, Li X, Ruddy DA, Wang Y, Ong R, Chow P, Qiu S, Tam A, Rakiec DP, et al. 2015. FGFR-Mediated Reactivation of MAPK signaling attenuates antitumor effects of Imatinib in gastrointestinal stromal tumors. Cancer Discov. 5(4):438–451. Available from: https://www.ncbi.nlm.nih.gov/pubmed/25673643. doi:10.1158/2159-8290.CD-14-0763.
- Li H, Yu B, Li J, Su L, Yan M, Zhang J, Li C, Zhu Z, Liu B. 2015. Characterization of differentially expressed genes involved in pathways associated with gastric cancer. PLoS One. 10(4):e0125013. Available from: https://www.ncbi.nlm.nih.gov/pubmed/25928635. doi:10.1371/journal.pone.0125013.
- Lian Y, Xiong F, Yang L, Bo H, Gong Z, Wang Y, Wei F, Tang Y, Li X, Liao Q, et al. 2018. Long noncoding RNA AFAP1-AS1 acts as a competing endogenous RNA of miR-423-5p to facilitate nasopharyngeal carcinoma metastasis through regulating the Rho/Rac pathway. J Exp Clin Cancer Res. 37(1):253. Available from: https://www.ncbi.nlm.nih.gov/pubmed/30326930. doi:10.1186/s13046-018-0918-9.
- Liang Y, Song X, Li Y, Su P, Han D, Ma T, Guo R, Chen B, Zhao W, Sang Y, et al. 2019. circKDM4C suppresses tumor progression and attenuates doxorubicin resistance by regulating miR-548p/PBLD axis in breast cancer. Oncogene. 38(42):6850–6866. Available from: https://doi.org/10.1038/s41388-019-0926-z. doi:10.1038/s41388-019-0926-z.
- Liu B, Pan CF, Yao GL, Wei K, Xia Y, Chen YJ. 2018. The long non-coding RNA AK001796 contributes to tumor growth via regulating expression of p53 in esophageal squamous cell carcinoma. Cancer Cell Int. 18:38. Available from: https://www.ncbi.nlm.nih.gov/pubmed/29568233. doi:10.1186/s12935-018-0537-8.
- Liu Z, Zhang H. 2017. LncRNA plasmacytoma variant translocation 1 is an oncogene in bladder urothelial carcinoma. Oncotarget. 8(38):64273–64282. Available from: https://www.ncbi.nlm.nih.gov/pubmed/28969069. doi:10.18632/oncotarget.19604.
- Milhem M, Deutsch JM. 2015. Imatinib Dosing in gastrointestinal stromal tumors (GISTs): when, How Much, and How long? Curr Clin Pharmacol. 10(4):311–320. Available from: https://www.ncbi.nlm.nih.gov/pubmed/26548908. doi:10.2174/1574884710666151020100518.
- Peterson TR, Laplante M, Thoreen CC, Sancak Y, Kang SA, Kuehl WM, Gray NS, Sabatini DM. 2009. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 137(5):873–886. doi:10.1016/j.cell.2009.03.046.
- Pickard MR, Mourtada-Maarabouni M, Williams GT. 2013. Long non-coding RNA GAS5 regulates apoptosis in prostate cancer cell lines. Biochimica et Biophysica Acta (BBA) - Mol Basis Dis. 1832(10):1613–1623. Available from: https://www.ncbi.nlm.nih.gov/pubmed/23676682. doi:10.1016/j.bbadis.2013.05.005.
- Ping GF, Xiong WC, Zhang LF, Li YR, Zhang YS, Zhao YL. 2018. Silencing long noncoding RNA PVT1 inhibits tumorigenesis and cisplatin resistance of colorectal cancer. Am J Transl Res. 10(1):138. Available from: <Go to ISI>://WOS:000425213900012.
- Qi B, Wang Y, Chen Z-J, Li X-N, Qi Y, Yang Y, Cui G-H, Guo H-Z, Li W-H, Zhao S. 2017. Down-regulation of miR-30a-3p/5p promotes esophageal squamous cell carcinoma cell proliferation by activating the Wnt signaling pathway. World J Gastroenterol. 23(45):7965–7977. Available from: https://www.ncbi.nlm.nih.gov/pubmed/29259372. doi:10.3748/wjg.v23.i45.7965.
- Sang Y, Chen B, Song X, Li Y, Liang Y, Han D, Zhang N, Zhang H, Liu Y, Chen T, et al. 2019. circRNA_0025202 regulates Tamoxifen sensitivity and tumor progression via regulating the miR-182-5p/FOXO3a Axis in breast cancer. Mol Ther. 27(9):1638–1652. doi:10.1016/j.ymthe.2019.05.011.
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. 2005. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 307(5712):1098–1101. doi:10.1126/science.1106148.
- Shao Y, Ye M, Jiang X, Sun W, Ding X, Liu Z, Ye G, Zhang X, Xiao B, Guo J. 2014. Gastric Juice long Noncoding RNA used as a tumor Marker for Screening gastric cancer. Cancer. 120(21):3320–3328. Available from:<Go to ISI>://WOS:000344164100011. doi:10.1002/cncr.28882.
- Shen SN, Li K, Liu Y, Yang CL, He CY, Wang HR. 2019. Down-regulation of long noncoding RNA PVT1 inhibits esophageal carcinoma cell migration and invasion and promotes cell apoptosis via microRNA-145-mediated inhibition of FSCN1. Mol Oncol. 13(12):2554–2573. Available from: <Go to ISI>://WOS:000485575400001. doi:10.1002/1878-0261.12555.
- Soreide K, Sandvik OM, Soreide JA, Giljaca V, Jureckova A, Bulusu VR. 2016. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol. 40:39–46. Available from: https://www.ncbi.nlm.nih.gov/pubmed/26618334. doi:10.1016/j.canep.2015.10.031.
- von Mehren M, Randall RL, Benjamin RS, Boles S, Bui MM, Ganjoo KN, George S, Gonzalez RJ, Heslin MJ, Kane JM, et al. 2018. Soft Tissue sarcoma, version 2.2018, NCCN clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 16(5):536–563. Available from: https://www.ncbi.nlm.nih.gov/pubmed/29752328. doi:10.6004/jnccn.2018.0025.
- Wei F, Jing Y-Z, He Y, Tang Y-Y, Yang L-T, Wu Y-F, Tang L, Shi L, Gong Z-J, Guo C, et al. 2019. Cloning and characterization of the putative AFAP1-AS1 promoter region. J Cancer. 10(5):1145–1153. Available from: https://www.ncbi.nlm.nih.gov/pubmed/30854123. doi:10.7150/jca.29049.
- Wilusz JE, Sunwoo H, Spector DL. 2009. Long noncoding RNAs: functional surprises from the RNA world. Genes Dev. 23(13):1494–1504. Available from: https://www.ncbi.nlm.nih.gov/pubmed/19571179. doi:10.1101/gad.1800909.
- Wu DP, Li Y, Zhang HX, Hu XG. 2017. Knockdown of lncrna PVT1 enhances Radiosensitivity in Non-small cell lung cancer by Sponging Mir-195. Cell Physiol Biochem. 42(6):2453–2466. Available from:<Go to ISI>://WOS:000415239800026. doi:10.1159/000480209.
- Yang X, Chen Y, Chen L. 2017. The Versatile role of microRNA-30a in human cancer. Cell Physiol Biochem. 41(4):1616–1632. Available from: https://www.ncbi.nlm.nih.gov/pubmed/28359057. doi:10.1159/000471111.
- Zeidler R, Lipp M, Joos S, Delecluse H-J, Bornkamm GW, Klobeck G, Vuillaume M, Lenoir GM. 1994. Breakpoints of Burkitt's lymphoma t(8;22) translocations map within a distance of 300 kb downstream of MYC. Genes Chromosomes Cancer. 9(4):282–287. Available from: https://www.ncbi.nlm.nih.gov/pubmed/7519050. doi:10.1002/gcc.2870090408.
- Zhao L, Kong H, Sun H, Chen Z, Chen B, Zhou M. 2018. LncRNA-PVT1 promotes pancreatic cancer cells proliferation and migration through acting as a molecular sponge to regulate miR-448. J Cell Physiol. 233(5):4044–4055. Available from: https://www.ncbi.nlm.nih.gov/pubmed/28657147. doi:10.1002/jcp.26072.
- Zhu S, Mao J, Shao Y, Chen F, Zhu X, Xu D, Zhang X, Guo J. 2015. Reduced expression of the long non-coding RNA AI364715 in gastric cancer and its clinical significance. Tumour Biol. 36(10):8041–8045. Available from: https://www.ncbi.nlm.nih.gov/pubmed/25971582. doi:10.1007/s13277-015-3543-7.