Abstract
Reports of snails’ resistance to niclosamide appeared recently and finding new molluscicides becomes necessary. We investigated the molluscicidal effects of Cucurbita maxima seed extracts on Biomphalaria pfeifferi, and Bulinus globosus snails under laboratory conditions. For B. pfeifferi, we tested seed extracts on juvenile and adult snails while only adult B. globosus were available for testing. Snails were exposed to water and crude ethanol extracts for 72 h and significant concentration-dependent mortality rates were observed. The number of B. pfeifferi juveniles collected was not enough for a comprehensive investigation against both solvents. We, therefore, tested them against water extracts only. A lethal concentration of 0.02 mg/mL killed 50% of the snails (LC50) for both water and ethanol extracts on adult B. pfeifferi snails. Our results suggest that pumpkin seed extracts have a significant molluscicidal effect on B. pfeifferi and B. globosus snails. The LC50 values for all the extracts in B. pfeifferi and B. globosus snails are within the threshold set for potential molluscicides by the World Health Organisation. We propose that C.maxima seed extracts be considered as potential molluscicidal agents in Schistosomiasis transmission control.
Introduction
Schistosomiasis (also known as Bilharzia) is a helminthic infection caused by a digenean trematode of the genus Schistosoma (Katsurada Citation1904). The disease leads to an estimated 200 000 deaths per year globally and at least 229 million people required treatment for the disease in 2018 alone (World Health Organisation (WHO) Citation2020). Like all digenetic trematodes, the intermediate host for schistosomes is the snails. In Zimbabwe, the snail vectors are Bulinus globosus for Schistosoma haematobium and Biomphalaria pfeifferi for Schistosoma mansoni (Chimbari Citation2012). Although Schistosomiasis is one of the most persistent Neglected Tropical Disease (NTD), treatment and disease control are based on the use of a single drug, praziquantel (PZQ), otherwise called biltricide (Sokolow et al. Citation2013).
Controlling or preventing morbidity in subjects using PZQ has not been entirely successful in restricting transmission in high-risk areas, as there have been reports of PZQ schistosomal resistance (Ismail et al. Citation1999; Qi and Cui Citation2012; Doenhoff et al. Citation2014; Augusto et al. Citation2017). WHO has set a roadmap that includes the elimination of Schistosomiasis as a public health problem by the year 2030 (WHO Citation2020) and efforts to achieve this goal using Mass Drug Administration (MDA) alone have proved inefficient. To address the inadequacy of current Schistosomiasis control endeavors and move towards its elimination, there is an urgent need to improve existing intervention measures (Molyneux et al. Citation2017; Krauth et al. Citation2019; Engels and Zhou Citation2020). As a responsive measure, in the light of its call to reach the elimination milestone, WHO discusses Schistosomiasis management through the ecological control of the intermediate host population of Schistosoma snails from the Biomphalaria and Bulinus genus (WHO Citation2014; Augusto et al. Citation2017). In 2017, WHO published an operational manual for the field use of molluscicides in Schistosomiasis control (WHO Citation2017) and supports member states in the implementation of snail control activities (WHO Citation2020). It is, therefore, largely agreed that the regulation of the snail population is an essential part of the control of Schistosomiasis (Bossier et al. Citation2019).
Molluscicide programmes have been employed in the control of Schistosomiasis for years (WHO Citation1961; McCullough Citation1980). Niclosamide is recommended by the WHO as the only chemical molluscicide to be used for snail control (WHO Citation1998) despite concerns of resistance of Oncomelania snails to the molluscicide (Dai Li and Wang Citation2014, Citation2018). The toxicity of niclosamide to non-target organisms such as fish is also of great concern. This is because the majority of the population that live in Schistosomiasis endemic areas rely on fish as a source of protein and income (WHO, Citation2020). The high cost of niclosamide also precludes its use in the endemic areas as they are usually characterised by low-income and high levels of poverty. Studies that will facilitate the development of new, affordable molluscicides with potentially low toxicity to non-target organisms are crucial (Sokolow et al. Citation2018). WHO, and other researchers, therefore, recommend further studies on plant molluscicides as these tend to be cost-effective and environmentally friendly (Coelho and Cadeira Citation2016; Augusto et al. Citation2017). The growing interest in plant-based molluscicides is also influenced by the fact that they degrade very rapidly when released into the environment, despite them being highly toxic to the snails (WHO Citation1983).
There has been a progression of several reports on the efficacy and molluscicidal activity of extracts from many researchers including Lemma et al. (Citation1972) who discovered the molluscicidal activity of butanol extracts of Phytolacca dodecandra on Biomphalaria snails and Rug and Ruppel (Citation2000) who studied the molluscicidal activity of Jatropha curcas on Biomphalaria glabrata, Bulinus natalensis & Bulinus. trunctatus snails. Ojewole (Citation2004) also found 14 of plants indigenous to South Africa to possess molluscicidal activity on B. pfeifferi and Bulinus africanus. El-sherbini et al. (Citation2009) studied the lethal molluscicidal capability of some Solanum species on Biomphalaria alexandrina snails and their vulnerability to infection with Schistosoma. Victor (Citation2015) studied the molluscicidal effects of Ocimum americanum, Brideli amicrantha and Chenopodium ambrosoides; Amalammar et al. (Citation2016) found the molluscicidal effects of Callistemon citrinus, Punica granatum and pumpkin on B. alexandrina and Augusto et al. (Citation2017) studied the double impact of Euphorbia milii latex on S. mansoni cercariae and their vectors, Biomphalaria snails.
Pumpkins (Cucurbita maxima) are known not only for their edible fruit but also for their several health benefits and thus have been used for a long time in traditional medicine in many countries such as Turkey and China (Young Kim et al. Citation2012). Pumpkin seeds have been used in different parts of the world as a traditional medicine for the treatments of gastrointestinal parasites such as anthelmintic (Ayaz et al. Citation2015), urinary dysfunctions, hyperplasia of the prostate, dysuria, cardiovascular disease, enuresis, and lowering blood glucose (Medjakovic et al. Citation2016; Lestari and Meiyanto Citation2018). Among the studies that have been done on pumpkin seeds, their anthelmintic potential has proved to be a success on S. mansoni (Beshay et al. Citation2018). However, there is a dearth of literature on the molluscicidal effects of pumpkin seeds on the vector snails. A successful trial of pumpkin seeds as a molluscicide would mean a double impact on both the vectors and the cercarial stage of the S. mansoni parasite in freshwater. Molluscicidal plant extracts may offer affordable, locally produced, biodegradable, and effectual control means in the rural parts of low-income countries where Schistosomiasis is prevalent (Hamed Citation2010).
In this study, we show that C. maxima seed water and ethanol crude extracts have molluscicidal effects against B. globosus and B. pfeifferi. To our knowledge, our work represents the first investigation to assess the molluscicidal activity of C. maxima extracts on the planorbid snails of the B. pfeifferi and B. globosus species. The work thus opens an opportunity for further research on the development of cost-effective alternatives for the control of Schistosomiasis based on natural compounds.
Materials and methods
Study site
The bio-assays and the seed extraction process were carried out in the Chinhoyi University of Technology biology and chemistry laboratories, respectively.
Collection of pumpkin seeds and snail vectors
Organic pumpkins were bought from a local supermarket in Chinhoyi, Zimbabwe. The pumpkins were thoroughly washed and cut to separate the seeds from the fruit.
B. globosus and B. pfeifferi snails were sampled using a sweep net in October 2018 at Madzorera dam in Murombedzi, Zimbabwe. Both snail species were identified using morphological keys according to Krauss (Citation1848) and Morelet (Citation1866). For B. pfeifferi, both adults and juveniles were obtained. However, only adults were obtained for B. globosus. The snails were kept in open plastic bottles and covered with moist cotton wool to keep them alive before reaching the laboratory.
Preparation of pumpkin seed ethanolic extracts
Pumpkin seeds were sun-dried for 72 h to a moisture content of 12.4%. Approximately 600 g of the seeds were milled into a fine powder using a mortar and pestle. The maceration technique was used to obtain the ethanolic crude extract. Thereafter, 900 ml of ethanol was added to 300 g of refined pumpkin seed powder and left in a dark cupboard for 7 days. At the end of this period, the mixture was filtered through a 0.1 mm Whatman filter paper grade using an EC vacuum pump (WP6122050) and then concentrated to dryness using a Buchi Rotavapor (R205) at 78°C to obtain pure crystals of the extract. The crystals were weighed and a total yield of 5 g was obtained. The crystals were dissolved in distilled water and the resulting solution of 100 mg/mL concentration was considered as the pure extract.
Preparation of pumpkin seeds water extracts
Approximately 600 ml of water was added to 300 g of fine pumpkin seed powder and left in a dark cupboard for 7 days. The mixture was filtered on 0.1 mm Whatman filter paper grade using an EC vapour pump (WP6122050) and the filtrate was concentrated to dryness using the Buchi Rotavapor (R205) to yield 8 g of crystals. The crystals were dissolved in 80 ml distilled water and the solution of 100 mg/ml concentration was considered as the pure extract.
Snail rearing
The snails were reared under laboratory conditions in plastic aquaria of 5 L holding capacity measuring 13 × 12 cm. The aquaria were provided with fresh water from the dams from which the snails were taken after every 2 days. No mud, sand, nor any other substratum was put in the aquaria. The laboratory in which they were kept was maintained at a room temperature of 25°C with natural fluctuations of +/−2°C for the length of the research. The snails were fed on oven-dried lettuce leaves (Chimbari and Shiff, Citation2008). The snails were allowed to acclimatise to laboratory conditions for 5 days.
Shedding of snails
To ensure that only healthy snails are used, cercariae were shed from the snails as described by El-sherbini et al. (Citation2009), with modifications. Briefly, after being exposed to the dark for 8 h, snails were placed in 300 ml plastic bottles filled with non-chlorinated water and placed in direct sunlight for 8 h after which a drop of water from each of the bottles was then viewed under the light microscope to check for the presence of cercariae.
Molluscicidal activity assay
During the study, the snails were kept under normal diurnal lighting and room temperature. Snails were organised into 2 classes, based on their developmental stage and shell diameter. Snails with a shell diameter of less than 45 mm were considered to be juveniles while those with shell diameters above 45 mm were considered to be adults (Ciomperlik et al., Citation2013). Snails were deprived of food during the molluscicidal assays. Before the molluscicidal assays, preliminary molluscicidal assay tests were done to determine the minimum effective concentration. A range of 5 concentrations were randomly assayed; 20%; 40%; 60%; 80% and 100% of the ethanol and water extracts. A lethal effect in 2 h among all the concentrations was observed and, therefore, serial dilutions of the lowest concentration (20%) were used for the molluscicidal assays. A maximum of 6 serial dilutions of 20% of the pure water and ethanol extracts were made as per WHO guidelines (WHO Citation1983). The final concentrations of the water and ethanol extract serial dilutions were 20 mg/mL; 2 mg/mL; 0.2 mg/mL; 0.02 mg/mL; 0.002 and 0.0002 mg/mL to give 6 treatments for each solvent. A 0, 1 dilution of Thunder (Imidaclopride + Betacyfluthrine 100 + 45 g/L derivative) was prepared and used as positive control and plain dam water was used as a negative control. Thunder is currently used as a molluscicide in Zimbabwe. A second positive control of absolute ethanol was used to factor into consideration the effects of residual ethanol in the ethanol extracts. Hence, inclusive of the Thunder and the 2 controls, 15 treatments were evaluated. The molluscicidal activity assays were set up simultaneously including all treatments and controls. Therefore, the controls were common for all assays.
Snails were sorted into 3 groups as follows: B. pfeifferi adults, B. pfeifferi juveniles and B. globusus adults. The snails from each group were assigned to a treatment and this was replicated 3 times. For the treatments, 10 ml of the 6 dilutions of pumpkin seeds extracts were mixed with 90 ml of dam water from where the snails were sampled. This was done to minimise the number of limiting factors that could affect the snails’ metabolism during the trial experiment. The duration of exposure to the molluscicide dilutions and control was 3 days. After the first 24 h, the number of molluscs withdrawn into their shells, immobile and unresponsive to vigorous action was recorded. Snails that were unresponsive to forceful, mechanical stimulation or probing were considered as dead. To further assure that the snails were indeed dead, they were placed in distilled water and observed for 2 h.
LC 50 determination and statistical analysis
Mortality percentages (LC50) were plotted against the log-transformed values of the extract concentrations using Graph pad Prism version 7.0 software (Finney, Citation1971) with a 95% confidence limit. The non-linear regression lines obtained from this data were used to determine the LC50 values.
A multifactor Analysis of Variance (ANOVA) was used to determine the significance of the effects of the C. maxima crude extracts on snail mortality. The ANOVA model selected allowed us to test the significance of the treatments whilst accounting for species and age. All the possible interactions were included in the model to determine whether the treatment effects varied with snail age and/or species. Non-significant interactions were discarded. An assessment of the model assumptions using plots on the residuals revealed that the assumptions were satisfied. Pairwise comparisons among the treatments were done using Tukey HSD to compare C. maxima crude extracts treatments and the respective concentrations against the positive control, Thunder. Data were considered statistically significant at P=0.05. All tests were done using IBM SPSS Statistics (Version 25) Tables and .
Table 1. Showing design of the experiment.
Table 2. Showing LC50 values of pumpkin seed extract on the 5 snail classes.
Results
Generally, C. maxima crude extracts induced a significant molluscicidal activity (P<0.00005; F=27.762; df=14) (Figure ). Furthermore, C. maxima crude extracts had the same molluscicidal activity regardless of species. However, the age of the snail influenced the efficacy of the C. maxima crude extracts (P=0.002, F=9.778; df=1). The mean mortalities of snails also significantly differed according to the solvent used to prepare the C. maxima crude extracts (P<0.00005; F=16.714; df=4). Ethanol-based extracts had significantly higher mean snail mortality compared to the water-based extracts. In addition, positive control of ethanol had a molluscicidal activity that was the same that of Thunder (P=0.311). Four ethanol-based concentrations, 20, 2, 0.2 and 0.02 mg/mL had mean snail mortalities that were not significantly different from Thunder (P>0.05). Three C. maxima water-based concentrations, 20, 2 and 0.2 mg/mL had mean snail mortality that was comparable to the positive control (Figure ). Based on Pairwise comparisons using Tukey’s HSD, the water extracts 20, 2 and 0.2 mg/mL had snail mortalities that were not significantly different from Thunder (P>0.05) (Figures ).
Figure 1. Molluscicidal activity of ethanol-based extracts and water-based extracts concentration of C. maxima seeds on B. pfeifferi and B. globosus snails. The asterisk indicates significance at p <0.05 compared to the positive control Thunder. The letter ‘a’ indicates significance at p <0.05 compared to the negative control. Error bars represent the standard error.
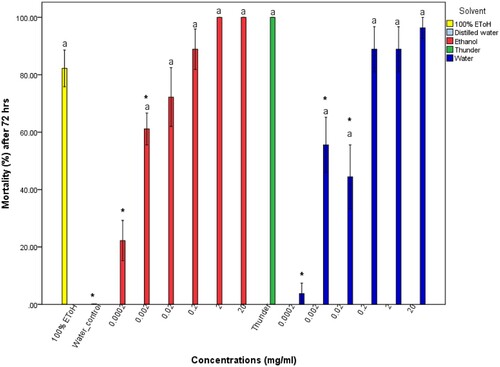
Figure 2. Molluscicidal activity of ethanol extracts of pumpkin seeds on adult B. globosus snails. The asterisk indicates significance at p <0.05 compared to the positive control Thunder. Error bars represent standard error.
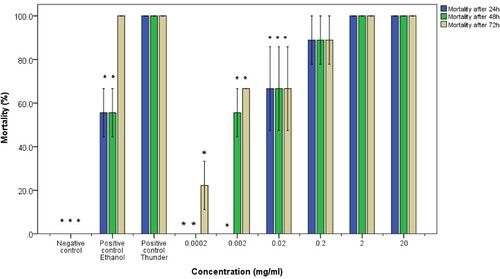
Figure 3. Molluscicidal activity of ethanol extracts of pumpkin seeds on adult B. pfeiferri snails. The asterisk indicates significance at p <0.05 compared to the positive control Thunder. Error bars represent standard error.
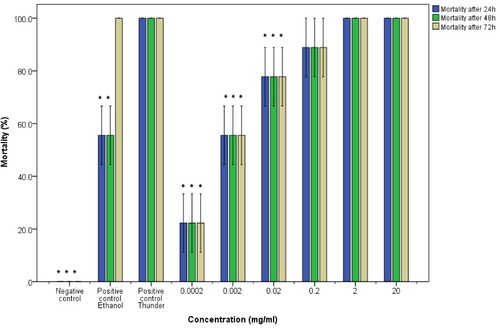
Figure 4. Molluscicidal activity of water extracts of pumpkin seeds on adult B. globosus snails. The asterisk indicates significance at p <0.05 compared to the positive control Thunder. Error bars represent standard error.
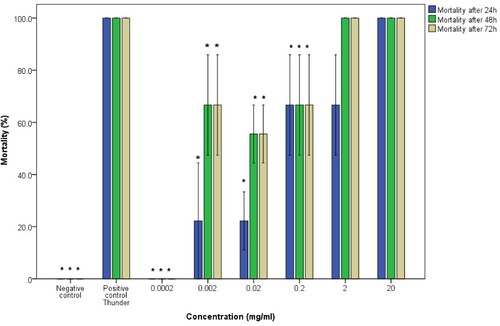
Results also showed that there was no significant difference between the survival rates of juvenile and adult B. pfeifferi snails exposed to water extracts (U=123.5; P=0.197; CI = 95%). Furthermore, there was no significance in the difference of species exposed to the water extracts (i.e. B. pfeifferi and B. globosus) (U=314.5; P=0.854; CI = 95%). The mortalities of B. pfeifferi adult snails were concentration-dependent and the molluscicidal activity of water extracts decreased with concentration with 0.0002 mg/mL showing no activity at all (Figure ). B. pfeifferi juvenile snails that were exposed to water extracts of pumpkin seeds did not show uniform concentration-dependent mortalities with 0.2 mg/mL and 0.002 mg/mL dilutions causing abnormally higher mortalities than the subsequent stronger more concentrated dilutions (Figure ). The average mortalities of the snails exposed to the positive and negative controls were uniform in all the trials.
Figure 5. Molluscicidal activity of water extracts of pumpkin seeds on adult B. pfeifferi snails. The asterisk indicates significance at p <0.05 compared to the positive control Thunder. Error bars represent standard error.
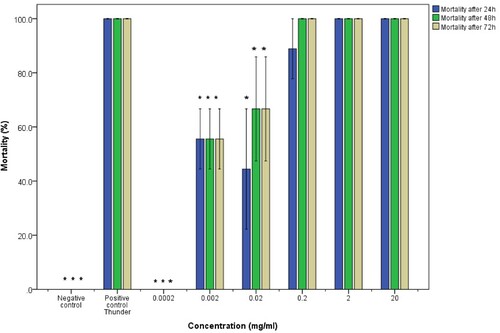
Figure 6. Molluscicidal activity of water extracts of pumpkin seeds on juvenile B. pfeifferi snail. The asterisk indicates significance at p <0.05 compared to the positive control Thunder. Error bars represent standard error.
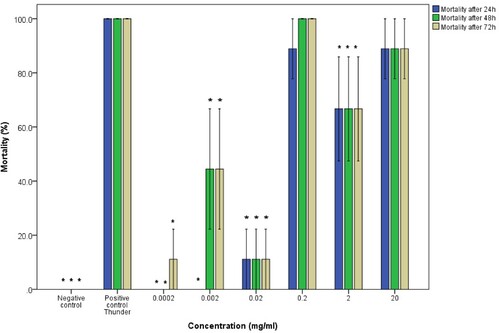
The results also showed that there was no significant difference between the effects of the water and ethanol extracts on adult B. pfeifferi (P = 0.875; CI=95%). The mortalities of B. pfeifferi adult snails exposed to ethanol extracts were concentration-dependent and the molluscicidal activity decreased with the extract concentration as shown by 0.0002 mg/mL showing no activity (Figure ). Water extracts of pumpkin seeds induced concentration-dependent mortalities on the B. pfeifferi juvenile snails with the lowest concentration of 0.0002 mg/mL causing mortality.
Discussion
The search for bioactive plant components that can be used as non-conventional molluscicides and anti-helminths has received considerable attention in recent times because of the increasing development of resistance to chemical synthetic molluscicides in snail populations. At present, there is an expanded consideration for the use of new molluscicides which are profoundly successful, rapidly biodegradable, more affordable, and easily accessible with uncomplicated application procedures (Jia et al. Citation2019). However, scientific evidence to validate the use of plant-based molluscicides remains limited and extensive investigations may help in understanding their properties and safety.
In the present study, we investigated the potential of pumpkin seed crude extracts molluscicide potential against adult and juvenile B. pfeifferi and adult B. globosus snails. We observed 100% mortalities in adult B. pfeifferi and B. globosus exposed to the high concentrations of both water and ethanol extracts. The potent molluscicidal activity exhibited by the C.maxima seed extracts in the present study is consistent with the high anthelminthic activity of Cucurbita moschata seed extracts (Marie-Magdeleine et al. Citation2009; Marie-Magdeleine et al. Citation2011). According to Marie-Magdeleine et al. (Citation2009), the phytochemical analysis suggested that non-proteic amino acid cucurbitin was responsible for helminth mortalities through intoxication. Therefore, although there is paucity in literature on the molluscicidal activity of C. maxima seed extracts on snails, it is plausible that the mortalities observed in our study may be attributed to cucurbitin. The susceptibility of B. pfeifferi and B. globosus snails to the extracts could be attributed to the fact that they have no operculum; thus, their cephalopodia were continuously in contact with the molluscicide during the assays (He et al. Citation2017).
Molluscicides can induce death by disrupting physiological processes for example, a decrease in the heart rate, swelling of tissues, and change in the water balance (McCullough Citation1980; Clark and Appleton Citation1996). Furthermore, He et al. (Citation2017) demonstrated that molluscicidal activity and mechanism of toxicity of a salicylanilide ester derivative against B. pfeifferi species could be based on an effect on neurohypophysis transmission and energy metabolism. There, however, is no current evidence to support that a common mechanism of action is responsible for these actions. Studies that evaluated the molluscicidal activity of three mangrove species (Avicennia schaueriana, Laguncularia racemosa and Rhizophora mangle) on the biological activities of B. glabrata show that sapponins and tannins are essential for effectual molluscicidal activity (Mendes et al. Citation2018). Further evidence is presented by studies on the molluscicidal activity of hydroalcoholic extracts of Jatropha gossypiifolia Linnaeus, 1753 on B. glabrata (Filho et al. Citation2014). The presence of these saponins and tannins in C. maxima seeds (Muchirah et al. Citation2018) could be a major contribution to their molluscicidal activity. However, further research is needed to discover all the specific compounds responsible for the molluscicidal activity of pumpkin seeds.
Solvents play an important role in the extraction of phytocompounds and this may influence the activity of the compounds (Babbar et al. Citation2014). Accordingly, our results on the molluscicidal activity of C. maxima seeds showed that ethanol-based extracts overall had a higher molluscicidal activity compared to the water-based extracts. Consistently, several studies have also highlighted that ethanol extracts generally induce higher mortalities than water extracts (Singh and Singh Citation1998; Tripathi and Singh Citation2000; El-sherbini et al. Citation2009). In addition, given that ethanol at 5% concentration can be used as an anaesthesia in physiological studies of snails (Gilbertson and Wyatt Citation2016; d’D’ovidio et al. Citation2019), a 100% concentration of ethanol will likely be lethal. This explains why the positive control of ethanol was as effective as to the Thunder.
It is important to note that three C. maxima water extract concentrations (20, 2 and 0.2 mg/mL) exhibited a molluscicidal efficacy significantly similar to Thunder. Furthermore, there were no mortalities recorded for the negative control of water. This suggests that the significant mortalities observed from the snails treated with the C. maxima water extracts can be attributed to compounds from the C. maxima seeds. As such this can be an indication that water is capable of extracting phytochemical compounds concentrations that are potent. This is advantageous because the ultimate goal is to attain effective active ingredients that can be used at the most convenient and cheapest way possible by the communities.
In general, a combined analysis of B. pfeifferi and B. globosus mortalities demonstrated a significant interaction between the C. maxima extracts and the age of the snail. Susceptibility to plant molluscicides is influenced by size. In some cases, adult snails have been more susceptible compared to juveniles because adults have a large body which offers a large surface for absorption of plant molluscicide extracts (Rawi et al. Citation2011). In contrast, Syombua et al. (Citation2013) and Obare et al. (Citation2016) found juvenile B. pfeifferi snails to be more susceptible to toxic effects of extract than adults. In the present, there was no significant difference between the survival rates of juvenile and adult B. pfeifferi snails exposed to C. maxima water extracts. We postulate that lack of consensus as to whether juveniles are more susceptible or vice versa, maybe to differences in the potency of plant molluscicides.
In conclusion, our results suggest that pumpkin seeds have a significant molluscicidal effect on B. pfeifferi and B. globosus snails. Throughout the study, high concentrations of both ethanolic and water extracts of pumpkin seeds showed substantial molluscicidal activities with LC50 values that were well below the upper threshold of 40 mg/L set for a potential molluscicide by the WHO (WHO, Citation2017). We demonstrated a huge potential of formulating an effective yet affordable and locally sourced pumpkin seed extract that can be an adopted molluscicide to be used as a snail control strategy in the reduction of Schistosomiasis transmission. Therefore, we propose that pumpkin seed extracts be categorised as one of the natural products that be considered as molluscicidal agents in a bid to control the transmission of Schistosomiasis. However, the elucidation of the molluscicidal activity requires studies that reveal details regarding the phytochemical profile of the plant. We, therefore, recommend future studies to include chromatographic analysis to analyse the phytochemical profile of the water and ethanol extracts of the pumpkin seed. Such an approach would allow researchers to identify more applications of the pumpkin seed extracts, such as the development of new drugs, and seeking molluscicidal compounds from plants which might impact transmission control of not only Schistosomiasis but other NTDs whose parasites are vectored by aquatic snails. Also it is important that further studies be carried out to test the toxicity of the pumpkin seed extracts on other taxonomic groups, e.g. invertebrates, small fish and others aquatic organisms to ensure that the extracts would not cause more ecological harm in the process of controlling snail populations.
Statement of informed consent
There are no human subjects in this article and informed consent is not applicable.
Articulation of human and animal rights
Ethical clearance to conduct this study was granted by the Biology Department of the Chinhoyi University of Technology.
Acknowledgements
We thank the Chinhoyi University of Technology for funding this research.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing is not applicable to this article as no new data were created or analysed in this study. The paper can be understood without any further data sets other than the ones provided in the tables and figures.
Additional information
Funding
References
- Amalammar ,El-Hefnawy N, Mahmoud S, Hodasabry, Fathyiagawish, Aly I , Lailarefahy. 2016. Evaluation of Callistemon citrinus, Punica granatum and Pumpkin against molluscicidal and free larval stages of Schistosoma mansoni. International Journal of Biology , Pharmacy and Allied Sciences.
- Augusto R, Tetreau G, ChanMarie-Laure P, Balieu W, Claudia M, Santos P, Grunau C. 2017. Double impact: natural molluscicide for schistosomiasis vector control also impedes the development of Schistosoma mansoni cercariae into adult parasites. PLoS Negl Trop Dis. doi:10.1371/journal.pntd.0005789.
- Ayaz E, Gokbulut C, Coşkun H, Turker A, Ozsoy Ş, Ceylan K. 2015. Evaluation of the anthelmintic activity of pumpkin seeds (Cucurbita maxima) in mice naturally infected with aspiculuris tetraptera. Journal of Pharmacognosy and Phytotherapy. doi:10.5897/JPP2015.0341.
- Babbar N, Oberoi H, Sandhu S, Bhargav V. 2014. Influence of different solvents in the extraction of phenolic compounds from vegetable residues and their evaluation as natural sources of antioxidants. J Food Sci Technol. 51:2568–2575.
- Beshay A, Rady A, Afifi A, Mohamed A. 2018. Schistosomicidal, antifibrotic and antioxidant effects of Cucurbita pepo L. seed oil and praziquantel combined treatment for Schistosoma mansoni infection in a mouse model. J Helminthol. 93(3):286–294. doi:10.1017/S0022149X18000317.
- Bossier J, Mouahid G, Moné H. 2019. Schistosoma spp. In: J.B. Rose, B. JiménezCisneros, editor. Globalwaterpathogenproject. E.Lansing, MI: UNESCO; http://www.waterpathogens.org (Robertson, Helminths) http://www.waterpathogens.org/book/shistosoma Michigan StateUniversity, https://doi.org/10.14321/waterpathogens.45.
- Chimbari M. 2012. Enhancing Schistosomiasis control strategy for Zimbabwe: building on past experiences. J Parasitol Res. doi:10.1155/2012/353768.
- Chimbari M, Shiff C. 2008. A laboratory assessment of the potential molluscicidal potency of Jatropha curcas aqueous extracts. Afr J Aquat Sci. 33(3):269–273. doi:10.2989/AJAS.2008.33.3.10.622.
- Ciomperlik M, Robinson D ,Gibbs I, Fields A, Stephens T, Taylor B. 2013. Mortality to the Giant African Snail, Lissachatina fulica (Gastropoda: Achatinidae), and Non - Target Snails using Select Molluscicides. Florida Entomologist. 96 doi:10.1653/024.096.0257.
- Clark T, Appleton C. 1996. The physiological effects of aqueous suspensions of plant molluscicides on helisoma duryi (gastropoda: planorbidae). J Molluscan Stud. 62(4):459–476.
- Coelho P, Cadeira R. 2016. Critical analysis of molluscicide application in schistosomiasis control programs in Brazil. Infect Dis Poverty. doi:10.1186/s4024901601536.
- Dai J., et al. 2018. Sensitivity of Oncomelania hupensis to niclosamide: A nation-wide survey in China. doi:10.3390/ijerph110303086.
- Dai Li Y, Wang W. 2014. Resistance to niclosamide in Oncomelania hupensis, the intermediate host of Schistosoma japonicum: should we be worried? Parasitol. 142:332–340. Pmid: 25003984. doi:10.1017/S0031182014000870.
- Doenhoff M, John R, Gerald K, Coles Donato C. 2014. The resistance of Schistosoma mansoni to praziquantel is there a problem? CioliPlumX Metrics. doi:10.1016/S0035-9203(02)90405-0.
- D’ovidio D, Monticelli P, Santoro M, Adami C. 2019. Immersion anaesthesia with ethanol in African giant land snails (acathina fulica). Heliyon. 5:e01546.
- El-sherbini G, Zayed R, El-Sherbini E. 2009. Molluscicidal activity of some Solanum species extracts against the Snail Biomphalaria alexandrina. J Parasitol Res. doi:10.1155/2009/474360.
- Engels D, Zhou X. 2020. Neglected tropical diseases: an effective global response to local poverty-related disease priorities. Infect Dis Poverty. 9:10. doi:10.1186/s40249-020-0630-9.
- Filho A, France C, Oliveira D, Mendes, R, Goncalves J, Rosa I. 2014. Evaluation of the molluscicidal potential of hydroalcoholic extract of Jatropha gossypiifolia linnaeus, 1753 on Biomphalaria glabrata (1818). Magazine of the Institute of Tropical Medicine of Sao Paulo (Print). 56:505–510.
- Finney D.J. 1971. Probit analysis. England: Cambridge University Press.
- Gilbertson C, Wyatt J. 2016. Evaluation of euthanasia techniques for an invertebrate species, land snails (succinea putris). J Am Assoc Lab Anim Sci. 55:577–581.
- Hamed M. 2010. Strategic control of schistosome intermediate host. Asian Journal of Epidemiology. 3:123–140. doi:10.3923/aje.2010.123.140.
- He P, Wang W, Sanogo B, et al. 2017. Molluscicidal activity and mechanism of toxicity of a novel salicylanilide ester derivative against Biomphalaria species. ParasitesVectors. 10:383. doi:10.1186/s13071-017-2313-3.
- Hotez P, Fenwick A. 2009. Schistosomiasis in Africa: an emerging tragedy in our new global health decade. PLoS Negl Trop Dis. 3:e485. doi:10.1371/journal.pntd.0000485.
- Ismail M, Botros S, Metwally A, William S, Farghally A, Tao L, Day T, Bennett J. 1999. Resistance to praziquantel: direct evidence from Schistosoma mansoni isolated from Egyptian villagers. doi:10.4269/ajtmh.1999.60.932.
- Jia T, Wang W, Sun L, et al. 2019. Molluscicidal effectiveness of Luo-Wei, a novel plant-derived molluscicide, against Oncomelania hupensis, Biomphalaria alexandrina and Bulinus truncatus. Infect Dis Poverty. 8(27). doi:10.1186/s40249-019-0535-7.
- Katsurada F. 1904. The etiology of a parasitic disease. Iji Shimbun. 669:1325–1332.
- Krauss B. 1848. Gastropoda: Planorbidae exposed to predatory and non - predatory fish. J Molluscan Stud 70. doi:10.1093/mollusc/70.4.353.
- Krauth S, Balen J, Gobert G, Lamberton P. 2019. A call for systems Epidemiology to tackle the complexity of schistosomiasis, Its control, and Its elimination. Tropical Medicine and Infectious Disease. 4(1):21. doi:10.3390/tropicalmed4010-021.
- Lemma A, Brody G, Newell G, Parkhurst R, Skinner W. 1972. Studies on the molluscicidal properties of endod (phytolaccadodecandra): I. increased potency with butanol extraction. J Parasitol. 58(1):104–107. doi: 10.2307/3278251.
- Lestari B, Meiyanto E. 2018. A review: The emerging nutraceutical potential of pumpkin seeds. Indonesian Journal of Cancer Chemoprevention. 9(2):92–101. ISSN: 2088–0197., DOI: 10.14499/indonesianjcanchemoprev9iss2pp92-101.
- Marie-Magdeleine C, Hoste H, Mahieu M, Varo H, Archimède H. 2009. In vitro effects of Cucurbita moschata seed extracts on haemonchus contortus. Vet Parasitol. 161:99–105.
- Marie-Magdeleine C, Mahieu H, Archimède H. 2011. Pumpkin (Cucurbita moschata Duchesne ex poir.) seeds as an anthelmintic agent? In: Nuts and seeds in Health and disease prevention.Chapitre 110 1st Ed. Academic Press-Elsevier.978-0123-756-886
- McCullough F. 1980. Molluscicides in Schistosomiasis control. Bulletin of the World Health Organisation. 58(5):681–689. doi:10.1016/0048-3575(76)90014-6.
- Medjakovic S, Hobiger S, Ardjomand-Woelkart K, Bucar F, Jungbauer A. 2016. Pumpkin seed extract: cell growth inhibition of hyperplasic and cancer cells, independent of steroid hormone receptors. Science Direct Journals. doi:10.1016/j.fitote.2016.03.010.
- Mendes R, Filho A, Nogueira A, Araujo K, Franca C, Carvalho I, da Silva N, Azevedo A, Rosa I. 2018. Evaluation of molluscicidal activity of three mangrove species (Avicennia schaueriana, Laguncularia racemosa and Rhizophora mangle) and their effects on the bioactivity of Biomphalaria glabratta (Say 1818). Rev.Inst.Med Trop. S. Paulo. 60. doi:10.1590/s1678-9946201860007
- Molyneux D, Savioli L, Engels D. 2017. Neglected Tropical diseases: progress towards addressing the chronic pandemic. Lancet. 389(100066):312–325. doi:10.1016/S0140-6736 (16)3071-4.Epub2016Sept 14PMID:27639954.
- Morelet. 1866. Predator avoidance in Bulinus globosus and B.tropicus. J Molluscan Stud. 70 doi:10.1093/molluscs/70.4.353
- Muchirah P, Waihenya R, Muya S, Abubakar L, Ozwara H, Makokha A. 2018. Characterization and anti-oxidant activity of Cucurbita maxima Duchesne pulp and seed extracts. The Journal of Phytopharmacology. 7(2):134–140.
- Obare B, Yole D, Nonoh J, Lwande W. 2016. Molluscicidal activity of selected plant extracts against adult and juvenile Biomphalaria pfeifferi. Journal of Biology, Agriculture and Healthcare. 6(22). http://www.iiste.org/.
- Ojewole J. 2004. Indigenous plants and Schistosomiasis control in South Africa: molluscicidal activity of some Zulu medicinal plants. Latin American and Caribbean Bulletin of Medicinal and Aromatic Plants. 3:8–22.
- Qi L, Cui J. 2012. A Schistosomiasis Model with Praziquantel Resistance School of Mathematical Sciences, Anhui University, Hefei 230601, China College of Science, Beijing University of Civil Engineering and Architecture, Beijing 100044, China. doi: 10.1159/000393137.
- Rawi S, Al-Hazmi M, Al Nassr F. 2011. Comparative study of the molluscicidal activity of some plant extracts on the snail vector of Schistosoma mansoni, Biomphalaria alexandrina. International Journal of Zoological Research. 7(2):169.
- Rug M, Ruppel A. 2000. Toxic activities of the plant Jatropha curcas against intermediate snail hosts and larvae of schistosomes. Trop Med Int Health. 6:423–430. doi:10.1046/j.1365-3156.2000.00573.x. PMID: 10929142.
- Singh S, Singh D. 1998. Molluscicidal activity of nerium indicum bark. Braz J Med Biol Res. 31:951–954.
- Sokolow S, Lafferty K, Kuris A. 2013. Regulation of laboratory populations of snails. (Biomphalaria and Bulinus spp.) By river prawns, macro brachium spp. (decapoda, palaemonidae): implications for control of schistosomiasis. Acta Trop. 132:64.
- Sokolow S, Wood C, Jones I, Lafferty K, Kuris A, Hsieh M, De Leo G. 2018. To reduce the global burden of Human schistosomiasis, Use ‘Old fashioned’ snail control. Trends Parasitol. 34(1):23–40. doi: 10.1016/j.pt.2017.10.002.
- Syombua E, Yole D, Musila F, Kutima, H, Kareru P. 2013. Assessment of Molluscicidal, cercaricidal and miracicidal activities of crude extracts of azadiratcha indica and entada leptostachya. Journal of Biology, Agriculture and Healthcare.Vol.3, No.5. ISSN 2224-3208 (Paper). ISSN 2225-093X (Online).
- Tripathi S, Singh D. 2000. Molluscicidal activity of Punica granatum bark and canna indica root. Braz J Med Biol Res. 33:1351–1355.
- Victor M. 2015. Assessment of Molluscicidal, cercaricidal and miracicidal activities of crude extracts of Ocimum americanum. Bridelia Micrantha and Chenopodium Ambrosoides. 5(22):1–8.
- WHO. 1961. Molluscicides. Second report of the WHO Expert Committee on Bilharziasis. World Health Organisation Technical Report Series.http://apps.who.int/iris/bitstream/10665/40484/1/WHO_TRS_214.pdf.
- WHO. 1983. Reports of the scientific working group on plant molluscicides. Bulletin World Health Organisation. 61(6):927–929. http://apps.who.int/iris/handle/10665/60086.
- WHO. 1998. Report of the WHO informal consultation on Schistosomiasis control. Geneva 2-4 December. WHO/CDS/CPC/SIP/99.2.
- WHO. 2014. Report of the WHO Strategic and Technical Advisory Group for Neglected Tropical Diseases ; 8-9 April 2014. Geneva. Switzerland.
- WHO. 2017. Field use of molluscicides in Schistosomiasis control programmes: an operational manual for programme managers. Geneva: World Health Organisation. 2017. Licence: CC BY-NC-SA 3.0 IGO.
- WHO. 2020. Schistosomiasis. Accessed Dec 3, 2020. https://www.who.int/newsroom/fact-sheets/detail/schistosomiasis.
- Young Kim M, Jin Kim E, Young-Nam K, Choi C, Lee B. 2012. Comparison of the chemical compositions and nutritive values of various pumpkin (Cucurbitaceae) species and parts. Department of Food and Nutrition, College of Natural Sciences, Chung-Ang University. https://doi.org/10.4162/nrp.2012.6.1.21.
