Abstract
Avocado production is severely affected by anthracnose disease in the field and after harvest worldwide. This disease causes losses in the field due to fruit abortion and as post-harvest disease affecting quality and quantity during marketing and storage. The management of this disease has been through the use of chemicals that toxify human beings and contaminate the environment. So, the development of alternative bio-pesticide, which is environment-friendly, is imperative. Therefore, this study was carried out to find an alternative to fungicides currently being used in the control of Colletotrichum gloeosporioides. This was achieved by evaluating the effectiveness of plant extracts from tree marigold (Tithonia diversifolia) and garlic (Allium sativum) in the inhibition of Colletotrichum gloeosporioides in vitro. The antifungal activity of the extracts was determined by the evaluation of their effectiveness in inhibiting the fungal mycelial growth and sporulation on PDA media. Fungicide, Atracol 70WP used to control the disease, was used for comparison purposes. Data obtained were analysed by ANOVA and Fishers mean separation test using Gestart version 6 software. Concentrations of 80 g/l of Allium sativum and 120 g/l of Tithonia diversifolia were significantly effective as antracol fungicides at 2 g/l which is the recommended rate by the manufacturer in inhibiting mycelial growth and sporulation of Colletotrichum gloeosporioides. However, further studies are recommended to evaluate the effectiveness of the extracts under field conditions.
Introduction
Avocado (Persea americana) is a principal commercial fruit in Kenya for local and export markets. It originated in Central America and Southern Mexico (Chen et al. Citation2018) and is currently considered as one of the most nutritious fruits worldwide. The fruit is highly vulnerable to decay and has a very low shelf life due to the rapid ripening and softening that limits the storage (Chen et al. Citation2017; Sierra et al. Citation2019), handling and transport potential (Hurtado-Fernández et al. Citation2018; Mazhar et al. Citation2018). Avocado, growing in Kenya and other parts of the world, is faced with various challenges such as pests and diseases (Agrios Citation2005; Kimaru et al. Citation2018a; Kwon et al. Citation2020). Diseases that are a threat to avocados include bacterial soft rot caused by Pseudomonas syringae, stem end rot caused by Dothiorella dominicana and anthracnose caused by Colletotrichum gloeosporioides among others (Agrios Citation2005; Darvas and Kotze Citation1987; Snowdon Citation1990). Anthracnose is the most common and serious fungal disease in horticulture, and a serious post-harvest disease of avocados worldwide including Kenya (Sharma et al. Citation2017; Kimaru et al. Citation2018a). A lot of loss is associated with the disease despite the use of chemicals by farmers to control it (Kebede and Belay Citation2019). Furthermore, consumer concerns on the use of fungicides in the management of the disease due to possible exceedant of set maximum residue level limits upon the use of chemicals (Ramírez-Gil et al. Citation2020; Yeung et al. Citation2017). Therefore, the study was aimed at coming up with alternatives to fungicides that are environment-friendly and non-toxic to human beings and animals (Palou et al. Citation2016; Sarkhosh et al. Citation2017). Tithonia diversifolia (Hemsl) and Allium sativum L. extracts were used in this studies due to their antifungal activities on various pathogenic fungi (Taskeen-Un-Nisa et al. Citation2011; Ni’na et al. Citation2011; Agboola et al. Citation2016; Ajao and Moteetee Citation2017; Ewané et al. Citation2020). Furthermore, T. diversifolia was used traditionally for various ailments in human beings in many countries including Kenya (Ajao and Moteetee Citation2017). This study, therefore, evaluated plant extracts as a possible bio-pesticide to control the fungus in vitro.
Materials and methods
Isolation of Colletotrichum gloeosporioides
Fungus isolation was done at the Plant Sciences Laboratories in Kenyatta University, Kenya. Potato dextrose agar (PDA) was the standard media used to isolate the fungal pathogen from the infected avocado. The infected avocado fruit samples were collected from farmers’ field in Gatanga in Murang’a County. The infected avocado fruits were washed cleanly under a running tap, then dipped into 1% sodium hypochlorite to surface sterilize for 30 seconds. The fruits were then rinsed in sterile distilled water and wiped dry using sterile paper towels. The isolates of Colletotrichum were obtained from the infected avocado, using the direct plating method. A sterile scalpel was used to cut 5 mm by 5 mm sections from the edge of the lesion of the infected area of the avocado where there was active mycelial growth. These diseased sections were placed individually at the centre of a petridishes containing hardened sterile potato dextrose agar (PDA media) for mycelial growth at room temperature. Sub-culturing was done until pure isolates were obtained for use in further studies.
Fungal identification
Each isolate was subjected to macroscopic and microscopic examinations through which their morphological features were observed and recorded. The fungus, C. gloeosporioides, was morphologically identified based on cultural and microscopical characteristics using published fungal key (Freeman et al. Citation1998; Domsch et al. Citation1980; Nagamani et al. Citation2006; Kimaru et al. Citation2018b) and at molecular level by the use of two sets of primers, CgInt (5-GGGGAAGCCTCTCGCGG-3) specific to Colletotrichum gloeosporioides combined with the universal primer ITS4 (TCCTCCGCTTATTGATATGC) (Kimaru et al. Citation2018a; Serra et al. Citation2011; Robert et al. Citation2012; Kolainis et al. Citation2020). Pure cultures of Colletotrichum gloeosporioides were obtained and used in the study (Table ).
Table 1. Colletotrichum gloeosporiode isolates with their genebank accession number.
Preparation of extracts
Young fresh leaf samples of Tithonia diversifolia were collected from a farmer’s hedge in Kiambu County. The leaves were washed thoroughly using clean water and wiped dry using a blotting paper. The leaves were dried in an oven for 20 min at 80°C to reduce excess water. Samples of 120, 100 and 80 g from dried leaves were taken. The samples were ground and mixed with 1 litre of distilled water in a mortar using a pestle. The resultant extract was filtered through a fine muslin cloth and different concentrations of the extract were obtained. Similarly, purchased garlic bulbs were peeled off the thin paper like covering to expose the cloves that were surface sterilized using 70% ethanol. The cloves were rinsed by distilled water and dried in an oven for 20 min in 80°C. After drying, the cloves weighed 80, 60 and 30 g, respectively and were crushed using a pestle and mortar dissolved in 1 litre of water for each. These different concentrations of Tithonia diversifolia and Allium sativum were used to amend PDA to evaluate their effects on the growth of Colletotrichum gloeosporioides in vitro.
Effect of different concentrations of leaf extracts on mycelial growth of the Colletotrichum gloeosporioides
The PDA media was amended by mixing 1 ml extract with 9 ml molten PDA prior to solidification for each extract concentration after autoclaving at 121°C for 15 min. The extracts were thoroughly mixed with the medium in the sterile flask by shaking with hands gently (Dhingra and Sinclair Citation1985). About 15 ml of sterilized molten extract-amended PDA medium was poured into sterile petridishes, 9 cm in diameter. Each extract concentration was replicated three times. Similarly, PDA was amended using Antracol (70WP) fungicide, a product of Bayer E. A. Its active ingredient Propineb is a dithiocarbamate fungicide with the multisite activity characteristic of the group. Two grams of antracol was dissolved in one litre of molten PDA media before it solidified. Petridishes, filled with molten PDA amended with antracol 70WP, served as positive standard, while non-amended PDA mixed with distilled water served as controls.
Once solidified, 5-mm discs of PDA with actively growing mycelium of 7-day-old colony of the isolate of C. gloeosporioides were cut out with sterile cork-borer and placed in the centre of each plate. The inoculated plates were incubated between 22°C and 24°C on the sterilized hood/chamber. On daily basis, two diagonal measurements of mycelium growth were taken on each plate and recorded. Final measurements were taken when fungal growth in the control fully covered the plates.
Furthermore, the plates were flooded with distilled water to bring the spores into suspension. Sporulation capacity was determined by counting the number of spores using a haemocytometer.
Results
The radial mycelia growth for fungal cultures grown on media amended with T. diversifolia and A. sativum extracts and fungicide, antracol 70WP was significantly different P < 0.05 from the controls (non-amended media) (Table ). The mean radial diameter for the control was highest at 85 mm in diameter at day 10, while the mean radial diameter for antracol at 4 g/l concentration was lowest in 0 diameter at day 10 (Table ). Mycelial growth of the isolate followed a similar trend among treatments in relation to the concentrations of plant extracts and antracol fungicide. The higher the concentrations of extracts, the higher the inhibitory effect, while low concentrations had the lowest effects (Table ). 80 g/l of A. sativum and 120 g/l of T. diversifolia extracts had significantly the same inhibitory effect as 2 g/l of antracol, the recommended rate as per the manufacturer’s label. No fungal growth was recorded on PDA amended with 4 g/l of antracol.
Highest inhibition was observed by media amended with antracol at a concentration of 4 g/l and lowest inhibition was observed by media amended with antracol at a concentration of 1 g/l (Figure ). The recommended dose of antracol for the control of anthracnose (2 g/l) showed 90% inhibition. Of the two extracts (Garlic and tree marigold extracts), garlic showed higher inhibition (85%) at a concentration of 80 g/l compared to Tithonia diversifolia that exhibited 80% inhibition at its highest concentration of 120 g/l (Figure ).
The mean number of spores was significantly different at P < 0.05 among Colletotrichum isolates treated with different concentrations of T. diversifolia, A. sativum and antracol 70WP (Table ). The mean number of spores was not significantly different: for garlic 30 g/l at 1.902, Tithonia 80 g/l at 1.946 × 106 and Antracol 1 g/l at 1.953 × 106 (Table ). The mean number of spores was significantly different: for garlic 60 g/l at 1.326 and Tithonia 120 g/l at 0.587 (Table ). The mean number of spores was significantly different for Tithonia 100 g/l at 1.217 and antracol 4 g/l at 0.924 (Table ).
Table 2. Mean radial mycelial growth of Colletotrichum gloeosporioides isolates 10 days after inoculation.
Figure 1. Graph showing percentage inhibition of mycelial growth of Colletotrichum gloeosporioides on PDA amended with the T. diversifolia and A. sativum extracts and antracol 70 WP.
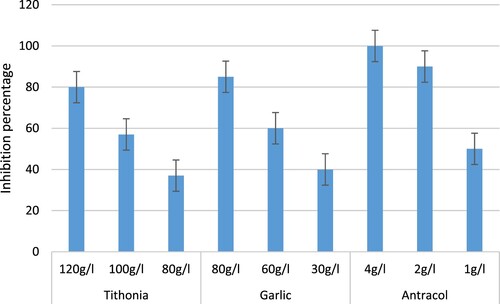
Table 3. Overall Mean number of spores per ml of Colletotrichum isolates.
Discussion
The results clearly showed that Allium sativum and Tithonia diversifolia have antifungal activity against Colletotrichum gloeosporioides on mycelial growth and sporulation (Table ). Similarly, Allium and Tithonia extracts were shown to have inhibitory effect on mycelial and sporulation of Colletotrichum gloeosporioides (Portz et al. Citation2008; Choudhary et al. Citation2017; Chen et al. Citation2018). These extracts were comparable to Antracol 70 WP, in their inhibition of Colletotrichum gloeosporioides in vitro. Inhibition of mycelial growth was observed to be increasing with an increase in the concentration of the extracts similar to antracol 70WP, fungicide. Similar findings were reported by Ilondu (Citation2012) and Ukech and Chiejina (Citation2012) that the increase in the antifungal activity was observed in the corresponding increase in the concentration of plant extracts.
The recommended dose of antracol of 2 g/l showed 90% inhibition, while 80 g/l of Allium sativum and 120 g/l of Tithonia diversifolia showed 85% and 80% inhibition, respectively. 4 g/l of antracol twice the recommended rate completely inhibited the mycelial growth of Colletotrichum gloeosporioides. There was also significant reduction in sporulation of the Colletotrichum gloeosporioides where the extracts were amended (Table ). Similar observations were made by Johnny et al. (Citation2010). Findings of this work could be an important step towards developing a biological control method better than the chemicals used in modern agriculture.
Public interest statement
The researcher focuses on post-harvest diseases of avocado in Kenya. The avocado has become a major fruit and economic earner for the country and at the world level. Quality and safety issues at local and export market call for research for addressing this. Post- harvest diseases being one of the major quality concerns motivated the researcher to undertake research project to study management/control options of these diseases in order to have quality, safe fruits acceptable by the consumers locally and internationally.
Acknowledgments
I thank the Almighty God for giving me the strength to carry on with this work. I am so grateful to my supervisor, Dr S. Kimaru, for his guidance, constructive comments and encouragement throughout the study. I am thankful to Plant Sciences Department, Kenyatta University for the chance to undertaking my research work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials. https://doi.org/10.6084/m9.figshare.13732153
References
- Agboola OO, Oyedeji S, Ajao A, Aregbesola O. 2016. Chemical composition and antimicrobial activities of essential oil extracted from Tithonia diversifolia (Asteraceae) flower. J Bioresourc Bioprod. 1(4):169–176.
- Agrios G. 2005. Plant pathology. 5th ed. Boston (MA): Elsevier Academic Press.
- Ajao AA, Moteetee AN. 2017. Tithonia diversifolia (Hemsl) A. Gray. (Asteraceae: Heliantheae), an invasive plant of significant ethnopharmacological importance: a review. S Afr J Bot. 113:396–403.
- Chen C, Liu CH, Cai J, Zhang W, Qi WL, Wang Z, Yang Y. 2018. Broad-spectrum antimicrobial activity, chemical composition and mechanism of action of garlic (Allium sativum) extracts. Food Control. 86:117–125.
- Chen J, Liu X, Li F, Li Y, Yuan D. 2017. Cold shock treatment extends shelf life of naturally ripened or ethylene-ripened avocado fruits. PloS one. 12(12):e0189991.
- Choudhary RS, Simon S, Bana SR. 2017. Efficacy of plant extracts against anthracnose (Colletotrichum lindemuthianum) of green gram (Vigna radiata L.). IJCS. 5(4):769–772.
- Darvas JM, Kotze JM. 1987. Avocado fruit diseases and their control in South Africa. South African avocado growers' association yearbook; Vol. 10. p. 117–119.
- Dhingra OD, Sinclair JB. 1985. Basic plant pathology methods. CRC Press.
- Domsch KH, Gams W, Anderson TH. 1980. Compendium of Soil Fungi. Vol. I & II. London: Academic Press.
- Ewané CA, Tatsegouock RN, Meshuneke A, Niemenak N. 2020. Field efficacy of a biopesticide based on Tithonia diversifolia against Black Sigatoka disease of plantain (Musa spp., AAB). Agric Sci. 11(08):730.
- Freeman S, Katan T, Shabi E. 1998. Characterization of colletotrichum species responsible of anthracnose diseases of various fruits. Plant Dis. 82:596–981.
- Hurtado-Fernández E, Fernández-Gutiérrez A, Carrasco-Pancorbo A. 2018. Avocado fruit – Persea americana. In: Exotic fruits. Academic Press; p. 37–48.
- Ilondu EM. 2012. Fungitoxic activity of leaf extracts from four Asteraceae against Sclerotium rolfsii Sacc., an isolate of sweet potato (Ipomoea batatas (L) Lam) vine rot disease. J Agric Biol Sci. 3(2):287–295.
- Johnny L, Yusuf UK, Nulit R. 2010. The effect of herbal plant extracts on the growth and sporulation of Colletotrichum gloeosporioides. J Appl Bioscien. 34:2218–2224.
- Kebede M, Belay A. 2019. Fungi associated with post-harvest avocado fruit rot at Jimma town, southwestern Ethiopia. J Plant Pathol Microbiol. 10(476):2.
- Kimaru SK, Monda E, Cheruiyot RC, Mbaka J, Alakonya A. 2018a. Morphological and molecular identification of the causal agent of anthracnose disease of avocado in Kenya. Int J Microbiol.
- Kimaru SK, Monda E, Cheruiyot RC, Mbaka J, Alakonya A. 2018b. Sensitivity of Colletotrichum gloeosporioides isolates from diseased avocado fruits to selected fungicides in Kenya. Advanc Agricul.
- Kolainis S, Koletti A, Lykogianni M, Karamanou D, Gkizi D, Tjamos SE. 2020. Specific primers that were used in the identification of Colletotrichum acutatum and Colletotrichum gloeosporioides isolates. Plos One. doi:10.1371/journal.pone.0233916.t001.
- Kwon J-H, Choi O, Lee Y, Kim S, Kang B, Kim J. 2020. Anthracnose on postharvest avocado caused by Colletotrichum kahawae subsp. ciggaro in South Korea, Canadian. J Plant Pathol. doi:10.1080/07060661.2019.1696891.
- Mazhar M, Joyce D, Hofman P, Vu N. 2018. Factors contributing to increased bruise expression in avocado (Persea americana M.) cv.‘Hass’ fruit. Postharvest Biol Technol. 143:58–67.
- Nagamani A, Kunwar IK, Manoharachary C. 2006. Hand book of Soil Fungi. New Delhi: I.K. International.
- Ni’na H, Meilin Y, Xiaoping Q, Qi L, Yuling D. 2011. The inhibitory effect of extract from Tithonia diversifolia on seven kinds of plant pathogens. Chines Agricult Sci Bull. 21.
- Palou L, Ali A, Fallik E, Romanazzi G. 2016. GRAS, plant-and animal-derived compounds as alternatives to conventional fungicides for the control of postharvest diseases of fresh horticultural produce. Postharvest Biol Technol. 122:41–52.
- Portz D, Koch E, Slusarenko AJ. 2008. Effects of garlic (Allium sativum) juice containing allicin on Phytopathora infestans and downy mildew of cucumber caused by Pseudoperonospora cubensis. In: The Downy Mildews-genetics, molecular biology and control. Dordrecht: Springer; p. 197–206.
- Ramírez-Gil JG, López JH, Henao-Rojas JC. 2020. Causes of Hass avocado fruit rejection in preharvest, harvest, and packinghouse: economic losses and associated variables. Agronomy. 10(1):8.
- Robert JM, Seijo TE, Hendricks K, Roberts PD. 2012. New report of Colletotrichum gloeosporioides causing postbloom fruit drop on citrus in Bermuda. Can J Plant Pathol. 34(2):187–194. doi:10.1080/07060661.2012.670137.
- Sarkhosh A, Vargas AI, Schaffer B, Palmateer AJ, Lopez P, Soleymani A, Farzaneh M. 2017. Postharvest management of anthracnose in avocado (Persea americana Mill.) fruit with plant-extracted oils. Food Packag Shelf Life. 12:16–22.
- Serra IMRS, Menezes M, Coelho RSB, Ferraz GMG, Montarroyos AVV, Martins LSS. 2011. Molecular analysis in the differentiation of Colletotrichum gloeosporioides isolates from the cashew and mango trees. Braz Arch Biol Technol. 54(6):1099–1108. doi:10.1590/S1516-89132011000600004.
- Sharma G, Maymon M, Freeman S. 2017. Epidemiology, pathology and identification of Colletotrichum including a novel species associated with avocado (Persea americana) anthracnose in Israel. Sci Rep. 7(1):15839.
- Sierra NM, Londoño A, Gómez JM, Herrera AO, Castellanos DA. 2019. Evaluation and modeling of changes in shelf life, firmness and color of ‘Hass’ avocado depending on storage temperature. Food Sci Technol Int. doi:10.1177/1082013219826825.
- Snowdon AL. 1990. A colour atlas of post-harvest diseases and disorders of fruits and vegetables. Volume 1: general introduction and fruits. London: Wolfe Scientific Ltd.
- Taskeen-Un-Nisa WA, Bhat MY, Pala SA, Mir RA. 2011. In vitro inhibitory effect of fungicides and botanicals on mycelial growth and spore germination of Fusarium oxysporum. J Biopestic. 4(1):53–56.
- Ukech JA, Chiejina NV. 2012. Preliminary investigations of the cause of post-harvest fungal rot of tomato. IOSR J Pharm Biol Sci. 4(5):36–39.
- Yeung MT, Kerr WA, Coomber B, Lantz M, McConnell A. 2017. Case studies of trade problems related to MRLs. In: Declining international cooperation on pesticide regulation. Cham: Palgrave Macmillan; p. 61–84.
