Abstract
Undifferentiated pleomorphic sarcoma (UPS) is a highly aggressive cancer that most commonly occurs in the extremities and trunk but is extremely rare in the kidney. This study reports a 60-year-old woman with obstinate cough and fever as the first symptoms and an initial diagnosis of inflammatory lung disease. However, none of the symptoms improved after one month of treatment. A repeated chest computed tomography (CT) scan suggested a huge renal mass in the left kidney; therefore, a radical nephrectomy was finally performed in the urology department. And the postoperative pathological examinations revealed UPS. Interestingly, the bothersome symptoms of the patient magically disappeared on the 2nd day after the surgery. During a follow-up of 32 months, there was no sign of recurrence. We found that early diagnosis together with thorough surgical treatment is currently the most effective approach to prolong the survival time of patients. In addition, postoperative radiotherapy and chemotherapy could be alternative options to benefit patients.
Introduction
Undifferentiated pleomorphic sarcoma (UPS), formerly known as malignant fibrous histiocytoma (MFH), was first reported by O'Brien and Stout in 1964 (O'Brien and Stout Citation1964). UPS accounts for about 20% of all soft tissue sarcomas, which commonly occurs in the extremities and trunk. UPS of the kidney is extremely rare (Kearney et al. Citation1980). UPS has four distinct histomorphological patterns: inflammatory subtype, storiform-pleomorphic subtype, giant cell subtype, and myxoid subtype (Altunkol et al. Citation2014). Besides, UPS has no characteristic clinical manifestations and imaging features, it is diagnosed mainly based on histology and immunohistochemistry, and is prone to metastasis and recurrence, so early detection is particularly important (Vuruskan et al. Citation2019). The patient complained of obstinate cough and fever for 2 days, with no significant improvement after 1 month of medication, followed by a chest CT scan which discovered, by some accident, that a mass occupied in the upper pole of the left kidney. This tortuous diagnostic and therapeutic process was informative and deepened our understanding of inflammatory undifferentiated pleomorphic sarcoma and paraneoplastic cough.
Case report
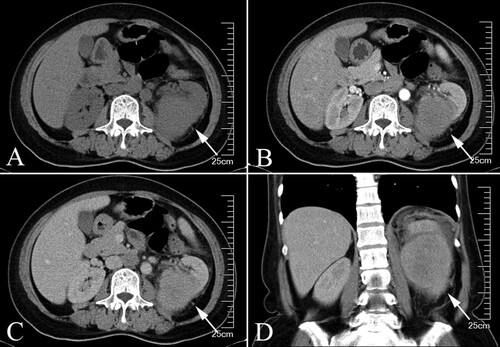
A 60-year-old female patient complains of an irritating dry cough, fever and poor appetite for 2 days. Physical examination was unremarkable with no positive signs. Instead, a chest X-ray showed a slight pulmonary infection in both lungs (Figure ). After one month of symptomatic treatment in the respiratory department, including cough suppression and antibiotic therapy, the above symptoms did not improve and a repeated CT of the lungs unexpectedly revealed a left suprarenal mass. Hematological analysis showed leukocytosis (32.6 × 103 µL) with thrombocytosis (364 × 103 µL) and decreased hemoglobin (7.1 g/dL), along with an elevated C-reactive protein level (36.5 mg/L). Urinalysis showed a number of WBC (36/hpf) and RBC (65/hpf) in the urine. Abdominal CT scan suggested a left renal mass (100 × 80 × 60 mm) with peripheral exudate, which was considered as a tumorous lesion with rupture and hemorrhage (Figure A–D). After assessing the condition, we decided to perform a radical left nephrectomy first.
Figure 1. Preoperative X-ray. A chest X-ray examination showed that there was a little inflammation in both lungs.
Figure 2. Preoperative Contrast-enhanced CT, the tumor was indicated by the arrows. (A) transverse plane (B) arterial phase (C) venous phase (D) Coronal plane.
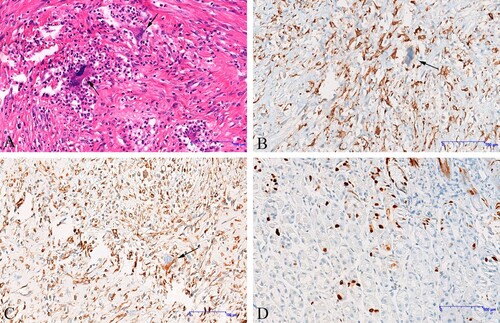
After the operation, the patient was given anti-inflammation medication and nutritional supplements. Interestingly, the persistent cough that had plagued the patient for a month stopped on the second day after the operation, and the blood white blood cell dropped to 9.4×103 µL. Postoperative pathology suggests negative histopathological margins. The results of hematoxylin and eosin staining (H&E) showed the cell morphology varies from round-like to spindle-shaped, and multinucleated tumor giant cells with abundant cytoplasm, large, darkly stained nuclei, distinct nucleoli and visible nuclear divisions are also seen. In combination with CD68 and Vimentin-positive expression, the diagnosis was inflammatory undifferentiated pleomorphic sarcoma (Figure A–D).
Figure 3. Immunohistochemistry (IHC) findings. (A) The morphology of tumor cells is diverse, and the giant cells of polynuclear tumors are abundant, the cytoplasm is abundant, the nucleus is large, deep staining, and the nucleolus is obvious; Nuclear splitting is easy to see, indicated by the arrows (hematoxylin and eosin, ×200). (B) Neoplastic cells are positive for CD68, nuclear splitting is easy to see, indicated by the arrows (IHC, ×200). (C) Neoplastic cells are positive for Vimentin, nuclear splitting is easy to see, indicated by the arrows (IHC, ×200) (D) Ki67 was examined as a proliferation marker of tumor cells.
In addition, the patient and her family refused chemotherapy and radiotherapy, so we did not proceed with further treatment. After follow-up of 32 months, the patient's hemoglobin rose to 12.8 g/dL, her leucocytes fell to 3.66 × 103 L and her weight increased by 6 kg.
Discussion
UPS originates from primitive undifferentiated interstitial cells and has no clear direction of differentiation (Schaefer and Fletcher Citation2018; Zhu and Hao Citation2018). UPS mainly occurs in the elderly aged from 50 to 70 years old, and incidence is higher in male than that in female. Besides, in a few cases, it will occur in children, but the invasiveness is lower (Henderson and Hollmig Citation2012). Although the 5-year overall survival rate of UPS is 60%, the metastasis-free survival rate is only 30%, and the most frequent metastatic site of UPS is the lung (Delisca et al. Citation2015; Jo and Doyle Citation2016; Vodanovich et al. Citation2019). As one of the subtypes of UPS, inflammatory undifferentiated pleomorphic sarcoma’s common histological morphology is diffuse and significant inflammatory infiltration, mainly neutrophils or eosinophils, and has nothing to do with infection and tissue necrosis. There are medium or large anaplastic tumor cells mixed inflammatory background, the nucleus is pleomorphic and the cell atypia is significant (Gu et al. Citation2015). Patients are often accompanied by peripheral leukocytosis and rare leukemia-like reaction.
The patient had not improved after 1 month of medication for obstinate cough and fever and had a leukaemia-like reaction with a reduced nutritional status. Therefore, in the absence of distant metastases, we decided to remove the patient's left renal tumor first. Two days after the surgery, the patient's cough and fever disappeared and her blood leukocytes returned to normal, fully indicating that the symptoms of pulmonary inflammation were closely related to the left renal tumor. The classic triad of kidney cancer presents with hematuria, back pain and an abdominal mass. However, some rare paraneoplastic manifestations of kidney cancer such as stiff person syndrome, limbic encephalitis, hypertension, Cushing syndrome, cerebellar ataxia and peripheral neuropathy have been reported (Agrawal and Sahni Citation2015; Sullivan Citation2016). Intractable cough is also one of the rare paraneoplastic syndromes of renal tumors and is a manifestation of non-metastatic renal cancer (Estfan and Walsh Citation2008). In this case, the following evidence supports the view that the UPS caused the cough. Firstly, there was no obvious coughing disorder other than the tumor. Secondly, the cough disappeared quickly after the tumor was removed. Cough can be produced by tumor cells or by cytokines or growth factors produced by the immune system. Prostaglandins secreted by kidney cancer, particularly prostaglandin E2, have been shown to play a role in the cough reflex (Maher et al. Citation2009). In addition, alterations in the renin-angiotensin system may be another area of research. Angiotensin-converting enzyme inhibitors can block the breakdown of bradykinin, which leads to coughing (Patel et al. Citation2017). Standard treatment of paraneoplastic cough with peripheral anesthetics and opioids is usually sufficient, but sometimes other drugs, such as diazepam, may be needed (Estfan and Walsh Citation2008). The cough will, of course, be relieved after the operation.
The diagnosis of UPS mainly depends on path morphology and immunohistochemistry. UPS is characterized by a typical immunopositive response to CD68 and vimentin, which distinguishes it from other renal sarcomas (Bairwa et al. Citation2017). The CT scan of renal UPS showed a large solid tumor, usually with necrotic or hemorrhagic areas and adjacent invasion, and the attenuation on enhanced CT scan was slightly higher than that of skeletal muscle (Karaosmanoğlu et al. Citation2015). The low signal area reflecting the fiber composition identified on the T2-weighted image is identified as an important feature of renal UPS (Kwak et al. Citation2003). The poor prognostic factors of UPS were age, tumor size, tumor necrosis, high mitotic count, degree of invasion, and distant metastasis (Winchester et al. Citation2018).
For renal UPS treatment, the key lies in early detection and timely treatment. Age, tumor size, incisal margin, and tumor stage are independent prognostic factors for UPS (Chen et al. Citation2019). The few reported cases of renal UPS with details are summarized in Table . In addition to surgery, it has been reported that radiotherapy may have a certain effect on renal UPS. Eroğlu et al. (Citation2005) reported a case of a patient who took radiotherapy after surgery and no tumor recurrence was found after follow-up of 15 months. When the tumor can not be removed completely or the cutting edge is positive, radiotherapy is an important adjuvant therapy (Shah et al. Citation2016). Proton or carbon ion-ion therapy is also effective for the unresectable or incomplete resection of UPS, but due to the limited number of postoperative radiotherapy cases of renal UPS, there is still a lack of large sample data (Demizu et al. Citation2017; Guan et al. Citation2019). Because of the small number of cases and a short follow-up period, the effect of chemotherapy after renal UPS is not clear (Gronchi et al. Citation2017). At present, most of them use doxorubicin and ifosfamide as first-line drugs. Recently, immunotherapy is effective for several types of advanced tumors, but it remains to be further explored for UPS (Uehara et al. Citation2015; Miwa et al. Citation2017; Keung et al. Citation2018; Nathenson et al. Citation2018).
Table 1. Literature review of undifferentiated pleomorphic sarcoma originating from kidney.
In conclusion, renal UPS is a highly malignant tumor with no specific clinical presentation, prone to metastasis and recurrence, and a poor prognosis. Complete surgical resection is currently the most effective treatment. Postoperative radiotherapy and chemotherapy may improve the survival cycle of patients and further clinical validation is needed.
Acknowledgements
Weiguo Chen and Feng Zhou conceived and designed this case report. Xiaojie Ang and Junjun Zhang wrote the initial draft of the report. Xu Zekun and Zhiyu Zhang provided academic help. Zhixin Ling provided us with a lot of advice and assisted us in the revision together. All authors have read and approved the final version of the manuscript. Ethics approval: Written informed consent for surgery was obtained from the patient. Written informed consent for publication of the present report was obtained from the patient.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Agrawal A, Sahni S, Iftikhar A, Talwar A. 2015. Pulmonary manifestations of renal cell carcinoma. Respir Med. 109:1505–1508.
- Altunkol A, Savas M, Ciftci H, Gulum M, Yagmur I, Bitiren M. 2014. Primary giant cell malignant fibrous histiocytoma-associated with renal calculus. Can Urol Assoc J. 8:E193–E195.
- Bairwa S, Sangwaiya A, Ansari M, Jindal A, Singla S, Yadav A. 2017. Malignant fibrous histiocytoma arising from renal capsule: an extremely rare entity. Indian J Pathol Microbiol. 60:402–404.
- Chen S, Huang W, Luo P, et al. 2019. Undifferentiated pleomorphic sarcoma: long-term follow-up from a large institution. Cancer Manag Res. 11:10001–10009.
- Chen CH, Lee PS, Han WJ, Shen KH. 2003. Primary giant cell malignant fibrous histiocytoma of the kidney with Staghorn calculi. J Postgrad Med. 49:246–248.
- Delisca GO, Mesko NW, Alamanda VK, et al. 2015. MFH and high-grade undifferentiated pleomorphic sarcoma – what's in a name? J Surg Oncol. 111:173–177.
- Demizu Y, Jin D, Sulaiman NS, et al. 2017. Particle therapy using protons or carbon ions for unresectable or incompletely resected bone and soft tissue sarcomas of the pelvis. Int J Radiat Oncol Biol Phys. 98:367–374.
- Ebrahimtabar F, Shafi H, Ranaee M, Darzi MM. 2019. A rare case of primary malignant fibrous histiocytoma: a sarcoma of the kidney. BMC Urol. 191:45.
- Eroğlu M, Bakirtaş H, Cimentepe E, Unsal A, Ataoğlu O, Balbay MD. 2005. Malignant fibrous histiocytoma arising from the renal capsule. Urol Int. 75:368–370.
- Estfan B, Walsh D. 2008. The cough from hell: diazepam for intractable cough in a patient with renal cell carcinoma. J Pain Symptom Manage. 36:553–558.
- Ghosh A, Dwivedi US, Kumar A. 2008. Inflammatory malignant fibrous histiocytoma of kidney: a case report. Pathol Res Pract. 204:857–861.
- Gronchi A, Ferrari S, Quagliuolo V, et al. 2017. Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): an international, open-label, randomised, controlled, phase 3, multicentre trial. Lancet Oncol. 18:812–822.
- Gu J, Zhang S, Wu X, et al. 2015. Malignant fibrous histiocytoma of visceral organs: clinicopathologic features and diagnostic value of ezrin and HMG-CoA reductase. Int J Clin Exp Pathol. 8:2876–2887.
- Guan X, Gao J, Hu J, et al. 2019. The preliminary results of proton and carbon ion therapy for chordoma and chondrosarcoma of the skull base and cervical spine. Radiat Oncol. 14:206.
- Gupta R, Gupta S, Aggarwal D, Singh S. 2008. Primary pleomorphic undifferentiated sarcoma of the kidney: a rare renal tumor. Indian J Pathol Microbiol. 51:573–576.
- Henderson MT, Hollmig ST. 2012. Malignant fibrous histiocytoma: changing perceptions and management challenges. J Am Acad Dermatol. 67:1335–1341.
- Hwang SS, Park SY, Park YH. 2010. The CT and F-FDG PET/CT appearance of primary renal malignant fibrous histiocytoma. J Med Imaging Radiat Oncol. 54:365–367.
- Jo VY, Doyle LA. 2016. Refinements in sarcoma classification in the current 2013 World Health Organization classification of tumors of soft tissue and bone. Surg Oncol Clin N Am. 25:621–643.
- Karaosmanoğlu AD, Onur MR, Shirkhoda A, Ozmen M, Hahn PF. 2015. Unusual malignant solid neoplasms of the kidney: cross-sectional imaging findings. Korean J Radiol. 16:853–859.
- Kearney MM, Soule EH, Ivins JC. 1980. Malignant fibrous histiocytoma: a retrospective study of 167 cases. Cancer. 45:167–178.
- Keung EZ, Tsai J-W, Ali AM, et al. 2018. Analysis of the immune infiltrate in undifferentiated pleomorphic sarcoma of the extremity and trunk in response to radiotherapy: rationale for combination neoadjuvant immune checkpoint inhibition and radiotherapy. Oncoimmunology. 7:e1385689.
- Kim SJ, Ahn BC, Kim SR, et al. 2002. Primary malignant fibrous histiocytoma of the kidney. Yonsei Med J. 43:399–402.
- Kwak H-S, Kim C-S, Lee J-M. 2003. MR findings of renal malignant fibrous histiocytoma. Eur Radiol. 13(Suppl 6):L245–L246.
- Maher SA, Birrell MA, Belvisi MG. 2009. Prostaglandin E2 mediates cough via the EP3 receptor: implications for future disease therapy. Am J Respir Crit Care Med. 180:923–928.
- Miwa S, Nishida H, Tsuchiya H. 2017. Current status of immunotherapy for sarcomas. Immunotherapy. 9:1331–1338.
- Nathenson MJ, Conley AP, Sausville E. 2018. Immunotherapy: a new (and old) approach to treatment of soft tissue and bone sarcomas. Oncologist. 23:71–83.
- O'Brien JE, Stout AP. 1964. Malignant fibrous xanthomas. Cancer. 17:1445–1455.
- Patel VR, Morganstern BA, Kavoussi LR. 2017. Persistent cough as a paraneoplastic presenting symptom in six patients with renal cell carcinoma. Asian J Urol. 4:10–13.
- Schaefer I-M, Fletcher CDM. 2018. Recent advances in the diagnosis of soft tissue tumors. Pathology. 50:37–48.
- Shah C, Verma V, Takiar R, et al. 2016. Radiation therapy in the management of soft tissue sarcoma: A clinician's guide to timing, techniques, and targets. Am J Clin Oncol. 39:630–635.
- Singh SK, Mandal AK, Agarwal MM, Das A. 2006. Primary renal inflammatory malignant fibrous histiocytoma: a diagnostic challenge. Int J Urol. 13:1000–1002.
- Sullivan S. 2016. Paraneoplastic cough and renal cell carcinoma. Can Respir J. 2016:5938536.
- Uehara T, Fujiwara T, Takeda K, Kunisada T, Ozaki T, Udono H. 2015. Immunotherapy for bone and soft tissue sarcomas. Biomed Res Int. 2015:820813.
- Vodanovich DA, Spelman T, May D, Slavin J, Choong PFM. 2019. Predicting the prognosis of undifferentiated pleomorphic soft tissue sarcoma: a 20-year experience of 266 cases. ANZ J Surg. 89:1045–1050.
- Vuruskan BA, Ozsen M, Coskun B, Yalcinkaya U. 2019. Evaluation of incidence and histolopathological findings of soft tissue sarcomas in genitourinary tract: Uludag University experience. Int Braz J Urol. 45:68–73.
- Winchester D, Lehman J, Tello T, et al. 2018. Undifferentiated pleomorphic sarcoma: factors predictive of adverse outcomes. J Am Acad Dermatol. 79:853–859.
- Zhu Y, Hao D. 2018. Tang X and Sun L: undifferentiated high-grade pleomorphic sarcoma of ethmoid sinus: a case report and literature review. Braz J Otorhinolaryngol. 84:389–392.
