Abstract
Human epidermal growth factor receptor 2 (HER2) proteins are overexpressed in a certain proportion of gastric cancer cases. Trastuzumab is a humanized monoclonal antibody that targets the HER2 receptor. Previous studies have demonstrated a synergistic interaction between trastuzumab and 5-fluorouracil/cisplatin in HER2-positive gastric cancer cells. However, it is unclear whether it is rational to combine trastuzumab with other chemotherapy regimens in clinical practice. We evaluated the growth inhibitory effects of combinations of trastuzumab and cytotoxic chemotherapy drugs in HER2-positive gastric cancer cell lines. HER2 protein expression was evaluated by Western blotting in five gastric cancer cell lines. MTT assays were performed to evaluate the growth inhibitory effects of trastuzumab and four chemotherapeutic agents, epirubicin, cisplatin, 5-fluorouracil and docetaxel, both alone and in combination. To explore the molecular mechanisms underlying the synergistic interactions of trastuzumab and chemotherapeutic agent treatment, the protein levels of phospho-protein kinase B (pAkt) were determined by Western blotting. We showed that trastuzumab synergizes with the cytotoxic effect of epirubicin in HER2-positive gastric cancer cells. Further investigation showed that the trastuzumab-epirubicin combination decreased the expression of pAkt. Altogether, our results provide preclinical evidence for the optimization of this combination regimen in the treatment of HER2-positive gastric cancer patients.
1. Introduction
Gastric cancer is the fifth most commonly diagnosed cancer and the third leading cause of cancer-related death worldwide (Bray et al. Citation2018). It has high incidence rates in Japan, China, Latin America, and some former Soviet European countries (Bray et al. Citation2018). Unfortunately, patients with gastric cancer are usually diagnosed at an advanced stage, and the prognosis of advanced gastric cancer remains poor. Median overall survival (OS) for advanced gastric cancer is 10–12 months (Smyth et al. Citation2016; Zaanan et al. Citation2018).
Chemotherapy is the main treatment option for advanced gastric cancer. Several drugs have shown good single-agent activity. These drugs include fluoropyrimidines (5fluorouracil, capecitabine, S1, and trifluridine–tipiracil), epirubicin, platinums, taxanes and irinotecan. The classically active drugs are considered cytotoxic agents that show 20%-30% response rates when used as first-line treatment (Cunningham et al. Citation2008). Evidently, there is a clear need for more active new agents and regimens.
Signal transduction by growth factors and their receptors plays an essential role in the carcinogenic process. The HER2 protein, which is encoded by ERBB2 located on human chromosome 17q12 (Tal et al. Citation1988), is a growth factor receptor that has receptor tyrosine kinase activity (Yarden and Sliwkowski Citation2001). It doesn’t have a direct ligand. It acts through the formation of heterodimer with other ErbB family receptors. Furthermore, it’s homodimers can spontaneously form in HER2-overexpressing cells. HER2 has been frequently detected in many human cancers. It participates in cell proliferation, apoptosis, angiogenesis and lymphangiogenesis. Overexpression of HER2 protein plays an important role in cancer development and progression (Cross and Dexter Citation1991; Hung and Lau Citation1999).
Trastuzumab is a humanized monoclonal antibody targeted against the extracellular domain of the HER2 oncogene, which is amplified and overexpressed in 12-13% of gastric cancers in China (Huang et al. Citation2013; Sheng et al. Citation2013). The TOGA trial showed that trastuzumab in combination with chemotherapy drugs significantly increased overall survival in patients with HER2-positive advanced gastric cancer compared with chemotherapy alone, and this improvement was particularly significant in patients with high HER2 expression. Moreover, trastuzumab did not increase the incidence of adverse events associated with chemotherapy (Bang et al. Citation2010).
However, the addition of trastuzumab to other validated chemotherapy regimens for advanced gastric cancer has not been tested prospectively. To acquire better methods for treating gastric cancer, we investigated the effect of trastuzumab in combination with cytotoxic chemotherapy drugs on gastric cancer cell lines with high HER2 expression and explored the intrinsic mechanisms. Antibody–drug conjugates (ADC) are designed to deliver cytotoxic drugs specifically to tumor cells. Some ADC consisting of trastuzumab linked to chemotherapy drug have shown efficacy in HER2-positive cancer (Verma et al. Citation2012). The chemotherapy drug which was chosen can be a candidate drug for ADC.
2. Materials and methods
2.1. Materials
Trastuzumab was purchased from Roche Pharmaceutical Ltd., Switzerland. Four chemotherapeutic drugs, namely, cisplatin, 5-fluorouracil, epirubicin and docetaxel, were purchased from Sigma-Aldrich Corporation, USA. 5-Fluorouracil and docetaxel were dissolved in DMSO and prepared into 1 mg/ml solution concentration. Epirubicin and cisplatin were dissolved in 0.9% sodium chloride and prepared into 1 mg/ml solution concentration. In every test, the drugs were diluted to the desired concentration by Roswell Park Memorial Institute (RPMI) 1640 medium. Anti-GAPDH (no. G8795) and anti-HER-2 (no. SAB4500789) antibodies were purchased from Sigma-Aldrich Corporation, USA. Anti-Akt (no. 4691) and anti-phospho-Akt (S473; no. 4060) antibodies were purchased from Cell Signaling Corporation, USA.
2.2. Cell lines and culture
Five gastric cancer cell lines (NCI-N87, SGC7901, AGS, MGC803, and MKN-28) were evaluated for HER2 protein expression. The human gastric cancer cell lines were provided by the Shanghai Institute of Cell Biology and the Chinese Academy of Sciences, China. The cells were maintained in RPMI 1640 supplemented with 10% foetal bovine serum (Gibco Corporation, USA) and 1% penicillin and streptomycin in a humidified incubator at 37 °C and 5% CO2.
2.3. In vitro drug sensitivity test for trastuzumab and cytotoxic chemotherapy drugs
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay was used for the in vitro cytotoxic chemotherapy drug sensitivity test. This assay observes the ability of viable cells to convert a soluble tetrazolium salt, MTT, into an insoluble formazan precipitate. In brief, gastric cancer cells taken from the exponential phase of cultures were added to sterile 96-well flat-bottomed microtiter plates (1×104 cells/per well) in 100 µl of medium, which were incubated at 37°C and 5% CO2 for 1 d. (1) Trastuzumab (0.1, 1, 10, 100, and 1000 µg/mL), epirubicin (0.01, 0.1, 1, 10, and 100 µM), cisplatin (0.01, 0.1, 1, 10, and 100 µM), 5-fluorouracil (0.1, 1, 10, 100, and 1000 µM) and docetaxel (0.01, 0.1, 1, 10, and 100 µM) were diluted at various concentrations and added to every 4 plates and then incubated for 1 d. The control was 200 µl of culture medium alone. MTT, 5 mg/ml in 20 µl of PBS, was added to each plate and incubated for 4 h. The medium was removed from the plate. DMSO (dimethyl sulfoxide, Sigma, USA) was added to each plate, and the plates were agitated on a plate shaker for 10 min to dissolve the formazan. The absorbance of formazan solutions was measured at 490 nm using a Thermo Multiskan FC Microplate Reader (Thermo Scientific Corporation, USA). IC50 values were derived for each drug in each cell line by calculating growth curves. (2) Based on the IC50 of a single agent, a drug sensitivity test for the combination of trastuzumab and chemotherapeutic drugs was administered. The chemotherapeutic drugs were selected at concentrations of IC50. Trastuzumab was selected at its clinically achievable concentration, 100 µg/ml. Trastuzumab and chemotherapeutic drugs were combined and incubated for 1 d. All results are the mean values of experiments repeated in triplicate. The percentage of cytotoxicity (growth inhibition) was measured by the following equation: % Cytotoxicity= (Experimental absorbance - Raw absorbance)/(Control absorbance - Raw absorbance)*100.
2.4. Protein extraction and estimation
NCI-N87, SGC7901 and MKN-28 cells were incubated with 0.1 µM epirubicin and 100 mg/L trastuzumab at 3 time points (0, 12, and 24 h). To isolate the proteins, cells collected from the cell culture plates were washed in phosphate-buffered saline (PBS) and lysed in cell lysis buffer. The cell lysates were incubated on ice for 30 min and centrifuged at 12,000 rpm for 30 min at 4°C. The supernatant was collected, and the concentration of total protein was evaluated by bicinchoninic acid (BCA) protein assay.
2.5. Western blot analysis
For western blot analysis, 20 µg of total protein from each sample was separated by SDS-PAGE and transferred onto polyvinylidene fluoride (PVDF) membranes at 400 mA for 1.5 h. After transfer, the membranes were blocked with blocking solution and then incubated with the primary antibody at 4°C overnight. The membranes were then washed with Tris-buffered saline with Tween 20(TBST) three times and incubated with horseradish peroxidase-conjugated secondary antibody (1:5000) for 1 h at room temperature. After washing three times in TBST, the membranes were subjected to Electro-Chemi-Luminescence (ECL) which was detected by Amersham Imager 600 (General Electric Company, USA) and quantified through ImageJ analysis. GAPDH was used as an internal loading control.
2.6. Statistical analysis
The data are expressed as the mean ± SD. The significance of the difference between the groups was analysed by Student’s t-test. A P value of less than 0.05 was considered statistically significant. All experiments were repeated at least three times. All statistics were analysed using SPSS software, version 18.0.
3. Results
3.1. HER-2 expression in gastric cancer cell lines by western blotting
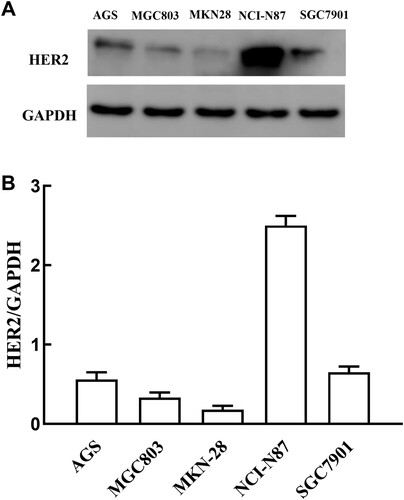
The expression of HER2 protein was analysed in five gastric cancer cell lines (AGS, MGC, MKN28, SGC7901 and NCI-N87) by western blotting. Of the five cell lines, NCI-N87 showed strong positivity of HER2 expression; SGC7901 showed moderate expression; the AGS and MGC gastric cancer cell lines showed weak expression; and the MKN28 gastric cancer cell line showed very weak expression (Figure ). Therefore, NCI-N87 and SGC7901 were selected as HER2-positive expression cell lines, and MKN28 was selected as a HER2-negative expression cell line.
3.2. In vitro drug sensitivity test for single drug treatment: trastuzumab or a kind of chemotherapeutic drug
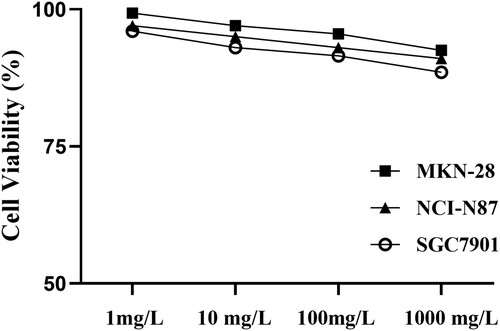
After the MTT assay was performed with 1 d of exposure of NCI-N87, SGC7901 and MKN28 to trastuzumab at various concentrations (1 mg/L, 10 mg/L, 100 mg/L, and 1000 mg/L) in vitro, the growth inhibition was almost negligible in the aforementioned cancer cell lines regardless of HER2 protein expression (Figure ). Our study was an experiment in vitro, and some important effects of trastuzumab could not be exerted, such as antibody-dependent cell-mediated cytotoxicity (ADCC). The clinical mean plasma concentration of trastuzumab was 79-123 mg/L, so we chose 100 mg/L as the experimental concentration.
Figure 2. Growth inhibitory effects of trastuzumab alone in gastric cancer lines. The in vitro MTT assay after 1 d of exposure to trastuzumab at various concentrations (1 mg/l, 10 mg/l, 100 mg/l, and 1000 mg/l).
For the four chemotherapeutic drugs, all cancer cell lines showed variable, dose-dependent sensitivity to the drugs, and the IC50 value (the drug concentration required for 50% growth reduction in the survival curve) was calculated (Table ).
Table 1. IC50 values of the four chemotherapy drugs in gastric cell lines.
3.3. In vitro drug sensitivity test for the combination of trastuzumab and chemotherapeutic drugs
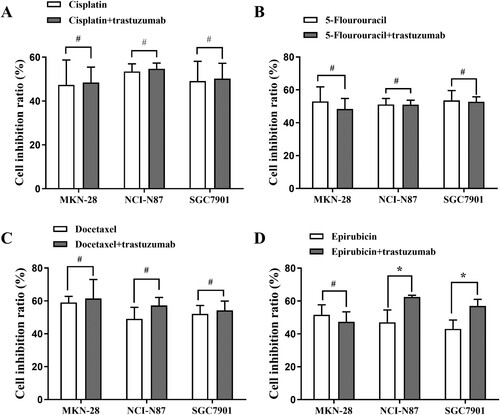
The cell growth inhibition rate was obtained by combining trastuzumab (100 mg/L) with each of the four chemotherapeutic drugs at IC50 individually for 1 d in the NCI-N87, SGC7901 and MKN-28 gastric cancer lines. In the NCI-N87 and SGC7901 cell lines, trastuzumab combined with epirubicin showed an increased cell inhibition rate compared with epirubicin alone (p < 0.05), but this effect was not observed in the MKN-28 cell line or with other drugs (Figure ).
Figure 3. Growth inhibitory effects of trastuzumab (100 mg/l) in combination with four chemotherapeutic drugs at IC50 for 1 d in the MKN-28, NCI-N87 and SGC7901 gastric cancer lines. (A): White column chart: cisplatin; black column chart: combination of trastuzumab and cisplatin; (B): white column chart: 5-Fu; black column chart: combination of trastuzumab and 5-Fu; (C) white column chart: docetaxel; black column chart: combination of trastuzumab and docetaxel; (D) white column chart: epirubicin; black column chart: combination of trastuzumab and epirubicin. Data points: mean values ± SD of the cell inhibition rate (%). Statistical analysis: Statistically significant differences between the two groups are shown with an asterisk (∗p < 0.05).
3.4. The mechanism of the synergistic effect of trastuzumab combined with epirubicin
Every cell line was divided into four groups: one group was used as the control group, one group was exposed to 0.1 µmol/L epirubicin, one group was exposed to 100 mg/L trastuzumab, and one group was exposed to 100 mg/L trastuzumab combined with 0.1 µmol/l epirubicin at three time points (0 h, 12, and 24 h). The protein levels of pAkt and Akt were determined by Western blotting.
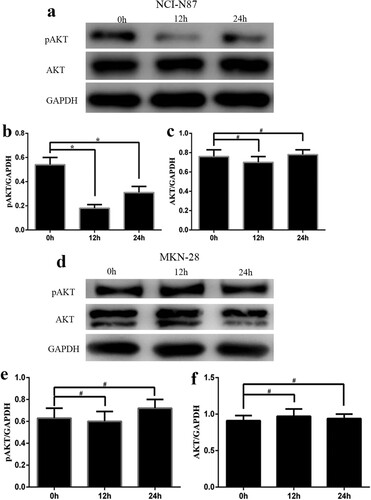
Trastuzumab inhibited active Akt as measured by antibodies specific to phospho-Ser473 Akt in the NCI-N87 and SGC7901 cell lines, without changes in total Akt. PAkt obviously decreased (p < 0.05) at 24 h after treatment in the combination group and trastuzumab group compared with the epirubicin group and control group. The expression of pAkt was lower in the combination group than in the trastuzumab group. PAkt did not change among the four groups in the M28 cell line (Figure ).
In the NCI-N87 cell line, which had high HER2 expression, pAkt was obviously decreased (p < 0.05) at 12 h and 24 h after treatment with trastuzumab and epirubicin, without changes in total Akt. However, in the M28 cell line, which had low HER2 expression, pAkt did not obviously change at 12 h or 24 h after treatment with trastuzumab and epirubicin, similar to total Akt (Figure ).
Figure 4. Protein levels of pAkt and Akt in the NCI-N87, MKN-28 and SGC7901 cell lines at 24 h after treatment. Every cell line was divided into four groups: (1) control group, (2) epirubicin group, (3) trastuzumab group, (4) trastuzumab+ epirubicin group. (A) Western blot assay of pAkt and Akt in the NCI-N87 cell line. The bar graph reflects the pAkt (B) and Akt (C) levels in the NCI-N87 cell line. (D) Western blot assay of pAkt and Akt in the MKN-28 cell line. The bar graph reflects the pAkt (E) and Akt (F) levels in the MKN-28 cell line. (G) Western blot assay of pAkt and Akt in the SGC7901 cell line. The bar graph reflects the pAkt (H) and Akt (I) levels in the SGC7901 cell line. Statistically significant differences between the two groups are shown with an asterisk (∗p < 0.05).
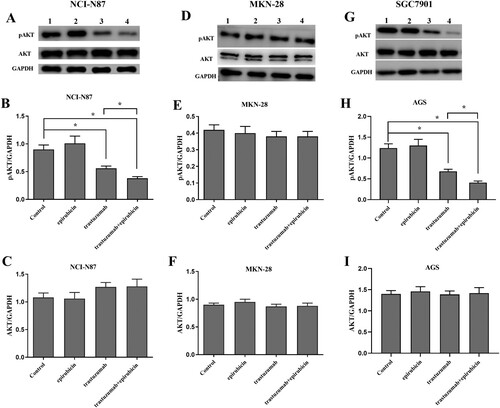
Figure 5. Protein levels of pAkt and Akt in the NCI-N87 and MKN-28 cell lines after treatment with trastuzumab and epirubicin at 3 time points (0, 12, and 24 h). (a) Western blot assay of pAkt and Akt in the NCI-N87 cell line. The bar graph reflects the levels of pAkt (b) and Akt (c) in the NCI-N87 cell line. (d) Western blot assay of pAkt and Akt in the MKN-28 cell line. The bar graph reflects the levels of pAkt (e) and Akt (f) in the MKN-28 cell line. Statistically significant differences between the two groups are shown with an asterisk (∗p < 0.05).
4. Discussion
In the current study, we explored the antitumor effect of combinations of trastuzumab and cytotoxic chemotherapy drugs in a HER2-positive gastric cancer cell line. Firstly, five gastric cancer cell lines, NCI-N87, SGC7901, AGS, MGC803, and MKN-28, were evaluated for HER2/neu protein expression. The NCI-N87, SGC7901 and M28 cell lines were selected for the following tests. Our data show that trastuzumab synergizes with the cytotoxic effect of epirubicin in HER2-positive gastric cancer cells. The trastuzumab-epirubicin combination inhibits the Akt signaling pathway.
HER2, a member of EGFR receptor family, is overexpressed in a wide range of cancers, including the biliary tract cancer, bladder cancer, colorectal cancer, breast cancer, ovarian cancer, non-small-cell lung cancer and gastric cancer (Oh and Bang Citation2020). After human epidermal growth factor receptor-2 (HER2) was first discovered in breast cancer cells, this protein has been actively researched in the breast cancer field. HER2 is overexpressed in approximately 25-30% of primary human breast cancers and is associated with aggressive tumor growth and poor clinical prognosis that includes resistance to therapy and a high chance of metastasis and recurrence (Paik et al. Citation1992; Ross et al. Citation2009; Arteaga et al. Citation2012). All HER2-positive breast cancer patients at every stage can benefit from anti-HER2 therapy (Slamon et al. Citation2001; Slamon et al. Citation2011). Accurate assessment of HER-2 expression levels is essential for identifying patients who will benefit from HER-2-targeted therapy. Currently, the criterion of high expression of HER2 protein is immunohistochemistry 2+ and FISH positive or immunohistochemistry 3 + .
The amplification or overexpression of the HER2 gene or protein also has been implicated in the development of gastric adenocarcinoma (Hechtman and Polydorides Citation2012). However, unlike in breast cancer, the prognostic significance of HER2 status in gastric cancer is unclear (Aizawa et al. Citation2014; Gu et al. Citation2014; Qiu et al. Citation2014; Kurokawa et al. Citation2015). While further studies are needed to assess the prognostic significance of HER2 status in gastric cancer, the addition of HER2 monoclonal antibodies to chemotherapy regimens is a promising treatment option for patients with HER2-positive metastatic disease.
Trastuzumab is a recombinant humanized monoclonal antibody against HER2 and has shown certain clinical benefits in some treatments of HER2-positive gastric cancer (Sheng et al. Citation2013; Shah et al. Citation2017). Trastuzumab therapy is being considered for patients with inoperable locally advanced, recurrent, or metastatic adenocarcinoma of the stomach.
In vitro, trastuzumab alone did not show obvious growth inhibition in any of the gastric cancer cells at the tested trastuzumab concentrations. This finding is similar to those of previous reports (Kasprzyk et al. Citation1992; Gong et al. Citation2004; Li et al. Citation2010). However, another three studies suggested the efficacy of trastuzumab alone in gastric cancer cell lines (Matsui et al. Citation2005; Fujimoto-Ouchi et al. Citation2007; Kim et al. Citation2008). Different drug exposure times or variations in cell lines may explain the inconsistent results.
Trastuzumab was approved in combination with chemotherapy as the first line of treatment in patients with metastatic HER2-overexpressing gastric cancer (Bang et al. Citation2010). Among chemotherapy agents, fluoropyrimidines, epirubicin, platinums, taxanes and irinotecan are conventionally administered in advanced gastric cancer (Wagner et al. Citation2010). We selected cisplatin, 5-fluorouracil, docetaxel and epirubicin to observe growth inhibition in gastric cancer cells for two reasons: (1) these chemotherapeutic drugs have been active in gastric cancer; and (2) the clinical characteristics, pharmacokinetics and pharmacodynamics of these drugs are well known. Fortunately, we found that the addition of trastuzumab to epirubicin enhanced the growth inhibition of HER2-positive gastric cancer cells. Our study also found that for the HER2-negative gastric cancer cell line, the addition of trastuzumab to chemotherapy did not change the growth inhibition effect. This finding indicated that trastuzumab should only be applied to HER2-positive gastric cancer, and strict screening for the right patients is essential.
Epirubicin, an anthracycline, can irreversibly bind to the electron-richgroups in DNA grooves through covalent bonds, thereby blocking DNA replication, transcription, and eventually leads to cell death. Epirubicin in combination with platinum and fluoropyrimidine demonstrated efficacy in the REAL 2 trial of advanced gastric cancer (Cunningham et al. Citation2008). In another study, the use of trastuzumab with doxorubicin, another anthracycline drug, increased cytotoxicity in HER2-overexpressing gastric cancer cells in vitro (Gong et al. Citation2004). Both epirubicin and trastuzumab have cardiotoxicity, which we are concerned about. It was reported an acceptable safety and efficacy profile in a small cohort treated with epirubicin -based chemotherapy with trastuzumab followed by trastuzumab maintenance (Sofia et al. Citation2014). In this small cohort, the decrease in EF was reversible and improved with administration of carvedilol.
The antitumor possible mechanisms of trastuzumab include inhibition of dimerization, induction of apoptosis, receptor internalization and/or degradation, inhibition of PI3 K/AKT and mammalian target of rapamycin (mTOR), and antibody-dependent cell-mediated cytotoxicity (ADCC) (Klapper et al. Citation2000). Among them, downregulating the autophosphorylation of HER2, followed by the inhibition of PI3K-Akt and Ras-Raf-Erk signal pathways is an important mechanism (Nahta and Esteva Citation2006). Unfortunately, despite Her2/neu overexpression, not all patients equally benefit from trastuzumab treatment because some patients have primary drug resistance. One of the main known mechanisms of resistance to anti-HER2 treatments is the aberrant activation of the PI3K-Akt and MAPK pathways (Tortora Citation2011). It was known that the inhibition of Akt and Erk phosphorylation can increase sensitivity to chemotherapy in gastric cancer (Oki et al. Citation2005; Liu et al. Citation2018). Our study demonstrated that trastuzumab in combination with epirubicin inhibited Akt phosphorylation levels further without changes in total Akt compared to trastuzumab alone in HER2-positive gastric cancer cells.
In conclusion, in different types of gastric cancer cell lines, the expression of HER2 protein is different. Trastuzumab synergizes with the cytotoxic effect of epirubicin in HER2-positive gastric cancer cells. Our findings suggest that the HER2 expression level can be a standard for the selection of chemotherapeutic agents that can be combined with trastuzumab more efficient in the treatment of gastric cancer.
Furthermore, new therapeutic agents have recently emerged. In particular, novel antibody – drug conjugates could yield interesting results. Ado-trastuzumab emtansine (T-DM1), trastuzumab deruxtecan (DS-8201a) and (vic-)trastuzumab duocarmazine (SYD985) are successful examples of targeting HER2 in some solid tumors (Xu et al. Citation2019). Among them, duocarmazine, which is similar to epirubicin, is also a DNA alkylating agent. Epirubicin may be a candidate drug for ADC after some chemical modifications.
Acknowledgements
This work was supported by grants from the Shandong Province Science and Technology Development Project Program (No. 2013GSF11845).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data is not available.
Additional information
Funding
References
- Aizawa M, Nagatsuma AK, Kitada K, Kuwata T, Fujii S, Kinoshita T, Ochiai A. 2014. Evaluation of HER2-based biology in 1006 cases of gastric cancer in a Japanese population. Gastric Cancer. 17(1):34–42.
- Arteaga CL, Sliwkowski MX, Osborne CK, Perez EA, Puglisi F, Gianni L. 2012. Treatment of HER2- positive breast cancer: current status and future perspectives. Nat Rev Clin Oncol. 9:16–32
- Bang Y-J, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, et al. 2010. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastroesophageal junction cancer (ToGA): a phase 3, open-label, randomized controlled trial. Lancet. 376 (9742): 687–697.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. 2018. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 68(6):394–424.
- Cross M, Dexter TM. 1991. Growth factors in development, transformation, and tumorigenesis. Cell. 64(2):271–280.
- Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR. 2008. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 358(1): 36–46.
- Fujimoto-Ouchi K, Sekiguchi F, Yasuno H, Moriya Y, Mori K, Tanaka Y. 2007. Antitumor activity of trastuzumab in combination with chemotherapy in human gastric cancer xenograft models. Cancer Chemother Pharmacol. 59(6), 795–805.
- Gong SJ, Jin CJ, Rha SY, Chung HC. 2004. Growth inhibitory effects of trastuzumab and chemotherapeutic drugs in gastric cancer cell lines. Cancer Lett. 214(2):215–224.
- Gu J, Zheng L, Wang Y, Zhu M, Wang Q, Li X. 2014. Prognostic significance of HER2 expression based on trastuzumab for gastric cancer (ToGA) criteria in gastric cancer: an updated meta-analysis. Tumour Biol. 35(6):5315–5321.
- Hechtman JF, Polydorides AD. 2012. HER2/neu gene amplification and protein overexpression in gastric and gastroesophageal junction adenocarcinoma: a review of histopathology, diagnostic testing, and clinical implications. Arch Pathol Lab Med. 136:691–697.
- Huang D, Lu N, Fan Q, Sheng W, Bu H, Jin X, Li G, Liu Y, Li X, Sun W, et al. 2013. HER2 status in gastric and gastroesophageal junction cancer assessed by local and central laboratories: Chinese results of the HER-EAGLE study. PLoS One. 8(11):e80290.
- Hung MC, Lau YK. 1999. Basic science of HER-2/neu: a review. Semin Oncol. 26 (4 Suppl 12). 51–59.
- Kasprzyk PG, Song SU, Di Fiore PP, King CR. 1992. Therapy of an animal model of human gastric cancer using a combination of anti-erbB-2 monoclonal antibodies, Cancer Res. 52:2771–2776.
- Kim SY, Kim HP, Kim YJ, Oh DY, Im SA, Lee D, Jong HS, Kim TY, Bang YJ. 2008. Trastuzumab inhibits the growth of human gastric cancer cell lines with HER2 amplification synergistically with cisplatin. Int J Oncol. 32(1), 89–95.
- Klapper LN, Waterman H, Sela M, Yarden Y. 2000. Tumor-inhibitory antibodies to HER-2/ErbB-2 may act by recruiting c-Cbl and enhancing ubiquitination of HER-2. Cancer Res. 60, 3384–3388.
- Kurokawa Y, Matsuura N, Kimura Y, Adachi S, Fujita J, Imamura H, Kobayashi K, Yokoyama Y, Shaker MN, Takiguchi S, et al. 2015. Multicenter largescale study of prognostic impact of HER2 expression in patients with resectable gastric cancer. Gastric Cancer. 18(4):691–697.
- Li XL, Yi SQ, Xu JM, Zhang Y, Yingying-Feng, Chen W, Song ST. 2010. The sequence-dependent cytotoxic effect of trastuzumab in combination with 5-fluorouracil or cisplatin on gastric cancer cell lines. Cancer Invest. 28(10):1038–1047.
- Liu N, Zhu M, Linhai Y, et al. 2018. Increasing HER2 α2,6 sialylation facilitates gastric cancer progression and resistance via the Akt and ERK pathways. Oncol Rep. 40(5):2997–3005.
- Matsui Y, Inomata M, Tojigamori M, Sonoda K, Shiraishi N, Kitano S. 2005. Suppression of tumor growth in human gastric cancer with HER2 overexpression by an anti-HER2 antibody in a murine model. Int J Oncol. 27(3), 681–685.
- Nahta R, Esteva FJ. 2006. Herceptin: mechanisms of action and resistance. Cancer Lett. 232(2); 123–138.
- Oh DY, Bang YJ. 2020. HER2-targeted therapies - a role beyond breast cancer. Nat Rev Clin Oncol. 17(1):33–48.
- Oki E, Baba H, Tokunaga E, Nakamura T, Ueda N, Futatsugi M, Mashino K, Yamamoto M, Ikebe M, Kakeji Y, Maehara Y. 2005. AKT phosphorylation associates with LOH of PTEN and leads to chemoresistance for gastric cancer. Int J Cancer. 117(3): 376–380.
- Paik S, Burkhard E, Lippman ME. 1992. Clinical significance of erbB2 protein overexpression. Cancer Treat Res. 61:181–191.
- Qiu M, Zhou Y, Zhang X, Wang Z, Wang F, Shao J, Lu J, Jin Y, Wei X, Zhang D, et al. 2014. Lauren classification combined with HER2 status is a better prognostic factor in Chinese gastric cancer patients. BMC Cancer. 14:823.
- Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. 2009. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 14:320–368.
- Shah MA, Xu RH, Bang YJ, Hoff PM, Liu T, Herráez-Baranda LA, Xia F, Garg A, Shing M, Tabernero J. 2017. HELOISE: phase IIIb randomized multicenter study comparing standard-of-care and higher-dose trastuzumab regimens combined with chemotherapy as first-line therapy in patients With human epidermal growth factor receptor 2-positive metastatic gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol. Aug 1;35(22):2558–2567.
- Sheng WQ, Huang D, Ying JM, Lu N, Wu HM, Liu YH, Liu JP, Bu H, Zhou XY, Du X. 2013. HER2 status in gastric cancers: a retrospective analysis from four Chinese representative clinical centers and assessment of its prognostic significance. Ann Oncol. 24(9): 2360–2364.
- Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, Mackey J, Glaspy J, Chan A, Pawlicki M, et al. 2011. Adjuvant trastuzumab in HER2-positive breast cancer. N. Engl. J. Med. 365:1273–1283.
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, et al. 2001. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 344:783–792.
- Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D. 2016. Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 27: v38–v49.
- Sofia P, Arturo LB, Muaiad K, et al. 2014. Successful use of trastuzumab with anthracycline-based chemotherapy followed by trastuzumab maintenance in patients with advanced HER2-positive gastric cancer. Anticancer Res. Jan;34(1):301–306.
- Tal M, Wetzler M, Josefberg Z, Deutch A, Gutman M, Assaf D, Kris R, Shiloh Y, Givol D, Schlessinger J. 1988. Sporadic amplification of the HER2/neu protooncogene in adenocarcinomas of various tissues. Cancer Res. 48(6):1517–1520.
- Tortora G. 2011. Mechanisms of resistance to HER2 target therapy. J Natl Cancer Inst Monogr. 2011(43): 95–98.
- Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, Pegram M, Oh D-Y, Diéras V, Guardino E, et al. 2012. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 367:1783–1791.
- Wagner AD, Unverzagt S, Grothe W, Kleber G, Grothey A, Haerting J, Fleig WE. 2010. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 3:CD004064
- Xu Z, Guo D, Jiang Z, Tong R, Jiang P, Bai L, Chen L, Zhu Y, Guo C, Shi J, Yu D. 2019. Novel HER2-targeting antibody-drug conjugates of trastuzumab beyond T-DM1 in breast cancer: trastuzumab deruxtecan(DS-8201a) and (Vic-)trastuzumab duocarmazine (SYD985). Eur J Med Chem. 183:111682.
- Yarden Y, Sliwkowski MX. 2001. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2(2):127–137.
- Zaanan A, Bouché O, Benhaim L, Buecher B, Chapelle N, Dubreuil O, Fares N, Granger V, Lefort C, Gagniere J, et al. 2018. Gastric cancer: French intergroup clinical practice guidelines for diagnosis, treatments and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO). Dig Liver Dis. 50:768–779.