?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Sepsis, a life-threatening organ dysfunction, continues to cause considerable morbidity. Reliable animal models to evaluate therapeutic options are urgently required, however, some of the most established ones are technically challenging. We herein present a murine model of abdominal sepsis based on the intraperitoneal (i.p.) injection of a defined inoculum of stool specimens (IPSI). Fecal bacterial compositions of male C57BL/6N mice were used for experiments. Dilutions of suspended stool (1:3, 1:6, 1:9) were injected i.p. into recipient mice. Clinical symptoms, cytokine expression, and mortality were assessed up to 48 h following IPSI. Storage at 4°C for 72 h exerted only minimal effect on bacterial composition, while deep freezing substantially impacted bacterial viability. After 9 h following IPSI, a dose-dependent, significant increase in clinical score was visible in all stool-injected mice. The same was observed for expression levels of pro-inflammatory cytokines IL-6 and CCL-2. Mortality rate of septic mice ranged from ∼30% (1:9) to 100% (1:3) within 48 h, depended on the dilution of stool inoculum. The IPSI model ensures experimental stability while mimicking a pathophysiological relevant stimulus similar to other established models. Characterization of microbial composition, minimal invasiveness and easy technical handling allow a good reproducibility and disease severity can be titrated.
Introduction
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection (Seymour et al. Citation2016; Singer et al. Citation2016). Despite advances in its management, sepsis continues to cause considerable morbidity. In 2017, an estimated 48.9 million incident cases of sepsis were recorded worldwide, and 11 million sepsis-related deaths were reported, representing 19.7% of all global deaths (Rudd et al. Citation2020). The treatment of sepsis is generally supportive in addition to antimicrobial substances to eliminate the pathogen. Although tremendous progress has been made in our understanding of the pathophysiology of sepsis, no definitive therapies exist to treat this condition.
Murine models have largely been used in sepsis research to better understand the pathophysiological changes during disease progression and to develop therapeutic options. Sadly, clinical studies of human sepsis relying on these preclinical observations failed to demonstrate efficacy of any pharmacological intervention. Although murine studies are criticized for this and caution should be exercised in translating conclusions from animal models to humans, it is indisputable that animal models have tremendously enhanced our understanding of sepsis. In addition, former clinical intervention trials were often based on heterogenic cohorts resulting in a considerable noise-to-signal ratio, making any demonstration of clinical efficacy difficult. Hence, the missing success in the establishment of new drugs against human sepsis is not just based on insufficient animal models but is rather system immanent due to the pleiomorphic character of this syndrome. On the other hand, murine models of sepsis can be powerful research tools to better understand certain aspects of the disease development and to explore new biomarkers – a requirement for personalized therapy and successful patient stratification. Hence, animal models are still indispensable for future sepsis research and there is an urgent need for pathophysiological relevant and reproducible animal models that mimic human disease.
The intra-abdominal sepsis model of cecal ligation and puncture (CLP) and its modifications including the colon ascendens stent peritonitis (CASP) are frequently used in mice (Hubbard et al. Citation2005). CLP is considered as the gold-standard for polymicrobial sepsis and has been used extensively over the past 30 years (Wichterman et al. Citation1980). These models create an abdominal focus similar to what is found in human abscess formation and peritonitis (Dejager et al. Citation2011). Importantly, CLP and CASP seem to reproduce several of the typical temporal responses to sepsis, characterized by overwhelming inflammation, activation of the coagulation system and circulatory decompensation with subsequent organ failure and death (Fink Citation2014). Unfortunately, both techniques are technically challenging, which creates a high degree of intra-individual and – laboratory variability and therefore complicating the interpretation of existing data (Buras et al. Citation2005; Rittirsch et al. Citation2009). In this context, we recently showed that the murine gut microbiota substantially varies among different animals and that these variations influence the phenotype of abdominal sepsis (Hilbert et al. Citation2017).
We herein report the characterization of a sepsis model based on the intraperitoneal (i.p) injection of a defined volume of feces/stool (IPSI) from donor mice by clinical observation and gene expression analysis. By diluting the fecal inoculum before injection, we generate septic mice showing dose-dependent clinical symptoms, together with upregulation of pro-inflammatory genes. Technical handling of the collected stool as well as aspects of storing are highlighted. Together, we present a murine sepsis model that combines advantages of setting an intra-abdominal and polymicrobial focus with the technical ease of a single injection. Because IPSI allows the characterization of the pathogenic cocktail before administration into mice, this technique has the potential to become a significant model of future sepsis research.
Materials and methods
Animals
Donor mice for stool preparation were purchased from Charles River (CR; Sulzfeld, Germany). All experiments were performed on male C57BL/6N mice at an age of about 8 weeks. All recipient mice employed in the present study were housed for 14 days in individually ventilated, pathogen-free cages with free access to water and standard rodent chow (SSNIFF) prior to challenge. The animal protocol was approved by the local committee for animal care (LANUV, Recklinghausen, Germany; animal protocol # 84-02.04.2013.A071) and is in accordance with the National Institutes of Health guidelines for use of live animals (NIH publication No. 85–23, revised 1996).
Preparation of feces
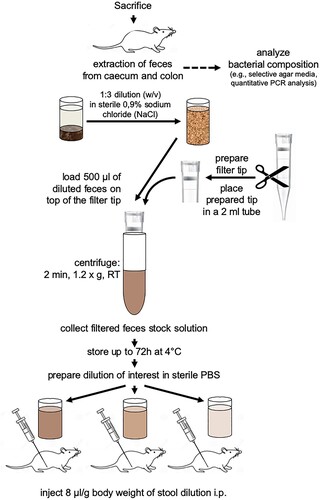
Immediately after arrival, donor mice were sacrificed by cervical dislocation. After median laparotomy, feces from the caecum and the complete colon were isolated under strict sterile conditions. One animal yielded approximately 200 mg of feces. Stool from one single mouse was immediately used for analysis of bacterial composition or feces from 5 donor mice were pooled for stimulation assays. The pooled feces were initially diluted 1:3 (w/v) with sterile 0.9% sodium chloride (NaCl). To remove undigested fibers and debris prior to intraperitoneal (i.p.) injection, diluted feces were filtered. Therefore, a 1000 µl filter tip (Axygen, Fisher Scientific, Schwerte, Germany) was cut 3 mm underneath the filter and placed into a sterile 2 ml reaction tube. 500 µl of diluted feces were filled onto the top of the filter and centrifuged for 2 min at 1.2 g and room temperature (Eppendorf centrifuge 5415R, Eppendorf, Hamburg). This was repeated for two times resulting in about 1.5 ml filtered 1:3 diluted feces (stock). Before application, this stock was also diluted with phosphate-buffered saline (PBS) to gain the 1:6 and 1:9 dilutions for respective experiments (Figure ).
Analysis of bacterial composition
For estimation of bacterial composition and quantity, feces were characterized by streak plate on ChromAgar Orientation plates (Mast Diagnostics, Reinfeld, Germany) as described earlier (Hilbert et al. Citation2017). Plates were incubated at 37°C for up to 48 h. Identification of different strains and groups of bacteria was made possible due to different coloring of colonies on ChromAgar plates. Typical appearance of bacteria are: Escherichia coli (E. coli) → dark pink to reddish, Enterococcus species (spp.) → turquoise blue, Klebsiella spp., Enterobacter spp., Citrobacter spp. → metallic blue, Proteus → brown halo, Pseudomonas → cream, translucent, Staphylococcus aureus (S. aureus) → golden, opaque, small. For technical reasons, Enterococcus spp., Klebsiella, Enterobacter, and Citrobacter spp. were summarized as KEEC group. Estimation of bacterial quantity was achieved by detection of bacterial growth in the first, second and third inoculation streak. Presence of bacteria only in the first inoculation streak means low, in the first and the second streak medium, and in first to third inoculation streak high number of bacteria. Filtered and characterized feces were then stored at 4°C, −20°C and −80°C up to 72 h. Prior to application, the stored feces were thawed at room temperature and again subjected to a quality control on ChromAgar Orientation plates.
Application of feces: intraperitoneal stool injection
Recipient mice were weighted, and stool suspension with the respective dilutions were injected i.p. using a 21 G injection cannula as described earlier (8 μl suspension/g body weight (BW)) (Hilbert et al. Citation2017). Sham mice received an equivalent volume of PBS. Mice were followed for up to 48 h with intensive clinical observation after 9, 15, 18, 21, and 24 h. Body weight and temperature were monitored. Moreover, a clinical numeric score adapted from Morton and Griffiths (Citation1985) and Hawkins (Citation2002) additionally covering coat care, occurrence of diarrhea, natural activity, and social behavior was used to describe the disease conditions of the mice (Table ).
Table 1. Clinical score sheet for the evaluation of disease level in mice.
Gene expression analysis
9h following IPSI, mice were sacrificed by cervical dislocation, spleens were removed, snap frozen in liquid nitrogen, and stored at −80°C until further analysis. Tissue was homogenized in Trizol™ reagent (Life Technologies, Carlsbad, CA, USA), and total RNA was extracted according to the manufacturers’ protocol. 2 µg of total RNA was reverse transcribed into cDNA using the high capacity cDNA reverse transcription kit (Applied Biosystems, Weiterstadt, Germany). Expression of IL-6 and CCL-2 was quantified by real-time PCR using Taqman probes (IL-6: Mm00446190_m; CCL2: Mm00441242_m1) on the Viia7 system (Applied Biosystems). Target gene expression was normalized to 18s RNA, and induction of IL-6 and CCL2 genes was calculated using the ΔΔCt method (Livak and Schmittgen Citation2001).
Analysis of mortality
Mice were subjected to IPSI using different dilutions of feces preparation (1:3, 1:6, 1:9), and survival was assessed up to 48 h. Kaplan-Meier curve was used for graphical presentation of the results.
Recording of clinical sepsis symptoms, splenic gene expression, and mortality rates was derived from independent experiments due to different observation periods (18–24 h for sepsis symptoms, 9 h for gene expression, and up to 48 h for mortality, as given above). Each experiment has been performed repeatedly and at least three times, resulting in the total n-numbers given in the Results section and the figure legends.
Statistical analyses
Statistical analysis and visualization of data was performed using GraphPad PRISM 5 (La Jolla, CA, USA). Data are presented as mean + SD. Results of gene expression analysis are shown as 2-ΔΔCt values, indicating the fold change in gene expression in treated animals vs. untreated control animals. For bacterial viability, significance was tested using the two-sided, unpaired Student’s t-test. Significance of dose- or time-dependent changes in the IPSI model was tested using one-way analysis of variance (ANOVA), followed by Dunnett´s multiple comparison test or Tukey’s multiple comparison test, respectively. Statistically significant differences were assumed at p-values < 0.05.
Results
Semi-quantitative analysis of stool
Feces from caecum and colon were isolated, and an injectable solution was prepared (Figure ). To characterize the initial composition if the murine feces composition, freshly isolated feces from 20 mice were inoculated separately on CHROMagar orientation plates and incubated for 48 h (Figure ). An overall low bacterial growth was shown for E. coli and S. aureus, and a medium growth was seen after streak plating for bacteria from the KEEC group. The overall bacterial growth for the respective bacteria detected after inoculation of fresh feces was set as 100%. All following analyses of filtration and storage effects were set in relation to the bacterial composition and quantity seen in fresh feces. To prepare an injectable solution, isolated feces were filtered to remove undigested material. No changes in qualitative microbiome composition occurred during filtration (data not shown). Next, we tested different storing conditions for filtered feces. While storage at 4°C for 72 h exerted only minimal effect on bacterial composition, storage at −20°C or −80°C substantially impacted viability of certain bacteria and thus overall feces composition (Figure (A–C)). In particular, storage at −80°C reduced the viability of E. coli significantly (Figure (A)), whereas the viability of S. aureus was not significantly affected (Figure (C)). Bacteria from the KEEC group were not affected by the different storage conditions (Figure (B)). Therefore, storage conditions of up to 72 h were set to 4°C for all further experiments.
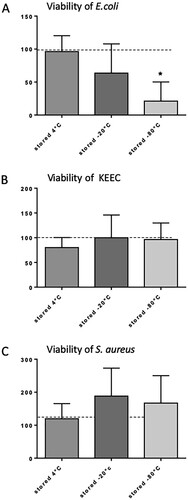
Figure 2. Quantity of selected bacteria in mouse feces. Bacterial quantity was estimated by streak plate on ChromAgar orientation plates. Freshly isolated feces from 20 single mice were plated on ChromAgar orientation plates and incubated for 48 h at 37°C. Quantity of indicated bacteria was deduced from appearance of colonies only in the first streak (1 = low bacterial growth), first and second streak (2 = medium bacterial growth), and first to third streak (3 = high bacterial growth). Mean + SD (n = 20). KEEC group subsume Enterococci, Klebsiella, Enterobacter and Citrobacter.
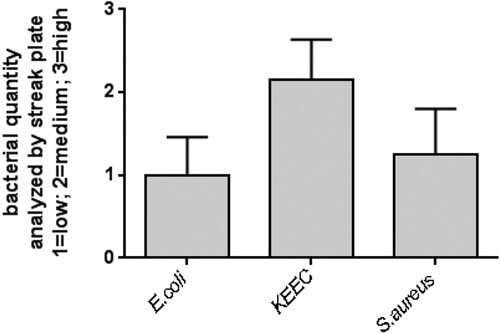
Figure 3. Influence of different storage conditions on the viability of selected bacteria. (A) E. coli; (B) bacteria from the KEEC group (subsume Enterococci, Klebsiella, Enterobacter and Citrobacter); (C) S. aureus. Feces were isolated, initially diluted 1:3 with sterile 0.9% sodium chloride (NaCl), filtered as described, and subsequently, stock solution samples were stored for 72 h under the indicated conditions. Viability is given in % of bacterial viability observed in freshly isolated feces. Mean + SD (n = 6–8), p < 0.05 vs. freshly isolated feces (two-sided, unpaired Student’s t-test).
Clinical symptoms
Dilution series of filtered feces in sterile PBS were prepared, and 200 µl of diluted feces were administered to each mouse (n = 16–24 animals per group). Sham animals received an equal volume of PBS. Independent from the dilution factor of the injected feces, body weight of all mice dropped significantly compared to control and sham animals. Mice lost about 3% of body weight within the first 18 h after application of feces when compared to initial body weight before starting the experiment (Figure (A)). However, no differences with respect to the amount of injected bacteria were detectable. In contrast, a dose-dependent reduction in body temperature was observed 18 h after injection of feces. Mice having received the highest number of bacteria (1:3 dilution) showed the maximum in temperature drop (27%, p < 0.001), in contrast to mice that received the 1:6 dilution of feces (5% drop, p < 0.05). Animals having received the lowest amount of bacteria (1:9 dilution) showed no significant changes in body temperature (Figure (B)).
Figure 4. Intraperitoneal feces injection dose-dependently influence the development of sepsis symptoms. Filtered stock solution feces were diluted 1:3; 1:6, and 1:9 with sterile PBS and injected intraperitoneally. Sham animals received an equal volume of PBS, control animals were not injected. Body weight and temperature were recorded 18 h after injection, clinical scores were recorded at the indicated time points (hours after feces injection, given on the X axis of panel C). (A) body weight in % of initial body weight; (B) temperature; (C) clinical scores. Mean + SD (n = 16–24 animals per group), p < 0.001 (ANOVA, Dunnett’s multiple comparison test (A;B), Tukey’s multiple comparison test (C)).
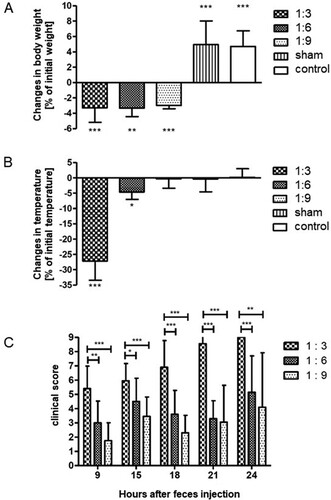
Mice were closely inspected according to the clinical score sheet (Table ). As early as 9 h after feces injection, a significant and dose-dependent increase of the clinical score (reflecting a more severe sepsis) was visible in all mice receiving feces (Figure (C)). After 9 h, the highest score was observed in mice who received the 1:3 feces dilution (5.5), followed by mice having received the 1:6 feces dilution (3.0) and mice that received the 1:9 feces dilution (1.8). Significant differences were observed between mice being injected the 1:3 feces dilution and mice that received the 1:6 dilution or the 1:9 dilution (p < 0.001; p < 0.01, respectively). Of note, as the clinical score of all treatment groups increased in a time-dependent manner, significant differences between the groups were continuously detectable. However, none of the mice that received the 1:3 diluted feces (i.e. the highest inoculum) survived the first 24 h (see below).
Gene expression of inflammatory mediators
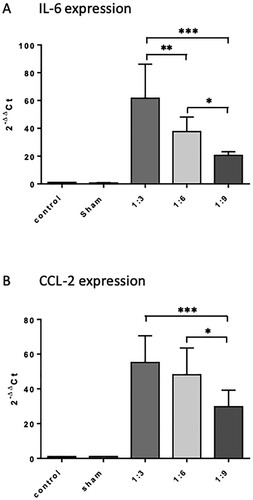
We analyzed the gene expression levels of IL-6 and CCL2 in spleen tissue 9 h after intraperitoneal injection of different feces dilutions (n = 4−8 animals per group). Feces injection induced a dose-dependent increase in IL-6 expression with significant differences between the treatment groups (p < 0.05, Figure (A)). Compared to the sham group, mice having received the lowest dilution (1:3) showed IL-6 mRNA induction of up to 61.6-fold vs. 37.6-fold (1:6) vs. 20.6-fold (1:9). Comparable results were obtained for the expression of CCL-2 (Figure (B)).
Figure 5. Influence of intraperitoneal feces injection on the expression levels of the pro-inflammatory cytokine IL-6 and chemokine CCL2 in the murine spleen. 9 h after feces injection, animals were sacrificed, spleens were removed, snap frozen in liquid nitrogen, and stored at −80°C until further use. Total RNA was extracted, reverse transcribed into cDNA, and the mRNA levels of IL-6 and CCL2 were quantified by real-time PCR. Target gene expression was normalized to expression of 18s RNA, and induction of target genes was calculated using the 2-ΔΔCT method. All treatment groups were significantly different from control and sham groups, differences between the feces dilutions used were significant when indicated. The graphs compare the expression of genes of interest in the treatment groups relative to the control (fold changes). Mean + SD (n = 4 −8 animals per group), p < 0.05 (ANOVA, Tukey multiple comparisons-test).
Overall survival
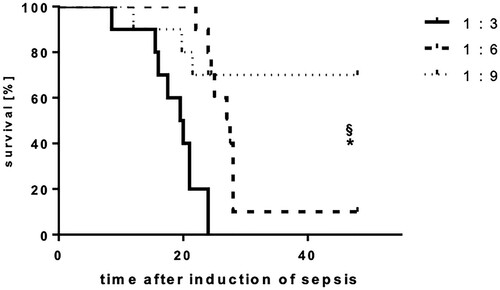
Mice that received one single injection of the 1:3 diluted feces preparation died within the first 24 h. The survival rate of mice having received the 1:6 feces dilution was significantly improved during the first 24 h (60%, p < 0.05), but further declined, so that only 10% of these mice survived longer than 48 h. In contrast, the survival rate of the group receiving the highest dilution of feces (1:9) was 90% after 24 h (p < 0.05) and 70% after 48 h (p < 0.01) (Figure ) (n = 12 animals per group).
Figure 6. Intraperitoneal feces injection dose-dependently influence the lethality of mice. Mice were subjected to IPSI, and survival was assessed up to 48 h. Mean (n = 12 animals per group), p < 0.05 (Student’s unpaired T-test). * indicates significant improvement of survival in comparison to the 1:3 group, § indicates significant improvement of survival in comparison to the 1:6 group.
Discussion
Sepsis, a life threating syndrome characterized by organ failure after infection, is the most common cause of death in hospitalized patients. Although many immunomodulatory therapies that based on findings observed in animal models have failed to improve sepsis outcome in clinical studies, preclinical in vivo experiments are still powerful research tools to analyze the pathophysiology of sepsis and to explore new therapeutic options (Kingsley and Bhat Citation2016; Osuchowski et al. Citation2018). Hence, the purpose of this study was the evaluation of a simple, non-invasive, reproducible, and controllable sepsis model with emphasis on technical practicability. Therefore, we chose to advance the existing model of cecal slurry which was initially described in rats (Lang et al. Citation1983; Sam et al. Citation1997). Our results show that i.p. administration of diluted feces can reproducibly induce sepsis with dose-dependent clinical symptoms (Figure ), systemic cytokine release (Figure ), and lethality (Figure ).
We previously demonstrated profound vendor effects on the murine fecal microbiota influencing sepsis models, concluding that variable intestinal microbiota must be considered when interpreting results from murine abdominal sepsis models (Hilbert et al. Citation2017). Our IPSI model is based on the collection of fecal samples from donor animals by median laparotomy and subsequent standardized homogenization and dilution. Thus, one advantage of this model is the generation of larger quantities of identical pathogenic inocula by pooling feces from different mice. This approach however raises the question how to store the stool before administration without influencing the microbial composition. Interestingly, we found that storing the fresh and filtered feces in 0.9% sodium chloride at −80°C decreases the viability of the gram-negative bacterium E. coli (Figure , p < 0.05). Similar results were obtained in a different context by Dan et al. (Citation1989). In that study, several enteropathogenic species including E. coli, which were isolated from human stool, were kept at −70°C without preservation medium. Many cultures started to lose viability as early as 3 months and there was no survival beyond 6–9 months. To stabilize bacterial viability in stool specimens, multiple groups have added glycerol to the storage medium (Gonnert et al. Citation2011; Starr et al. Citation2014). However, the injection of glycerol into mice can lead to unintended side effects and is an established agent to induce renal failure in various animal models (Kim et al. Citation2014; Nara et al. Citation2016; Al Asmari et al. Citation2017). Hence, the use of glycerol as stabilizing agent in freezing bacteria is unfavorable when studying sepsis. Barium, a likewise toxic agent that was described to be used for maintaining bacteria viability in frozen stocks before induction of sepsis, is also not suitable in this context (Lang et al. Citation1983; Gonnert et al. Citation2011). Interestingly, in the sepsis model used by Weinstein et al. (Citation1974), barium was added to the cecal inoculum in order to increase the inflammatory response in the peritoneum in rats. Hence, to avoid side effects related to the injection of any storage media, we diluted the feces in 0.9% sodium chloride. Since freezing the stool aliquots at −20°C or −80°C affected the bacterial composition (Figure ), we propose to store the feces at 4°C before injection. In our study, storing the stool samples at 4°C did not significantly alter the composition of the aerobic bacteria over a time period of 3 days. Other groups reported that storing feces at 4°C is even possible for up to 1 week without losing viability of the enteropathogens (Dan et al. Citation1989). Of note, we did not analyze the viability of anaerobic pathogens and can therefore not exclude that storing feces in the refrigerator significantly affects the composition of these pathogens.
Sepsis is a heterogeneous syndrome with significant variability in pathophysiology, clinical presentation, and outcome. Hence, this makes it difficult to model sepsis in animals. Several sepsis (or sepsis-mimicking) models have been proposed over the years, including (I) the administration of exogenous toxins such as lipopolysaccharide (LPS), (II) the administration of viable pathogens, and (III) the alteration of the endogenous protective barrier of the animal (Buras et al. Citation2005; Dejager et al. Citation2011). The LPS model involves the direct administration of LPS into the blood stream or peritoneum and was used in early days of sepsis research. Like our model, one advantage of this approach is the technical ease and the high degree of homogeneity following the injection of a defined dose of endotoxin. Although the administration of LPS induces a strong inflammatory response leading to the characteristic hyperdynamic cardiovascular state associated with sepsis, it fails to reflect the complexity of the human immune response during the disease progression (Gonnert et al. Citation2011). As a consequence, LPS injection is no longer considered a valid model that mimics human sepsis (Rittirsch et al. Citation2009; Dyson and Singer Citation2009). Live pathogens such as bacteria can be administered via intravenous, intraperitoneal, or intratracheal injection (Buras et al. Citation2005; Kingsley and Bhat Citation2016). The infusion of e.g. E. coli causes a pro-inflammatory immune response similar to what we observed in our study (Figure ), including sepsis symptoms like shock, activation of coagulation, and organ dysfunction (Fischer et al. Citation1992; Taylor Citation2001). However, this model lacks a focus from where bacteria are disseminated, which is pathognomonic for sepsis. Additionally, high doses of bacteria are needed to surpass host defense mechanism such as lysis by the complement system (Cross et al. Citation1993; Kingsley and Bhat Citation2016). By contrast, the herein presented IPSI model is a polymicrobial sepsis model that induces a diffuse peritonitis, resembling clinical problems such as perforated appendicitis and diverticulitis, and reflects the typical pathophysiological changes observed in polymicrobial sepsis (Gonnert et al. Citation2011; Hilbert et al. Citation2017). This is particularly demonstrated by the significant and dose-dependent increase in the expression levels of the pro-inflammatory genes IL-6 and CCL2, both of which have been shown to be highly upregulated in murine endotoxinemia and abdominal sepsis (Krakauer et al. Citation2010; Skirecki et al. Citation2019).
The frequently used CLP and CASP model likewise reproduce several of the typical responses to sepsis, characterized by an initial hyperdynamic response followed by decompensation, organ failure, and death (Wichterman et al. Citation1980; Zantl et al. Citation1998; Parker and Watkins Citation2001; Kingsley and Bhat Citation2016). Compared to IPSI, both CLP and CASP are based on the direct release of intestinal pathogens into the abdominal cavity. Although both techniques guarantee a pathophysiological relevant polymicrobial focus, since no processing of the endogenous feces is required (as in the IPSI model), several limitations need to be addressed. In contrast to IPSI, a surgery involving a midline laparotomy, the preparation of the intestine, and the injury of the gut via needle (CLP) or stent (CASP) is a prerequisite for both models (Stortz et al. Citation2017). This creates a large degree of undesired investigator variability (Wynn et al. Citation2007). Although the magnitude of sepsis severity following CLP/CASP can be modulated by altering needle/stent size, number of punctures, etc., it is difficult to standardize these techniques and to generate constant leakage of fecal contents. Furthermore, inflammatory response to the surgical trauma alone may be considerable, resulting in sham operations that will have to be performed mandatory. By comparison, the IPSI model used in the herein presented study is based on a single i.p. injection of a titrated inoculum dose. Depending on the dilution factor, we could generate a highly reproducible severity grade of sepsis with corresponding survival and systemic inflammation (Figures ). Especially doses with higher dilution factors such as 1:9 generate septic mice with a sustained inflammation and a mortality rate of 25% similar to what is reported in humans (Figure ) (Fleischmann et al. Citation2016; Rudd et al. Citation2020). There is a particular need to be able to model sepsis in its different stages and severities, e.g. to further study characteristic gene expression profiles and their prognostic significance and to link them with clinical symptoms. Others previously demonstrated immunopathologic alterations in animal models of sepsis of different severity or used fecal injection models to titrate mortality (Ebong et al. Citation1999; Maslove and Wong Citation2014; Shrum et al. Citation2014). However, as far as we know, our report is the first to provide a highly reproducible preclinical model of sepsis severity titration showing an impact on clinic, gene expression as well as on mortality. Moreover, repetitive i.p. injection may be performed to further modulate the septic host response.
Regardless of the model used, reproducible results rely on defined and invariant baseline conditions, that is to say primarily the bacterial composition of the feces being used to create an intra-abdominal focus (Hilbert et al. Citation2017). As we already demonstrated, this bacterial composition may on one hand vary over time, on the other, even the distributor the animals are purchased from may have a significant impact on the animals’ microbiota (Hilbert et al. Citation2017). We, therefore, previously provided in detail ways to control this variability when performing preclinical models of abdominal sepsis in small animals, comprising the use of adequate n-numbers for the experiments, the assessment of the microbial composition of the donor animals’ stool prior to injection or using specifically designed (so-called gnotobiotic) mice with known microbiota for defined issues. The herein presented IPSI model allows to conduct larger studies with more animals receiving the same pathogenic cocktail by pooling feces from several donor mice. Additionally, it is possible to link results to the exact bacterial composition by analyzing the feces via platforms such as RNA sequencing or microarray and to publish the composition as well, most probably resulting in higher reliability and scientific confirmability. Last, this approach could control experimental heterogeneity due to varying fecal microbiome.
Beside the route of infection, several factors need to be addressed to generate a translational and meaningful sepsis model. e.g. factors like co-morbidities, age, concurrent supportive therapies including mechanical ventilation, vasopressor use, and the administration of fluids and antibiotics should be considered when studying the pathophysiology of sepsis including testing intervention therapies. To address these important aspects, guidelines for animal models defined by the so-called Minimum Quality Threshold in Pre-Clinical Sepsis Studies (MQTiPSS) were recently proposed (Osuchowski et al. Citation2018). The intention of our study was to optimize the process of sepsis induction including the preparation, diluting, and storage of the feces. Of course, any kind of supportive therapy may be introduced and evaluated using this approach. Depending on the posited hypothesis, the herein proposed IPSI model must be specifically tailored and ‘reverse translated’ to its clinical counterpart including implementation of supportive care.
Of course, our herein presented data have to be interpretated while respecting some critical limitations. First, the total amount of bacteria (in terms of colony-forming units (CFU)) was not determined. We characterized the microbial composition of the stool being injected using qualitative methods, and in our opinion, this seems to be of particular importance, since the microbiota may significantly vary inter-individually (as we demonstrated previously). However, instead of counting CFUs, we used a rather simple way of serial dilution of the inoculum to achieve dose-dependent and reproducible gradations of sepsis severity. Other authors use a similar approach and describe the different stool content of their preparation in mg/ml instead of counting the CFU (Shrum et al. Citation2014). We kindly want to point out that other (even more invasive and complex) models of murine abdominal sepsis such as CLP or CASP likewise do not rest upon counting the CFUs of the inoculum. Second, additional assessment of cytokines in serum or plasma samples would have been useful for the interpretation of our results (as performed by Panda et al. (Citation2012) and Das et al. (Citation2019)). However, pro-inflammatory response was determined by assessing gene expression changes in the animals’ spleen tissue instead.
Taken together, we propose a sepsis model of intraperitoneal stool injection using pooled feces from donor mice without freezing and use of preservation media. The fecal specimens should be stored at 4°C and injected within three days. This approach guarantees stability of the enteropathogens and mimics a pathophysiological relevant stimulus similar to models like CLP or CASP. The advantages of the IPSI model are (I) the exact dosage of the feces by dilution and thereby controlling the disease severity; (II) the generation of larger quantities of the same polymicrobial trigger which controls for experimental heterogeneity when studying more mice; (III) the possibility to characterize the microbial composition before administration; (IV) the lack of any surgical trauma and anesthetics; and (V) the technical ease of this approach. All these features contribute to the adequate reproducibility of the herein presented IPSI model that is of significant importance for every preclinical sepsis model and that is illustrated by controlled standard deviations, as demonstrated by our data.
Authors’ contributions
T.H., F.S., S.S., N.C., C.B., M.P., S.F., O.B., and S.K. made substantial contributions to the conception or design of the work, or the acquisition, analysis, or interpretation of data for the work. T.H., F.S., C.B., M.P., S.F., O.B., and S.K. performed drafting the work or revising it critically for important intellectual content; T.H., F.S., S.S., N.C., C.B., M.P., S.F., O.B., and S.K. participated in final approval of the version to be published.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- Al Asmari AK, Al Sadoon KT, Obaid AA, Yesunayagam D, Tariq M. 2017. Protective effect of quinacrine against glycerol-induced acute kidney injury in rats. BMC Nephrol. 18(1):41.
- Buras JA, Holzmann B, Sitkovsky M. 2005. Animal models of sepsis: setting the stage. Nat Rev Drug Discov. 4(10):854–865.
- Cross AS, Opal SM, Sadoff JC, Gemski P. 1993. Choice of bacteria in animal models of sepsis. Infect Immun. 61(7):2741–2747.
- Dan M, Richardson J, Miliotis MD, Koornhof HJ. 1989. Comparison of preservation media and freezing conditions for storage of specimens of faeces. J Med Microbiol. 28(2):151–154.
- Das P, Panda SK, Agarwal B, Behera S, Ali SM, Pulse ME, Solomkin JS, Opal SM, Bhandari V, Acharya S. 2019. Novel Chitohexaose analog protects young and aged mice from CLP induced polymicrobial sepsis. Sci Rep. 9(1):2904.
- Dejager L, Pinheiro I, Dejonckheere E, Libert C. 2011. Cecal ligation and puncture: the gold standard model for polymicrobial sepsis? Trends Microbiol. 19(4):198–208.
- Dyson A, Singer M. 2009. Animal models of sepsis: why does preclinical efficacy fail to translate to the clinical setting? Crit Care Med. 37(1 Suppl):S30–S37.
- Ebong S, Call D, Nemzek J, Bolgos G, Newcomb D, Remick D. 1999. Immunopathologic alterations in murine models of sepsis of increasing severity. Infect Immun. 67(12):6603–6610.
- Fink MP. 2014. Animal models of sepsis. Virulence. 5(1):143–153.
- Fischer E, Marano MA, Van Zee KJ, Rock CS, Hawes AS, Thompson WA, DeForge L, Kenney JS, Remick DG, Bloedow DC. 1992. Interleukin-1 receptor blockade improves survival and hemodynamic performance in Escherichia coli septic shock, but fails to alter host responses to sublethal endotoxemia. J Clin Invest. 89(5):1551–1557.
- Fleischmann C, Thomas-Rueddel DO, Hartmann M, Hartog CS, Welte T, Heublein S, Dennler U, Reinhart K. 2016. Hospital incidence and mortality rates of sepsis. Dtsch Arzteblatt Int. 113(10):159–166.
- Gonnert FA, Recknagel P, Seidel M, Jbeily N, Dahlke K, Bockmeyer CL, Winning J, Lösche W, Claus RA, Bauer M. 2011. Characteristics of clinical sepsis reflected in a reliable and reproducible rodent sepsis model. J Surg Res. 170(1):e123–e134.
- Hawkins P. 2002. Recognizing and assessing pain, suffering and distress in laboratory animals: a survey of current practice in the UK with recommendations. Lab Anim. 36(4):378–395.
- Hilbert T, Steinhagen F, Senzig S, Cramer N, Bekeredjian-Ding I, Parcina M, Baumgarten G, Hoeft A, Frede S, Boehm O, Klaschik S. 2017. Vendor effects on murine gut microbiota influence experimental abdominal sepsis. J Surg Res. 211:126–136.
- Hubbard WJ, Choudhry M, Schwacha MG, Kerby JD, Rue LW, Bland KI, Chaudry IH. 2005. Cecal ligation and puncture. Shock Augusta Ga. 24(Suppl 1):52–57.
- Kim JH, Lee D-W, Jung MH, Cho H-S, Jeon D-H, Chang S-H, Park DJ. 2014. Macrophage depletion ameliorates glycerol-induced acute kidney injury in mice. Nephron Exp Nephrol. 128(1–2):21–29.
- Kingsley SMK, Bhat BV. 2016. Differential paradigms in animal models of sepsis. Curr Infect Dis Rep. 18(9):26.
- Krakauer T, Buckley MJ, Fisher D. 2010. Proinflammatory mediators of toxic shock and their correlation to lethality. Mediators Inflamm. 2010:517594:1-7.
- Lang CH, Bagby GJ, Bornside GH, Vial LJ, Spitzer JJ. 1983. Sustained hypermetabolic sepsis in rats: characterization of the model. J Surg Res. 35(3):201–210.
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods San Diego Calif. 25(4):402–408.
- Maslove DM, Wong HR. 2014. Gene expression profiling in sepsis: timing, tissue, and translational considerations. Trends Mol Med. 20(4):204–213.
- Morton DB, Griffiths PH. 1985. Guidelines on the recognition of pain, distress and discomfort in experimental animals and an hypothesis for assessment. Vet Rec. 116(16):431–436.
- Nara A, Yajima D, Nagasawa S, Abe H, Hoshioka Y, Iwase H. 2016. Evaluations of lipid peroxidation and inflammation in short-term glycerol-induced acute kidney injury in rats. Clin Exp Pharmacol Physiol. 43(11):1080–1086.
- Osuchowski MF, Ayala A, Bahrami S, Bauer M, Boros M, Cavaillon J-M, Chaudry IH, Coopersmith CM, Deutschman CS, Drechsler S, et al. 2018. Minimum Quality Threshold in Pre-Clinical Sepsis Studies (MQTiPSS): An International expert Consensus initiative for improvement of animal modeling in sepsis. Shock Augusta Ga. 50(4):377–380.
- Panda SK, Kumar S, Tupperwar NC, Vaidya T, George A, Rath S, Bal V, Ravindran B. 2012. Chitohexaose activates macrophages by alternate pathway through TLR4 and blocks endotoxemia. PLoS Pathog. 8(5):e1002717:1-17.
- Parker SJ, Watkins PE. 2001. Experimental models of gram-negative sepsis. Br J Surg. 88(1):22–30.
- Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. 2009. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 4(1):31–36.
- Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S, et al. 2020. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global burden of disease study. Lancet Lond Engl. 395(10219):200–211.
- Sam AD, Sharma AC, Law WR, Ferguson JL. 1997. Splanchnic vascular control during sepsis and endotoxemia. Front Biosci J Virtual Libr. 2:e72–e92.
- Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, Rubenfeld G, Kahn JM, Shankar-Hari M, Singer M, et al. 2016. Assessment of clinical Criteria for sepsis: For the third International Consensus Definitions for Sepsis and Septic Shock (sepsis-3). JAMA. 315(8):762–774.
- Shrum B, Anantha RV, Xu SX, Donnelly M, Haeryfar SMM, McCormick JK, Mele T. 2014. A robust scoring system to evaluate sepsis severity in an animal model. BMC Res Notes. 7:233.
- Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche J-D, Coopersmith CM, et al. 2016. The third International Consensus Definitions for Sepsis and Septic Shock (sepsis-3). JAMA. 315(8):801–810.
- Skirecki T, Drechsler S, Hoser G, Jafarmadar M, Siennicka K, Pojda Z, Kawiak J, Osuchowski MF. 2019. The fluctuations of leukocytes and circulating cytokines in septic humanized mice vary With outcome. Front Immunol. 10:1427.
- Starr ME, Steele AM, Saito M, Hacker BJ, Evers BM, Saito H. 2014. A new cecal slurry preparation protocol with improved long-term reproducibility for animal models of sepsis. PloS One. 9(12):e115705:1-15.
- Stortz JA, Raymond SL, Mira JC, Moldawer LL, Mohr AM, Efron PA. 2017. Murine models of sepsis and trauma: Can We bridge the Gap? ILAR J. 58(1):90–105.
- Taylor FB. 2001. Staging of the pathophysiologic responses of the primate microvasculature to Escherichia coli and endotoxin: examination of the elements of the compensated response and their links to the corresponding uncompensated lethal variants. Crit Care Med. 29(7 Suppl):S78–S89.
- Weinstein WM, Onderdonk AB, Bartlett JG, Gorbach SL. 1974. Experimental intra-abdominal abscesses in rats: development of an experimental model. Infect Immun. 10(6):1250–1255.
- Wichterman KA, Baue AE, Chaudry IH. 1980. Sepsis and septic shock–a review of laboratory models and a proposal. J Surg Res. 29(2):189–201.
- Wynn JL, Scumpia PO, Delano MJ, O’Malley KA, Ungaro R, Abouhamze A, Moldawer LL. 2007. Increased mortality and altered immunity in neonatal sepsis produced by generalized peritonitis. Shock Augusta Ga. 28(6):675–683.
- Zantl N, Uebe A, Neumann B, Wagner H, Siewert JR, Holzmann B, Heidecke CD, Pfeffer K. 1998. Essential role of gamma interferon in survival of colon ascendens stent peritonitis, a novel murine model of abdominal sepsis. Infect Immun. 66(5):2300–2309.