Abstract
Aloperine has been shown to exhibit tremendous pharmacological potential. The present study was designed to investigate the effects of aloperine against human liver cancer cells and to explore the underlying mechanism. The results showed significant (P < .05) upregulation of GROα (GRO1 oncogene) in liver cancer tissues and cell lines. However, the expression of GROα significantly (P < .05) declined in liver cancer cells treated with aloperine (5 µM) which was concomitant with the inhibition of proliferation. Silencing of GROα significantly (P < .05) inhibited the proliferation of the liver cancer via G2/M cycle arrest. Interestingly, aloperine also triggered G2/M cell cycle arrest of the liver cancer cells. Nonetheless, the growth inhibitory effects of aloperine on liver cancer cells were attenuated by GROα overexpression and the cancer cells did not show the arrest of cell division. Additionally, aloperine also suppressed the migration, invasion and epithelial to mesenchymal transition of the human liver cancer cells via inhibition of GROα expression. Collectively, the findings revealed growth inhibitory effects of aloperine via suppression of GROα expression and point toward its therapeutic potential.
Introduction
Liver cancer is listed among the most prevalent human cancers and is globally ranked as the third most lethal human cancer (Li and Wang Citation2016; Xu et al. Citation2020). Developing countries particularly have higher incidence of liver cancer (Shi et al. Citation2020 sep 30). Etiologically, the liver cancer is most commonly shown to result from viral infections of hepatitis B virus (HBV) and hepatitis C virus (HCV) which contribute for more than 80% of liver cancer cases (de Martel et al. Citation2015). Liver cancer has an extremely poor prognosis and accounts for more than half a million cancer related deaths worldwide (Boloker et al. Citation2018). The overall 5-year survival rate of liver cancer is marginally low and ranges between 15% and 17% (Siegel et al. Citation2019). Presently, the surgery is the main curative procedure for liver cancer (Anwanwan et al. Citation2020). However, the unfavorable outcomes of post-operative disease recurrence and acquisition of therapeutic resistance by liver cancer cells largely preclude the satisfactory clinical success (Chen et al. Citation2014). Besides, the liver cancer cells exhibit resistance to radiotherapy (Haraguchi et al. Citation2010). Hence, intensive scientific research efforts must be directed toward the exploration of potential therapeutic measures against liver cancer. Considering this, the current study worked out the therapeutic effectiveness of aloperine against human liver cancer cells, in vitro with an emphasis on understanding its mechanism of action. Aloperine is an alkaloid type of quinolizidine which is isolated from a medicinally important Chinese herb, Sophora alopecuroides L. (Zhou et al. Citation1989). Aloperine has been shown to exhibit anti-microbial, anti-inflammatory, neuro-protective, anti-viral and anti-cancer properties (Yuan et al. Citation2010; Lin et al. Citation2011; Lin and Lin Citation2011; Ma et al. Citation2015; Dang et al. Citation2016). Studies have implicated that aloperine shows anti-cancer activity against a number of human cancer cell types like bladder cancer, prostate cancer, thyroid cancer and breast cancer to name a few (Lee et al. Citation2018; Ling et al. Citation2018; Tian et al. Citation2018; Zhang et al. Citation2020). Moreover, the administration of human hepatocellular carcinoma cells with aloperine has been reported to induce apoptosis and G2/M cell cycle arrest by modulating PI3 K/AKt signaling pathway (Liu et al. Citation2019). To make further insight into its anti-cancer action against the liver cancer, the effects of aloperine treatment were investigated against the liver cancer cells in the present study. Aloperine was shown to repress the expression of GROα (or GRO1 oncogene), which was otherwise significantly upregulated in liver cancer tissues and cell lines. The proliferation of liver cancer cells declined significantly under aloperine treatment via G2/M cell cycle arrest which was modulated through GROα. Targeting of GROα by aloperine besides reduced the migration, invasion and epithelial to mesenchymal transition of liver cancer cells. Collectively, the results of the present study point toward therapeutic implications of aloperine in the management of liver cancer.
Materials and methods
Human tissues
A total of 79 paired liver cancer and normal adjacent tissue specimens were obtained from patients after surgical resection at the Affiliated Tumor Hospital of Nantong University, Nantong Tumor Hospital, Nantong, Jiangsu, 226,361, China under standard ethical guidelines and after proper consent signing by the patients. The tissues were immediately frozen using liquid nitrogen stored at −80°C for future analysis.
Cell lines and culture conditions
Human liver cancer cell lines (SNU-182, SNU-423, SNU-449 and HeppG2) and THLE-2 normal human liver epithelial cells were provided by the BeNa Culture Collection Biological Technology Co., Ltd. (Beijing, China). Eagle’s Minimum Essential Medium (EMEM; Gibco) supplemented with 10% FBS (Sigma-Aldrich) and 100 U/mL penicillin and 100 mg/mL streptomycin was used for the maintenance and in vitro culturing of cell lines at 37°C in an atmosphere of 95% O2 and 5% CO2 in a humidified CO2 incubator.
Transfection
The SNU-182 cancer cells were transfected with small interfering RNA oligos of GROα (si-GROα) for GROα transcriptional knockdown, while si-NC transfected cells served as negative control. Both si-GROα and si-NC constructs were obtained from RiboBio, Guangzhou, China. GROα overexpression was obtained by cloning its ORF into pcDNA3.1 overexpression vector and transfecting the same into SNU-182 cancer cells. The vector (pcDNA3.1) transfected cells were used as negative control. All the transfection procedures were carried out with the help of Lipofectamine 2000 reagent (Thermo Fisher Scientific) as per the manufacturer guidelines.
RNA isolation and gene expression analysis
For GROα gene expression analysis, total RNA was isolated with the help of Trizol reagent (Thermo Fisher Scientific) as per the manufacturer’s instructions from tissues and cell lines. Next, the RNA was reverse transcribed to synthesize first-strand cDNA using Revert Aid First Strand cDNA synthesis kit (Thermo Fisher Scientific). Using GADPH and actin as internal controls, qRT–PCR was performed using Power SYBR Green Master Mix (Applied Biosystems) on QuantStudio 5.0 PCR system (Applied Biosystems) to determine the expression of GROα. Finally, the relative expression of GROα gene was ascertained by 2−△△Ct method.
Western blotting
Total proteins were extracted from aloperine treated (5 µM) or control (untreated) SNU-182 and SNU-449 cells by digesting the cells with RIPA lysis buffer. BCA protein kit (Thermo Fisher Scientific) was used for the quantification of total proteins. Following quantification, 45 µg of proteins from each sample were loaded on to 10% SDS-PAGE gel, resolved electrophoretically and the gel was blotted to polyvinylidene fluoride (PVDF, Thermo Fisher Scientific) membrane. Non-fat milk (5%) was used for blocking of PVDF membrane which was then exposed to primary antibodies of GROα and -actin (anti-GROα and anti- actin, Abcam; 1.1000) overnight at 4°C. Following, an incubation of secondary anti-rabbit antibody (Abcam; 1:5000) was used for 2 h at room temperature. Odyssey Imaging System was finally used for membrane scanning and visualization of protein bands. -actin was used as an internal expression control.
CCK-8 proliferation assay
The si-GROα transfected and aloperine liver cancer cells were added into 96-well plates at a density of 2.5 × 104 per well. The cells were incubated at 37°C for 0, 24, 48, 72 or 96 h, respectively. At the indicated incubation periods, each well was added with 10 µL CCK-8 reagent (Beyotime, Shanghai, China) and 37°C incubation was prolonged for 2 h. At last, a micro-plate reader was used to measure the absorbance of cellular samples at 450 nm.
Cell cycle analysis
For cell cycle analysis, si-GROα transfected and aloperine (5 µM) treated SNU-182 liver cancer cells along with the corresponding negative control cells as well as the aloperine (5 µM) treated GROα overexpressing SNU-182 cancer cells were initially incubated, in triplicates, at 37°C for 24 h. The cells were then harvested through centrifugation, washed with phosphate-buffered saline (PBS) and fixed using 4% formaldehyde. The cells were subsequently stained with propidium iodide solution. Lastly, the cancer cell mitosis was analyzed with the help of a flow cytometer.
Transwell migration and invasion assays
For examining the migration of liver cancer cells, the suspension carrying 105 si-GROα transfected and aloperine (5 µM) treated SNU-182 liver cancer cells along with the corresponding negative control cells as well as the aloperine (5 µM) treated GROα overexpressing SNU-182 cancer cells was placed into the upper chamber of transwell insert which was separated from the lower chamber by a polycarbonate membrane. Serum free EMEM with 10% FBS was inoculated into the lower chamber. Following 24 h of incubation at 37°C, the polycarbonate membranes was washed with PBS and cells sticking to its upper surface were carefully removed. The cells which had migrated to the lower chamber were fixed with 70% ethanol and subsequently stained using 0.2% crystal violet for 20 min. Light microscope was used for visualizing the cells. Using five-randomly selected microscopic fields, the percent cell migration was deduced. For the invasion analysis, similar procedure was followed except for 100 µL Matrigel (BD Bio-Sciences) was used for pretreatment of the transwell chambers.
Statistical analysis
Each experiment was performed using three independent experiments and final results were expressed as mean ± standard deviation. Student’s t-test performed on Graphpad Prism7.0 was used for the analysis of difference between two treatment groups. The P < .05 taken as the measure of a statistically significant difference between two groups.
Results
GROα is upregulated in human liver cancer
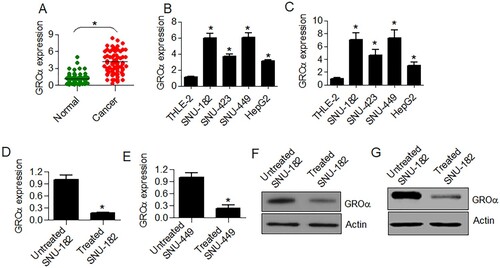
The transcript levels of GROα were analyzed from 79 paired liver cancer and normal adjacent tissues. The cancer tissues showed significantly (P < .05) higher GROα transcript levels (Figure (A)). Whether the same is true for the expression pattern of GROα alpha in cell lines, its expression was determined from four different liver cancer cell lines (SNU-182, SNU-423, SNU-449 and HepG2) and compared with that of THLE-2 normal liver cells. GROα expression was shown to be significantly higher (P < .05) in all the four cancer cell lines in comparison to the normal liver cells (Figure (B,C)). Moreover, SNU-182 cancer cells exhibited highest GROα transcript levels among all cancer cell lines and thus used for further experimentation.
Figure 1. GROα is upregulated in liver cancer and its expression is declined by aloperine. (A) Expression analysis of GROα from 79 paired liver cancer and normal adjacent tissues. (B) Analysis of transcript levels of GROα from liver cancer cell lines (SNU-182, SNU-423, SNU-449 and HepG2) and TLE-2 normal liver epithelial cells using GAPDH as reference (C) Analysis of transcript levels of GROα from liver cancer cell lines (SNU-182, SNU-423, SNU-449 and HepG2) and TLE-2 normal liver epithelial cells using actin as reference (D) Effect of aloperine (5 µM) treatment on GROα transcript levels in SNU-182 cells (E) Effect of aloperine (5 µM) treatment on GROα transcript levels in SNU-449 cells (F) Effect of aloperine (5 µM) treatment on GROα protein levels in SNU-182 cells (G) Effect of aloperine (5 µM) treatment on GROα protein levels in SNU-449 cells Three replicates were used for performing the experiments and the data is presented as mean ± SD (*P < .05).
Aloperine suppresses the expression of GROα in SNU-182 cells
The SNU-182 and SNU449 liver cancer cells were administered with 5 µM of aloperine and effect on GROα expression was assessed at both transcriptional and translational levels. The qRT–PCR analysis showed that the transcript levels of GROα declined by about 5 times under aloperine treatment in comparison to untreated SNU-182 and SNU-449 cells (Figure (D,E)). The GROα protein expression level also showed a considerable decrease in aloperine treated cancer cells with reference to SNU-182 untreated cells (Figure (F,G)).
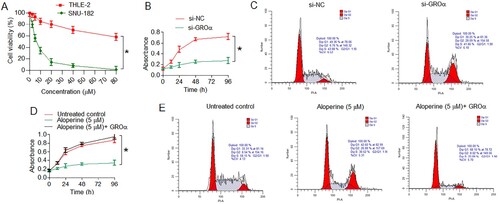
Aloperine inhibits the proliferation of SNU-182 cells via suppression of GROα
To examine the anti-proliferative effects of aloperine, SNU-182 cancer cells and THLE-2 normal liver cells were treated with different doses of aloperine (0–80 µM). SNU-182 cells suffered significant proliferation loss and proliferation rate decreased by about 50% at 5 µM aloperine which was taken as its IC50 against SNU-182 cancer cells (Figure (A)). In the contrary, aloperine was remarkably less toxic to THLE-2 normal cells and inhibited the normal cell growth with significantly less prominence (Figure (A)). Whether aloperine inhibited the proliferation of liver cancer cells via GROα, its transcriptional silencing was performed in SNU-182 cancer cells. The cancer cells down-regulating GROα showed significantly (P < .05) lower proliferation rates at different culture durations than that of the control transfected (si-NC) cells (Figure (B)). The flow cytometric investigation of cell cycle showed that SNU-182 cancer cells showed induction of G2/M cell cycle arrest under GROα knockdown (Figure (C)). Silencing of GROα increased the relative percentage of G2/M phase SNU-182 cells from 6.76 to 28. Interestingly, up-regulation of GROα over-shadowed the anti-proliferative effects of aloperine and SNU-182 cancer cells proliferated with comparable vigor as of the untreated cancer cells (Figure (D)). SNU-182 cancer cells treated with 5 µM aloperine also showed the induction of G2/M cell cycle arrest as under GROα knockdown, however the same was overcome by GROα overexpression in aloperine treated SNU-182 cancer cells (Figure (E)). The percentage of G2/M phase SNU-182 cells increased from 8.54% to 26.88% under 5 µM aloperine administration while it was only 8.02% for aloperine treated cancer cells overexpressing GROα (Figure (E)). Results are thus indicative that aloperine exerts anti-proliferative effects against the liver cancer cells, in vitro, through induction of G2/M cell cycle arrest mediated via down-regulation of GROα.
Figure 2. Aloperine inhibits the proliferation of liver cancer cells via GROα mediated G2/M cell cycle arrest. (A) Analysis of viability of SNU-182 and THLE-2 cells treated with aloperine at concentrations of 0–80 µM (B) assessment of proliferation rate of SNU-182 cancer cells down-regulating GROα with reference to control transfected cells at different culture durations (C) flow cytometric cell cycle analysis of SNU-182 cells transfected with si-GROα or si-NC (D) assessment of proliferation rate of SNU-182 cancer cells transfected with si-GROα or treated with aloperine (5 µM) and aloperine treated and overexpressing GROα with reference to corresponding negative control cells at different culture durations (E) flow cytometric cell cycle analysis of aloperine treated or aloperine treated and GROα over-expressing SNU-182 cells with reference to untreated cancer cells. Three replicates were used for performing the experiments and the data is presented as mean ± SD (*P < .05).
Aloperine inhibits the migration and invasion of SNU-182 cells via suppression of GROα
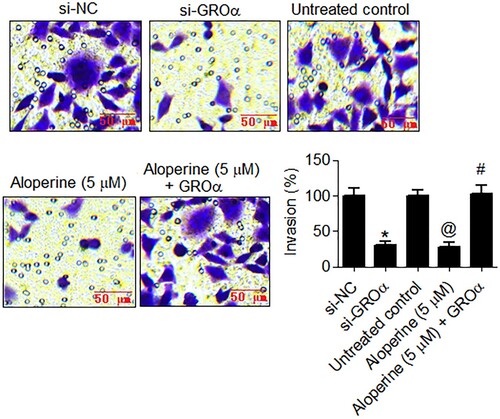
The anticancer potential of aloperine was also investigated in terms of its effects on motility of liver cancer cells via GROα targeting. The GROα down-regulating as well as aloperine treated (5 µM) SNU-182 cancer cells showed significant (P < .05) decline in migratory rate as compared to control transfected and untreated cancer cells, respectively (Figure ). The relative percent number of migratory cells decreased by more than 70% GROα silencing and aloperine treatment in comparison to the corresponding control SNU-182 cells. Similarly, the silencing of GROα and aloperine administration (5 µM) restrained the invasion of SNU-182 liver cancer cells, markedly and percent cell invasion declined by more than 70% in both cases (Figure ). However, the overexpression of GROα attenuated the inhibitory effects of aloperine on SNU-182 cancer cell migration and invasion (Figures and ). Together, the results indicate that aloperine inhibits the migration and invasion of liver cancer cells, in vitro, via GROα.
Figure 3. Aloperine targets GROα to inhibit migration of liver cancer cells. Examination of migration of si-GROα transfected, aloperine treated or aloperine treated GROα over-expressing SNU-182 cells by transwell chamber method with reference to corresponding negative control cells. Three replicates were used for performing the experiments and the data is presented as mean ± SD (*P < .05 for si-NC vs. si-GROα, @P for untreated control vs. Aloperine (5 µM) and #P < 0.05 for Aloperine (5 µM) vs. Aloperine (5 µM) + GROα).
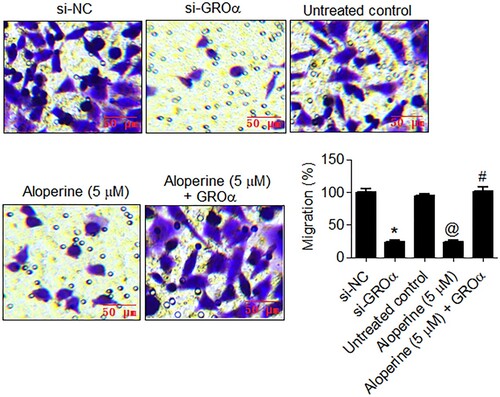
Figure 4. Aloperine targets GROα to inhibit invasion of liver cancer cells. Examination of invasion of si-GROα transfected, aloperine treated or aloperine treated GROα over-expressing SNU-182 cells by transwell chamber method with reference to corresponding negative control cells. Three replicates were used for performing the experiments and the data is presented as mean ± SD (*P < .05 for si-NC vs. si-GROα, @P for untreated control vs. Aloperine (5 µM) and #P < .05 for Aloperine (5 µM) vs. Aloperine (5 µM) + GROα).
Aloperine inhibited the epithelial to mesenchymal transition of the SNU-182 cells
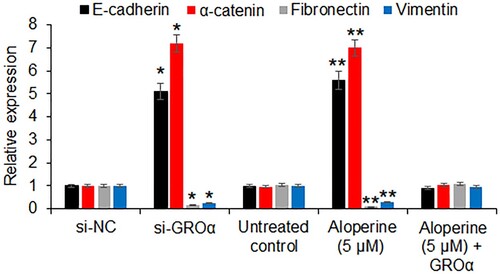
The effects of aloperine (5 µM) or GROα silencing was examined the expression of the epithelial to mesenchymal transition markers in SNU-182 cells. The qRT–PCR analysis revealed that the expression of E-cadherin and α-catenin was significantly increased under both aloperine treatment or GROα silencing. In contrary, the expression of fibronectin and vimentin was significantly (P < .05) suppressed upon aloperine treatment or GROα silencing (Figure ). Nonetheless, the effects of aloperine on the epithelial and mesenchymal transition markers were avoided by GROα overexpression.
Figure 5. Aloperine inhibits epithelial to mesenchymal transition. The qRT-PCR analysis showing the effect of GROα silencing and aloperine treatment on the expression of E-cadherin, α-catenin, fibronectin and vimentin. The experiments were performed in triplicate and expressed as mean ± SD (*P < .05 for si-NC vs. si-GROα and **P < .05 for untreated control vs. Aloperine (5 µM)).
Discussion
Liver cancer is counted among the highly frequent and aggressive type of human cancers. Alarmingly, the recent years have shown an increase in incidence rates of liver cancer (Liu et al. Citation2020). What is more, the satisfactory clinical outcomes are lagging for human liver cancer majorly because of its poor prognosis, post-therapeutic disease recurrence and inadequacy of currently used chemo- and radiotherapies (Haraguchi et al. Citation2010; Chen et al. Citation2014). Hence there is a necessity to explore and devise better and efficient treatment methods against this fatal malignancy (Anwanwan et al. Citation2020). In view of the huge pharmacological importance and anti-cancer potential of an alkaloid, aloperine; the current study investigated its therapeutic effectiveness against the human liver cancer cells, in vitro. Supporting its previous reported action against liver cancer cells, aloperine inhibited the proliferation of liver cancer cells in a dose-dependent manner with an observed IC50 value of 5 µM (Liu et al. Citation2019). The anti-proliferative effects of aloperine against the liver cancer cells were shown to be exerted via suppression of an oncogenic protein, GROα. GROα has been shown to exhibit transcriptional up-regulation in human cancers and has been shown to promote tumorogenesis and metastasis (Ogata et al. Citation2010). Concurrently, the liver cancer tissues and cell lines showed marked up-regulation of GROα. Transcriptional knockdown of GROα inhibited the liver cancer cell proliferation, remarkably and confirmed its oncogenic potential in liver cancer. Silencing of GROα-induced liver cancer cells triggered G2/M phase cell cycle arrest at the molecular level. Aloperine mediated targeting of GROα showed a similar cell cycle impeding effect on liver cancer cell mitosis. The mechanism by which aloperine suppresses the expression of GROα is still unclear. Whether aloperine directly blocks the transcription of GROα or some upstream genes that regulate the expression of GROα needs to be elucidated. Induction G2/M cell cycle arrest by aloperine has also been reported in case of human colon cancer and hepatocellular carcinoma cells (Zhang et al. Citation2014; Liu et al. Citation2019; Zhou et al. Citation2020). In another study, aloperine has been shown induce apoptosis in ovarian cancer cells via activation of reactive oxygen species (Qiu et al. Citation2020). Han et al. (Citation2021) showed that aloperine suppresses the growth of the colorectal cancer cells by modulating the circNSUN2/miR-296-5p/STAT3 pathway. Aloperine has also been shown to suppress the migration and invasion of the breast cancer cells via inhibition of the Ras signaling pathway (Tian et al. Citation2018). Chen et al. (Citation2018) also reported the inhibition of osteosarcoma cell invasion upon aloperine treatment. Given this background, the effects of aloperine were also examined on the migration, invasion and epithelial mesenchymal transition of the human oral cancer cells. The results indicated that aloperine has a potential to restrict the migration and invasion of liver cancer cells, in vitro. These anti-metastatic effects of aloperine were mimicked by GROα knockdown. Interestingly, aloperine was ineffective in exerting its ant-cancer effects on liver cancer cells overexpressing GROα which suggested that aloperine experienced anti-cancer activity against the liver cancer cells through GROα. Summing up, the current study provided an extension to the mechanistic action of aloperine against human liver cancer cells and revealed that aloperine targets GROα besides PI3 K/Akt signaling pathway in liver cancer cells to limit their proliferation, migratory vigor and invasiveness, in vitro conditions (Liu et al. Citation2019). However, the therapeutic applicability of aloperine against liver cancer needs to be validated in vivo systems. Although the toxicity of aloperine was evaluated against the normal cells, the toxicity of aloperine also needs to evaluated in vivo.
Conclusion
The results of the present study reveal significant up-regulation of GROα in liver cancer. GROα was confirmed to act as the molecular target of aloperine. Aloperine exhibited considerable anti-proliferative and anti-metastatic effect against the liver cancer cells via inhibition of GROα expression. The findings of the present studies point toward the potential of aloperine as a lead molecule for the management of liver cancer. However, further in vivo studies are recommended.
Acknowledgements
The authors acknowledge Affiliated Tumor Hospital of Nantong University, Nantong Tumor Hospital, Nantong, Jiangsu, 226361, China for providing laboratory facility.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
That data that supports the findings of this study are available at the figshare repository (https://figshare.com/) at https://figshare.com/s/ca5a26dbbff881374e86
Additional information
Funding
References
- Anwanwan D, Singh SK, Singh S, Saikam V, Singh R. 2020 Jan 1. Challenges in liver cancer and possible treatment approaches. Biochim Biophys Acta Rev Cancer. 1873(1):188314.
- Boloker G, Wang C, Zhang J. 2018 Mar. Updated statistics of lung and bronchus cancer in United States (2018). J Thorac Dis. 10(3):1158.
- Chen S, Jin Z, Dai L, Wu H, Wang J, Wang L, Zhou Z, Yang L, Gao W. 2018 Jan 1. Aloperine induces apoptosis and inhibits invasion in MG-63 and U2OS human osteosarcoma cells. Biomed Pharmacother. 97:45–52.
- Chen X, Liu HP, Li M, Qiao L. 2014 Nov 28. Advances in non-surgical management of primary liver cancer. World J Gastroenterol. 20(44):16630.
- Dang Z, Zhu L, Lai W, Bogerd H, Lee KH, Huang L, Chen CH. 2016. Aloperine and Its derivatives as a New class of HIV-1 entry inhibitors. ACS Med. Chem. Lett. 7:240–244. doi:10.1021/acsmedchemlett.5b00339.
- de Martel C, Maucort-Boulch D, Plummer M, Franceschi S. 2015 Oct 1. World-wide relative contribution of hepatitis B and C viruses in hepatocellular carcinoma. Hepatology. 62(4):1190–1200.
- Han W, Kong D, Lu Q, Zhang W, Fan Z. 2021. Aloperine inhibits proliferation and promotes apoptosis in colorectal cancer cells by regulating the circNSUN2/miR-296-5p/STAT3 pathway. Drug Des Devel Ther. 15:857.
- Haraguchi N, Ishii H, Mimori K, Tanaka F, Ohkuma M, Kim HM, Akita H, Takiuchi D, Hatano H, Nagano H, Barnard GF. 2010 Sep 1. CD13 is a therapeutic target in human liver cancer stem cells. J Clin Invest. 120(9):3326–3339.
- Lee YR, Chen SH, Lin CY, Chao WY, Lim YP, Yu HI, Lu CH. 2018 Jan. In vitro antitumor activity of aloperine on human thyroid cancer cells through caspase-dependent apoptosis. Int J Mol Sci. 19(1):312.
- Li L, Wang H. 2016 Sep 1. Heterogeneity of liver cancer and personalized therapy. Cancer Lett. 379(2):191–197.
- Lin Z, Huang CF, Liu XS, Jiang J. 2011 May. In vitro anti-tumour activities of quinolizidine alkaloids derived from Sophora flavescens Ait. Basic Clin Pharmacol Toxicol. 108(5):304–309.
- Lin WC, Lin JY. 2011 Jan 12. Five bitter compounds display different anti-inflammatory effects through modulating cytokine secretion using mouse primary splenocytes in vitro. J Agric Food Chem. 59(1):184–192.
- Ling Z, Guan H, You Z, Wang C, Hu L, Zhang L, Wang Y, Chen S, Xu B, Chen M. 2018. Aloperine executes antitumor effects through the induction of apoptosis and cell cycle arrest in prostate cancer in vitro and in vivo. Onco Targets Ther. 11:2735.
- Liu JS, Huo CY, Cao HH, Fan CL, Hu JY, Deng LJ, Lu ZB, Yang HY, Yu LZ, Mo ZX, Yu ZL. 2019 Aug 1. Aloperine induces apoptosis and G2/M cell cycle arrest in hepatocellular carcinoma cells through the PI3 K/Akt signaling pathway. Phytomedicine. 61:152843.
- Liu Z, Suo C, Mao X, Jiang Y, Jin L, Zhang T, Chen X. 2020 May 15. Global incidence trends in primary liver cancer by age at diagnosis, sex, region, and etiology, 1990-2017. Cancer. 126(10):2267–2278.
- Ma NT, Zhou R, Chang RY, Hao YJ, Ma L, Jin SJ, Du J, Zheng J, Zhao CJ, Niu Y, Sun T. 2015 Oct 1. Protective effects of aloperine on neonatal rat primary cultured hippocampal neurons injured by oxygen–glucose deprivation and reperfusion. J Nat Med. 69(4):575–583.
- Ogata H, Sekikawa A, Yamagishi H, Ichikawa K, Tomita S, Imura J, Ito Y, Fujita M, Tsubaki M, Kato H, Fujimori T. 2010 Dec 1. GROα promotes invasion of colorectal cancer cells. Oncol Rep. 24(6):1479–1486.
- Qiu M, Liu J, Su Y, Liu J, Wu C, Zhao B. 2020 Sep 1. Aloperine induces apoptosis by a reactive oxygen species activation mechanism in human ovarian cancer cells. Protein Pept Lett. 27(9):860–869.
- Shi JF, Cao M, Wang Y, Bai FZ, Lei L, Peng J, Feletto E, Canfell K, Qu C, Chen W. 2020 Sep 30. Is it possible to halve the incidence of liver cancer in China by 2050? Int J Cancer. 148(5):1051–1065.
- Siegel RL, Miller KD, Jemal A. 2019 Jan. Cancer statistics, 2019. CA Cancer J Clin. 69(1):7–34.
- Tian D, Li Y, Li X, Tian Z. 2018 Oct 1. Aloperine inhibits proliferation, migration and invasion and induces apoptosis by blocking the Ras signaling pathway in human breast cancer cells. Mol Med Rep. 18(4):3699–3710.
- Xu W, Li K, Song C, Wang X, Li Y, Bai X, Liang X, Deng W, Wang J, Liu J. 2020. Knockdown of lncRNA LINC01234 suppresses the tumorigenesis of liver cancer via sponging miR-513a-5p. Front Oncol. 10:571565.
- Yuan XY, Liu W, Zhang P, Wang RY, Guo JY. 2010 Mar 10. Effects and mechanisms of aloperine on 2, 4-dinitrofluorobenzene-induced allergic contact dermatitis in BALB/c mice. Eur J Pharmacol. 629(1-3):147–152.
- Zhang L, Liang J, Liu X, Wu J, Tan D, Hu W. 2020. Aloperine exerts antitumor effects on bladder cancer in vitro. Onco Targets Ther. 13:10351.
- Zhang L, Zheng Y, Deng H, Liang L, Peng J. 2014 Jun 1. Aloperine induces G2/M phase cell cycle arrest and apoptosis in HCT116 human colon cancer cells. Int J Mol Med. 33(6):1613–1620.
- Zhou CC, Gao HB, Sun XB, Shi HB, Liu W, Yuan HN, Wang ZX. 1989. Anti-inflammatory and anti-allergic action of aloperine. Acta Pharmacol. Sin. 10:360–365.
- Zhou H, Li J, Sun F, Wang F, Li M, Dong Y, Fan H, Hu D. 2020. A review on recent advances in aloperine research: pharmacological activities and underlying biological mechanisms. Front Pharmacol. 11. https://doi.org/10.3389/fphar.2020.538137.
