Abstract
This study aimed to investigate the effect of the miR-29c/PTEN axis on the PI3 K/Akt/NF-kB pathway in rats with severe pneumonia (SP). A total of 20 Sprague–Dawley rats were selected, of which 10 were randomly selected for modeling SP and included in the model group (MG) and the other 10 were included in the control group (CG). Expression levels of miR-29c and PTEN were measured in the peripheral blood and lung tissue. Pearson correlation coefficient analysis showed that miR-29c was positively correlated with PTEN in serum and lung tissue (r = 0.833 and 0.697, respectively; P < 0.05). The apoptosis rate was higher in the MG than in the CG (P < 0.05). Western blot analysis revealed that the relative expressions of PI3 K, Akt, and NF-kB proteins were higher in the MG than in the CG (P < 0.05). Protein expression in the miR-29c-mimics and sh-PTEN groups was higher than that in the other two groups, whereas the miR-29c-inhibitor group had the lowest protein expression (P < 0.05). MiR-29c/PTEN exhibited low expression levels in SP, which can promote pneumonia development by inhibiting the expression of PI3 K/Akt/NF-kB pathway proteins.
Introduction
Severe pneumonia (SP) is a relatively serious form of pneumonia, depending on the degree of local inflammation, spread of lung inflammation, and degree of systemic inflammatory response. SP is identified when a patient with pneumonia requires ventilation support owing to severe hypoxemia or acute respiratory failure or develops signs of circulatory failure such as hypotension, shock, and other organ dysfunction (Monsel et al. Citation2015; Haugen et al. Citation2017). In newborns and children, the disease results in circulatory system involvement, which is characterized by weak pulse, cardiac acceleration, dull heart sounds, exacerbated cyanopathy, and increased rales. Pale complexion, dark and clammy skin, decreased urine volume, decreased blood pressure, and prolonged capillary filling time are some of the signs observed during shock and peripheral circulation failure (Chisti et al. Citation2015; Guan and Jin Citation2016). Currently, the incidence of SP ranges from 3.9% to 45.0% worldwide (Kulnik et al. Citation2014). An increasing number of studies have shown that the incidence of SP is on the rise (Reyes et al. Citation2017). Compared with common pneumonia, SP is a high-risk disease and poses a threat to patient health (Montull et al. Citation2016). Currently, the pathogenesis of SP is not well-understood. Further understanding is particularly important for appropriate treatment.
Recently, the role of microRNA (mRNA) in various diseases has gradually gained clinical attention. Studies have demonstrated that mRNA is abnormally expressed in breast cancer, non–small-cell lung cancer, bone tumors, and other diseases, and is closely related to their occurrence (Abdullah et al. Citation2017; Hirai et al. Citation2017; Mancarella et al. Citation2019). Among them, miR-29c has been shown to play an important role in cancer as a tumor suppressor gene (Hezova et al. Citation2015). Previous studies have confirmed that miR-29c can regulate PTEN signaling pathway (Zou et al. Citation2015; Li et al. Citation2020). However, the effect of the miR-29c/PTEN molecular axis in SP remains to be clarified. Therefore, this study explored the mechanisms of miR-29c/PTEN axis action in SP to provide reference and guidance for future clinical diagnosis and treatment.
Materials and methods
Twenty clean-grade 6-week-old Sprague–Dawley rats, purchased from Beijing Charles River Laboratory Animal Technology Co., Ltd. (Certificate No.: SCXK (Beijing) 2016-0011), were selected as the experimental subjects. There were 10 males and 10 females, weighing 210 ± 20 g. All rats were kept in cages (5 in each cage) under normal feeding, light, temperature (29°C ± 2°C), and humidity (40%–50%) conditions.
Cells and animals
Mouse lung epithelial cells (MLE12) were purchased from the American Type Culture Collection (BNCC337698; Manassas, VA, USA) and cultured in DMEM medium at 37°C and 5% CO2.
Ten rats were randomly selected and included in the model group (MG) for SP modeling. A reference dose of Klebsiella pneumoniae bacteria solution (China Center of Industrial Culture Collection, Beijing, China) was diluted with sterile saline (2.4 × 1011/L) before inoculating the rats. Hemodynamics, blood gas analysis, and peripheral blood analysis of 2 ml fundus venous plexus of the rats were observed dynamically on the sixth day after inoculation. Modeling success was determined based on the simultaneous completion of the following four criteria: (1) manifestation of dysfunction, sluggishness, and other disturbances of consciousness; (2) shortness of breath (rapid movement of bilateral thoraces); (3) severe hypoxemia [arterial oxygen saturation (SaO2)] of <90% or arterial oxygen partial pressure of ≤60 mmHg; (4) chest X-ray examination showing diffuse oozing in both lungs. The other 10 rats left untreated were assigned to the control group (CG). Every procedure was approved by the Animal Care and Use Committee of Zibo Central Hospital.
When the growth and fusion of adherent cells reached 85%, 25% trypsin was added for digestion. Next, the cells were placed in the medium for further culture and passage prior to transfection. MiR-29c-mimics (overexpression sequence), miR-29c-inhibitor (inhibition sequence), miR-NC (negative control), sh-PETN (target overexpression), si-PETN (targeted inhibition), and NC (negative control) were transfected into the cells using the LipofectamineTM 2000 kit (Thermo Fisher Scientific) according to the kit instructions.
RT-qPCR Upon completion of modeling, all rats were sacrificed by cervical dislocation under 10% chloral hydrate (300 mg/kg) anesthesia to obtain venous blood and lung and myocardial tissue. No rats exhibited signs of peritonitis after the administration of 10% chloral hydrate. Myocardial tissue was homogenized with a homogenizer. Cellular apoptosis was detected by flow cytometry and mRNA expression in serum and tissue was detected using RT-qPCR. Total serum RNA was extracted and dissolved in 20 μL DEPC according to the Trizol reagent protocol and then reverse-transcribed using a reverse transcription kit. U6 was used as the internal reference for miR-29c, whereas β-actin was used for PTEN. After the reaction, real-time PCR amplification and fusion curves were confirmed and the relative quantification of target genes was calculated using the 2−△Ct method.
Western blotting
Protein expression of PI3 K/Akt/NF-kB in the two groups of rats was detected by western blotting. Total protein was extracted using the RIPA lysis method. Then, the protein concentration was determined using the BCA protein assay and adjusted to 4 μg/μL; it was electrophoresed on a 6%–12% sodium dodecyl sulfate polyacrylamide gel and transferred to a polyvinylidene fluoride membrane. After selecting the corresponding bands according to the target protein, the cells were blocked with a concentration of 5% skim milk powder for 2 h. After washing the membrane, 2 mL primary antibody (abcam, USA) with a dilution ratio of 1:1000 was added and stored at 4°C overnight. The secondary antibody (Jiangsu Beyotime Biotechnology Co., Ltd.; 1:10000) was incubated for 1 h following the same procedure, and then the developer was added in a darkroom for exposure. The polyvinylidene fluoride film was imaged using the Tocan240 automatic gel imaging system (Shanghai Tocan Biotechnology Co., Ltd.). Results were analyzed by grayscale using the Image Lab™ software (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Outcome measures in the two groups included the expression of miR-29c and PTEN in serum and tissue; expression of PI3 K, Akt, and NF-kB proteins in tissue; and apoptosis in lung tissue.
Statistical analysis
Statistical analysis was conducted using SPSS software, version 24.0 (IBM, Inc., Armonk, NY, USA). All graphical results were plotted using GraphPad 8 software. All data are expressed as means ± standard deviation. The t-test was used for intergroup comparisons, whereas univariate analysis of variance and the least significant difference post hoc test were employed for multigroup comparisons. The Pearson correlation coefficient was adopted for correlation analysis. P < 0.05 indicated statistical significance.
Results
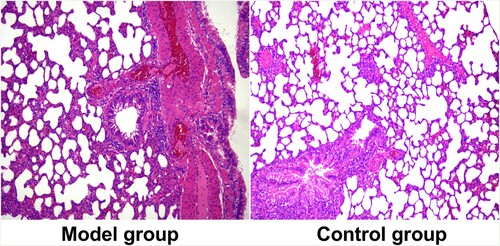
The success rate of modeling was 100%; all 10 MG rats met all 4 criteria. None developed peritonitis. The lung tissue sections of the two groups of rats are shown in Figure .
Figure 1. Lung tissue sections of rats in two groups. The HE staining figures show the model group and control group.
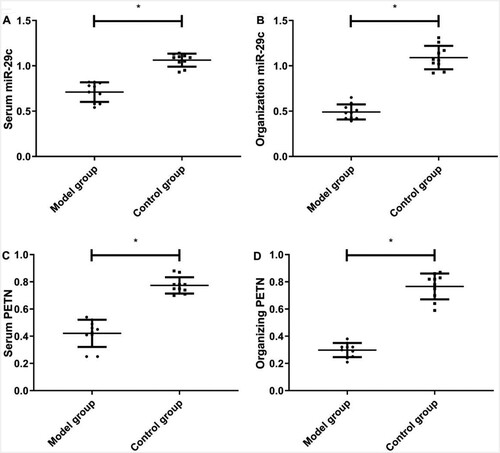
Expression levels of miR-29c and PTEN in the serum and tissue were lower in the MG than those in the CG (P < 0.05) (Figure ).
Figure 2. Expression of miR-29c and PTEN in serum and tissue of rats in the model and control groups. A) ELISA method for the comparison of miR-29c in serum; B) RT-qPCR method for comparison of miR-29c in lung tissue; C) ELISA method for the comparison of PTEN in serum; D) RT-qPCR method for comparison of PTEN in lung tissue. * indicates P <0.05, When model group compared with the control group.
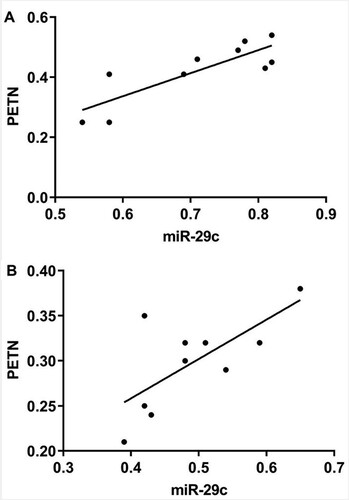
Pearson correlation coefficient analysis showed that miR-29c was positively correlated with PTEN in serum and lung tissue (r = 0.833 and 0.697, respectively; P < 0.05) (Figure ).
Figure 3. Correlation analysis between miR-29c and PTEN in rats with pneumonia. A) Correlation analysis of miR-29c and PTEN in serum of pneumonia rats; B) Correlation analysis of miR-29c and PTEN in lung tissue of pneumonia rats.
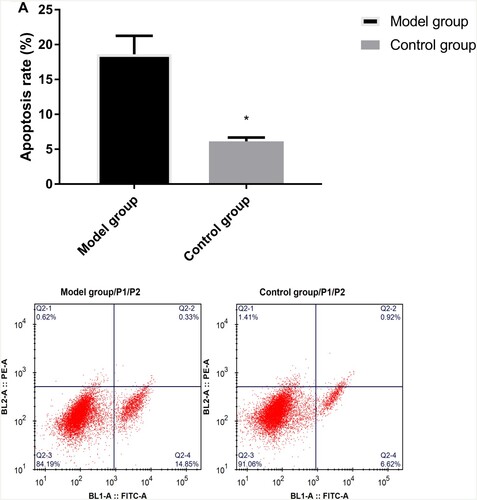
Flow cytometry demonstrated that the apoptosis rate was higher in the MG than those in the CG (P < 0.05) (Figure ).
Figure 4. Comparison of apoptosis rate between model and control groups by flow cytometry. * indicates P < 0.05. Flow cytometry method for the column and flow results for apoptosis between model and control groups.
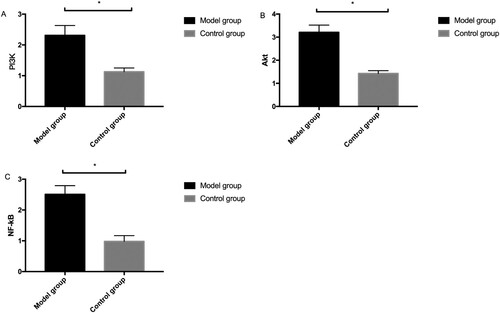
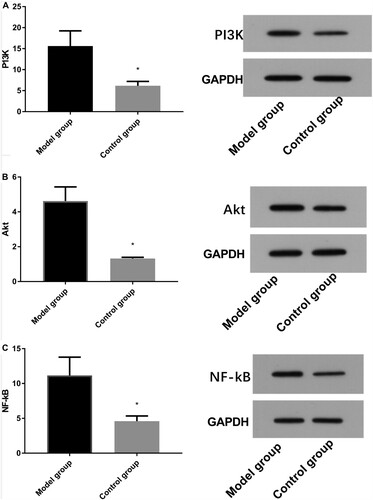
Western blot results demonstrated that the relative expression levels of PI3 K, Akt, and NF-kB proteins were higher in the MG than those in the CG (P < 0.05) (Figure ).
Figure 5. Comparison of protein expression between model and control groups by Western blot. A: Western blot method for column and protein figures for PI3 K expression (ab140307,1:1000) level. B: Western blot method for column and protein figures for Akt (ab38449,1:1000) expression level. C: Western blot method for column and protein figures for NF-kB (ab32536,1:1000) expression level.
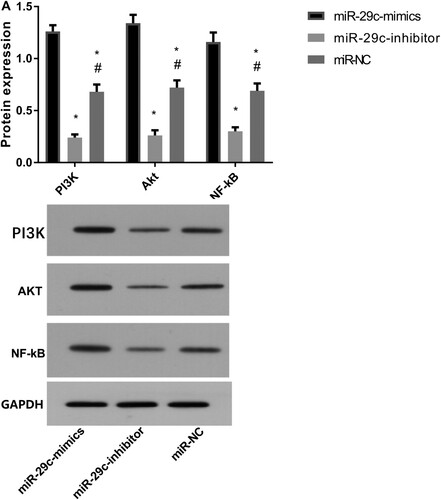
After transfection with miR-29c, the expression levels of PI3 K, Akt, and NF-kB proteins in lung epithelial cells were higher in the miR-29c-mimics group than those in the other two groups. Expression levels in the miR-29c-inhibitor group were the lowest (P < 0.05) (Figure ).
Figure 6. Protein expression after miR-29c transfection by Western blot. A: Western blot method for column figures for PI3 K(ab140307,1:1000), Akt(ab38449,1:1000), and NF-kB(ab32536,1:1000) proteins expression levels. B: The representative graph of protein expression figures for PI3 K, Akt, and NF-kB proteins expression. * indicates P < 0.05 when compared with the miR-29c-mimics group; # indicates P < 0.05 when compared with the miR-29c-inhibitor group.
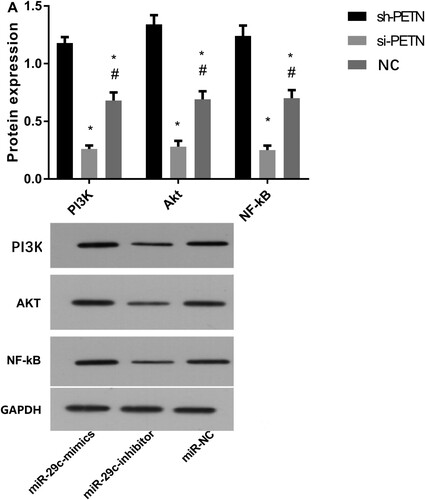
After the transfection of PTEN, the protein expression levels of PI3 K, Akt, and NF-kB proteins in lung epithelial cells were higher in the sh-PTEN group than those in the other two groups. Expression levels in the si-PTEN group were the lowest (P < 0.05) (Figure ).
Figure 7. Protein expression after PTEN transfection by Western blot. A) Western blot method for column figures for PI3 K(ab140307,1:1000), Akt(ab38449,1:1000), and NF-kB(ab32536,1:1000) proteins expression. B: The representative graph of protein expression figures for PI3 K, Akt, and NF-kB proteins expression. * indicates P < 0.05 when compared with the sh-PTEN group; # indicates P <0.05 when compared with the si-PTEN group.
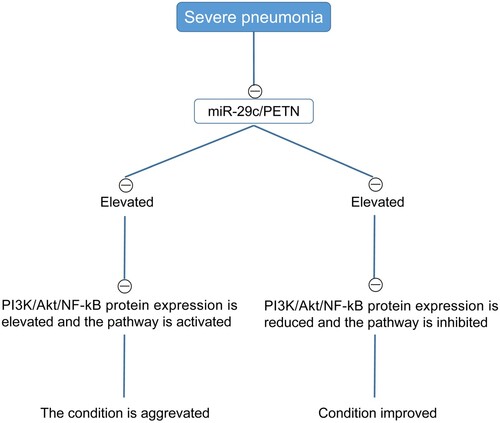
The levels of PI3 K, Akt, and NF-kB mRNA levelswere detected by RT-qPCR. The results showed that the mRNA expression levels of PI3 K, Akt, and NF-kB in the MG were higher than those in the CG (P < 0.05) (Figure ). Moreover, based on the above results, we observed that with the increase in miR-29c/PTEN level, PI3 K/Akt/NF-kB protein level increased, PI3 K/Akt/NF-kB pathway got activated, and the disease aggravated, whereas with the decrease in miR-29c/PTEN level, PI3 K/Akt/NF-kB protein level reduced, PI3 K/Akt/NF-kB pathway was inhibited, and the condition improved (Figure ).
Discussion
SP is a very common pediatric disease in clinical practice, and it is a leading cause of morbidity and mortality in children (Rudan et al. Citation2013). Current studies indicate that the occurrence of pneumonia is closely associated with the invasion of microorganisms such as fungi, bacteria, parasites, and viruses as well as the effects of inhalation and radiation (Liu et al. Citation2014). The severity of pneumonia is usually related to local severity, presence of spreading, and the degree of systemic inflammatory response (Mandourah et al. Citation2012). The development of SP indicates that the above conditions have deteriorated to such a level that it is very likely to cause a series of concurrent diseases that threaten a child’s life and health (Walker et al. Citation2013). Therefore, the key to improving patient prognosis lies in effectively controlling pneumonia development, which requires understanding of its pathogenic mechanisms. This study may be a breakthrough by revealing the role of the miR-29c/PTEN axis in SP.
MiR-29c and PTEN showed a decreasing trend in the peripheral blood and lung tissue in rats with SP, suggesting that they were involved in the initiation and development of SP. This is consistent with the findings of previous studies by Fang et al. (Fang et al. Citation2013) and Shan et al. (Shan et al. Citation2019) regarding the effects of miR-29c and PTEN on renal interstitial fibrosis and bladder cancer in rats. MiR-29c belongs to the miR-29s family and its expression is tissue-specific; it can regulate the development of colorectal cancer (Liu et al. Citation2016) and has been shown to inhibit the invasiveness and metastasis of nasopharyngeal carcinoma by targeting TIAM1 (Liu et al. Citation2013). However, the role of miR-29c and its mechanism in pneumonia has not been clarified. PTEN is mainly expressed in the cytoplasm and is found in almost 90% of normal tissues (Zuo et al. Citation2019). It acts as a tumor suppressor in neoplasms and shows a gradually decreasing trend during the transformation from normal tissues to cancerous tissues (Zhang and Zhang Citation2017), which is consistent with the results of this study. Jiang et al. (Jiang et al. Citation2014) previously pointed out that PTEN may have a relationship with pneumonia occurrence. However, similar to miR-29c, its mechanism in pneumonia remains elusive.
To determine the mechanism of action of miR-29c/PTEN in pneumonia, we detected the expression of PI3 K, Akt, and NF-kB proteins in the lung tissue of rats with (MG) and without SP (CG). Expression of PI3 K, Akt, and NF-kB proteins in the MG was notably less, suggesting that the influence of miR-29c/PTEN on pneumonia is realized through the PI3 K/Akt/NF-kB pathway. Previous studies found that miR-29c is implicated in bladder cancer via PI3 K/Akt (Fan et al. Citation2014), and PTEN and NF-kB are of great significance in colon cancer (Zhang et al. Citation2015). Moreover, it has been proved in the previous study that microRNA-4485 can improve SP by inhibiting the STAT3/PI3 K/AKT signaling pathway (Guo et al. Citation2020). Another study indicated that pinitol could reduce LPS-induced pneumonia in experimental animals, which may play a role by inhibiting the TLR-4 and NF-κB/IκBα signaling cascade (Fan et al. Citation2021). All these suggest that PI3 K, Akt, and NF-kB proteins are involved in the development of SP. Furthermore, we transfected miR-29c and PTEN into lung epithelial cells and found that the inhibition of miR-29c and PTEN resulted in markedly reduced levels of PI3 K, Akt, and NF-kB. Additionally, the levels of PI3 K, Akt, and NF-kB were significantly enhanced when miR-29c and PTEN were overexpressed. This confirmed our hypothesis that the mechanism of the miR-29c/PTEN axis in pneumonia was affected through the PI3 K/Akt/NF-kB pathway.
Several limitations of the study are worth mentioning. First, we did not perform dual-luciferase reporter assays to determine the targeting relationship between miR-29c/PTEN and PI3 K/Akt/NF-kB. However, we will conduct these experiments accordingly as soon as possible. In addition, because there are certain differences between animal models and humans, our results may not translate to human experiments. Moreover, we did not analyze the resistance of miR-29c/PTEN to pneumonia, which is another limitation. Furthermore, we did not investigate the potential molecular mechanism and pathways in which miR-29c/PTEN modulate PI3 K/Akt/NF-kB. In the future, we will conduct a more in-depth and comprehensive analysis to address the shortcomings of this study and obtain more representative experimental results for clinical reference.
In summary, miR-29c/PTEN exhibits low expression in SP, which can promote pneumonia development by inhibiting the expression of PI3 K/Akt/NF-kB pathway proteins. The miR-29c/PTEN axis may be a target to aid in the diagnosis and treatment of pneumonia in the future.
Data availability statement
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data is not available.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Abdullah C, Korkaya H, Courtneidge SA. 2017. SRC regulates proliferation in ER+ breast cancer cells by stabilizing MYC mRNA. FASEB J. 31(1_supplement):775.719–775.719.
- Chisti MJ, Salam MA, Smith JH, Ahmed T, Pietroni MA, Shahunja KM, Shahid AS, Faruque AS, Ashraf H, Bardhan PK, et al. 2015. Bubble continuous positive airway pressure for children with severe pneumonia and hypoxaemia in Bangladesh: an open, randomised controlled trial. Lancet. 386(9998):1057–1065.
- Fan Y, Song X, Du H, Luo C, Wang X, Yang X, Wang Y, Wu X. 2014. Down-regulation of miR-29c in human bladder cancer and the inhibition of proliferation in T24 cell via PI3K-AKT pathway. Med Oncol. 31(7):65.
- Fan Y, Wang J, Feng Z, Cao K, Xu H, Liu J. 2021. Pinitol attenuates LPS-induced pneumonia in experimental animals: possible role via inhibition of the TLR-4 and NF-κB/IκBα signaling cascade pathway. J Biochem Mol Toxicol. 35(1): e22622.
- Fang Y, Yu X, Liu Y, Kriegel AJ, Heng Y, Xu X, Liang M, Ding X. 2013. miR-29c is downregulated in renal interstitial fibrosis in humans and rats and restored by HIF-α activation. Am J Physiol Renal Physiol. 304(10):F1274–F1282.
- Guan Y, Jin D. 2016. Research on the Clinical Nursing Observation of Infant Severe Pneumonia. 2016 4th International Education, Economics, Social Science, Arts, Sports and Management Engineering Conference (IEESASM 2016).
- Guo L, Wang Q, Zhang D. 2020. MicroRNA-4485 ameliorates severe influenza pneumonia via inhibition of the STAT3/PI3K/AKT signaling pathway. Oncol Lett. 20(5):215.
- Haugen J, Chandyo R, Ulak M, Mathisen M, Basnet S, Brokstad K, Valentiner-Branth P, Shrestha P, Strand T. 2017. 25-hydroxy-vitamin d concentration is not affected by severe or non-severe pneumonia, or inflammation, in young children. Nutrients. 9(1):52.
- Hezova R, Kovarikova A, Srovnal J, Zemanova M, Harustiak T, Ehrmann J, Hajduch M, Svoboda M, Sachlova M, Slaby O. 2015. Diagnostic and prognostic potential of miR-21, miR-29c, miR-148 and miR-203 in adenocarcinoma and squamous cell carcinoma of esophagus. Diagn Pathol. 10(1):42.
- Hirai N, Sasaki T, Ohsaki Y. 2017. Novel ALK specific mRNA in situ hybridization assay for non-small cell lung carcinoma. Transl Lung Cancer Res. 9(2):257–268.
- Jiang C, Hu X, Alattar M, Zhao H. 2014. miRNA expression profiles associated with diagnosis and prognosis in lung cancer. Expert Rev Anticancer Ther. 14(4):453–461.
- Kulnik ST, Rafferty GF, Birring SS, Moxham J, Kalra L. 2014. A pilot study of respiratory muscle training to improve cough effectiveness and reduce the incidence of pneumonia in acute stroke: study protocol for a randomized controlled trial. Trials. 15(1):123.
- Li T, Gu J, Yang O, Wang J, Wang Y, Kong J. 2020. Bone marrow mesenchymal stem cell-derived exosomal miRNA-29c decreases cardiac ischemia/reperfusion injury through inhibition of excessive autophagy via the PTEN/Akt/mTOR signaling pathway. Circ J. 84(8):1304–1311.
- Liu X, Fu Q, Du Y, Yang Y, Cho W C. 2016. MicroRNA as regulators of cancer stem cells and chemoresistance in colorectal cancer. Curr Cancer Drug Targets. 16(9):738–754.
- Liu J, Liu F, Liu Y, Wang HW, Feng ZC. 2014. Lung ultrasonography for the diagnosis of severe neonatal pneumonia. Chest. 146(2):383–388.
- Liu N, Tang LL, Sun Y, Cui RX, Wang HY, Huang BJ, He QM, Jiang W, Ma J. 2013. MiR-29c suppresses invasion and metastasis by targeting TIAM1 in nasopharyngeal carcinoma. Cancer Lett. 329(2):181–188.
- Mancarella C, Manara MC, Mercurio AM, Scotlandi K. 2019. Insulin-like growth factor 2 (IGF-2) mRNA binding protein 3-mediated regulation of CD164-CXCR4 axis impacts aggressiveness of Ewing sarcoma. Front Oncol. 10(994).
- Mandourah Y, Al-Radi A, Ocheltree AH, Ocheltree SR, Fowler RA. 2012. Clinical and temporal patterns of severe pneumonia causing critical illness during Hajj. BMC Infect Dis. 12(1):117.
- Monsel A, Zhu YG, Gennai S, Hao Q, Hu S, Rouby JJ, Rosenzwajg M, Matthay MA, Lee JW. 2015. Therapeutic effects of human mesenchymal stem cell-derived microvesicles in severe pneumonia in mice. Am J Respir Crit Care Med. 192(3):324–336.
- Montull B, Menéndez R, Torres A, Reyes S, Méndez R, Zalacaín R, Capelastegui A, Rajas O, Borderías L, Martin-Villasclaras J. 2016. Predictors of severe sepsis among patients hospitalized for community-acquired pneumonia. PLoS One. 11(1):e0145929.
- Reyes LF, Restrepo MI, Hinojosa CA, Soni NJ, Anzueto A, Babu BL, Gonzalez-Juarbe N, Rodriguez AH, Jimenez A, Chalmers JD, et al. 2017. Severe pneumococcal pneumonia causes acute cardiac toxicity and subsequent cardiac remodeling. Am J Respir Crit Care Med. 196(5):609–620.
- Rudan I, O'Brien KL, Nair H, Liu L, Theodoratou E, Qazi S, Luksic I, Fischer Walker CL, Black RE, Campbell H, et al. 2013. Epidemiology and etiology of childhood pneumonia in 2010: estimates of incidence, severe morbidity, mortality, underlying risk factors and causative pathogens for 192 countries. J Glob Health. 3(1):010401.
- Shan G, Tang T, Xia Y, Qian HJ. 2019. MEG3 interacted with miR-494 to repress bladder cancer progression through targeting PTEN. J Cell Physiol. 235(2):1120–1112.
- Walker CLF, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, O'Brien KL, Campbell H, Black RE. 2013. Global burden of childhood pneumonia and diarrhoea. Lancet. 381(9875):1405–1416.
- Zhang L-L, Mu G-G, Ding Q-S, Li Y-X, Shi Y-B, Dai J-F, Yu H-G. 2015. PTEN represses colon cancer progression through inhibiting paxillin transcription via PI3K/AKT/NF-kB pathway. J Biol Chem jbc. M115:641407.
- Zhang Q, Zhang S. 2017. miR-214 promotes radioresistance in human ovarian cancer cells by targeting PETN. Biosci Rep. 37(4):BSR20170327.
- Zou H, Ding Y, Shi W, Xu X, Gong A, Zhang Z, Liu J. 2015. MicroRNA-29c/PTEN pathway is involved in mice brain development and modulates neurite outgrowth in PC12 cells. Cell Mol Neurobiol. 35(3):313–322.
- Zuo ML, Wang AP, Tian Y, Mao L, Song GL, Yang ZB. 2019. Oxymatrine ameliorates insulin resistance in rats with type 2 diabetes by regulating the expression of KSRP, PETN, and AKT in the liver. J Cell Biochem. 120(9):16185–16194.