Abstract
This study investigates the curative response of ethanol extract of Cassia sieberiana leaves (EECSL) on testosterone-induced benign prostatic hyperplasia (BPH). Thirty rats of 11–12 weeks old weighing 120–160 g were grouped into six of five rats each. Group 1 was the normal control; groups 2–6 were induced with testosterone (5 mg/kg body weight) for 21 days following standard procedures. Group 2 serves as BPH-control, groups 3 received finasteride while 4–6 received orally 100, 300, and 500 mg/kg of EECSL, respectively for 14 days. Biochemical and BPH indices were analyzed. Oral administration of EECSL showed a decrease in prostate weight, Prostate-specific antigen (PSA), testosterone and dihydrotestosterone (DHT), triacylglycerol (TAG), total cholesterol (TC), low-density lipoprotein (LDL), malondialdehyde (MDA), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) whereas an elevation in high-density lipoprotein (HDL), GSH and likewise antioxidant enzymes superoxide dismutase (SOD) and catalase (CAT) activities were recorded. The histology photomicrographs showed that EECSL restored lesions of testosterone induction in the treated groups. Our study reveals that EECSL exerts potent on BPH, hypolipidaemic, and antioxidant potentials and justifies the claims by the traditional doctors in BPH management.
1 Introduction
Benign prostatic hyperplasia (BPH) appears to be one of the most prevalent urological diseases among elderly men with a major impact on quality of life and a substantial economic burden. BPH is caused by the abnormal proliferation of epithelial and stromal cells in the prostate gland (Cartwright et al. Citation2014). The majority of the predisposing factors associated with BPH like age, genetic factors, sex hormones, and metabolic syndrome are the hallmark to the development of BPH (Aakula et al. Citation2016). Dihydrotestosterone (DHT) combines to androgen receptors and forms complex with testosterone by the action of 5α-reductase(s) activities which enhances protein synthesis and prostatic cell growth (Liao et al. Citation2012). Early signs of BPH are manifested through the narrowing of urethra excretion of urine and difficulty in completely empty of the bladder which may cause bladder inflation and hormonal imbalance (Yano et al. Citation2001).
Treatment and management of BPH usually involve surgical procedures which allow free expression of urine. Also, α-blockers and 5α-reductase inhibitors drugs that help in the reduction in prostate mass and muscle relaxation (Mustafa et al. Citation2016; Braeckman and Denis Citation2017). Nevertheless, these drugs have pose advanced side effects (Trost et al. Citation2013; Hagberg et al. Citation2016) and may impair low libido, ejaculation disorder, and erectile dysfunction (Bullock and Andriole Citation2006). This calls for a search for drugs that are potent and safe. Plant products are rich in therapeutic and medicinal constituents and are currently being explored for the management of BPH patients (Ejike and Ezeanyika Citation2011). It was estimated that about 30% of men diagnosed with BPH used complementary and alternative medicine (Ejike and Ezeanyika Citation2011; Rafieian-Kopaei Citation2012; Singh Citation2015).
Cassia sieberiana belongs to the family Fabaceae (Leguminosae-Caesalpinioiceae). The drumstick tree is a relatively unknown African plant species regarded as a weed (Singh Citation2017). The plant grows to like many other weeds until recently when researchers’ attention is being shifted to its medicinal values (Olapade et al. Citation2012). The plant extract possesses anti-bacterial and anti-inflammatory activity (Donkor et al. Citation2013). Previous studies showed that ethanolic root extract of C. sieberiana had an anti-parasitic effect, myorelaxant and anti-spasmodic activity (Duwiejua et al. Citation2007; Sy et al. Citation2009), anti-microbial activity against some bacteria and viruses (Sliva et al. Citation1997), analgesic and anti-inflammatory activity (Sy et al. Citation2009) and anti-malarial potentials of the leaf extract has been documented (Aliyu et al. Citation2013). In Nigeria, extracts of C. sieberiana plants have been used in folklore for the treatment of tooth pain, uterus inflammation, weakness of the bones, and joints (Sam et al. Citation2011). This study was aimed at investigating the potency of EECSL on testosterone-induced BPH in experimental animals so as to validate is use folklore medicine.
2. Materials and methods
2.1. Plant materials and extraction procedure
Fresh leaves of Cassia sieberiana were sourced from Odu Ajaruwa-Eke in Dekina Local Government Area of Kogi State. The plant sample was identified by Mr Alfred Ozioko of the Bio-resources Development and Conservation Programme (BDCP) Research Centre, Nsukka, Enugu State, Nigeria, with the identification number, Intercedd/162946. The leaves of C. sieberiana were washed in clean water, air-dried, and ground to powdered form. Six hundred grams of powdered C. sieberiana leaves were soaked in 1.7 L of absolute ethanol. The solution was filtered with Whatman No. 4 filter paper; the filtrate was concentrated to slurry using a rotary evaporator at pressure of 175 mbar and temperature of 40°C. The extract dose was prepared by weighing a specific quantity from the concentrate into the vehicle in a right proportion.
2.2. Animals
Thirty rats of 11–12 weeks old weighing 120–160 g sourced from the facility of Biological Sciences Faculty, University of Nigeria, Nsukka animal house. The animals were housed at room temperature and acclimatized for 14 days with a constant supply of water and commercial pellet rat feed (Standard Grower’s, Grand Cereals LTD, Enugu, Nigeria). The rat feed is composed of maize, sorghum, wheat, brewers dried grains, palm kernel meal, rice bran, groundnut cake, soya cake, full fat soya, lysine, methionine, limestone, bone meal, salt, vitamins/minerals premix, and antioxidants. The institution and national committee guidelines for the care and use of animals were strictly followed in this study (Indian Council of Medical Research Citation2001).
2.3. BPH induction
The rats were divided into six (6) of five (5) rats each. Group 1 was not induced nor treated (normal control), group 2 was induced but not treated (Untreated control), group 3 was induced and received 1 mg/kg b.w of finansteride (standard control) while groups 4, 5, and 6 were induced and received 100, 300, and 500 mg/kg b.w of EECSL (low, mid, and high dose), respectively. Group 1 and 2 received the vehicle throughout the duration of the study. An experimentally developed BPH model was created in the male rats by hypodermic injection of testosterone (5 mg/kg body weight (b.w)) for three (3) weeks as reported by Al-Trad et al. (Citation2019), with little adjustment. The PSA levels of the rats were determined to confirm that the induction was successful. The animals were treated with finansteride (group 3) and graded doses of EECSL for 14 days. We carried out a pilot LD50 study which showed that the leaf extract was safe up to 5000 mg/kg and this informed the choice of doses.
2.4. Treatment of BPH
Treatment with standard drug and different doses of extract commenced on the next day which represents the first day of treatment and it continued for 14 days. The oral administration (treatment) was done once per day by the use of gavages. Carboxymethyl cellulose (CMC) was used as the vehicle. The weight of the rats was recorded at the beginning of the experiment and every week for two weeks using an electronic weighing balance. The body weight gain was estimated by the subtraction of the final weight from the initial weight. The percentage prostate index and PSA differences were calculated by the difference in mean prostate index between the induced groups without treatment minus that of the treated group multiply by 100. After treatment, the rats were deprived of food and water overnight and sacrificed. Blood samples and tissues (prostate gland, testis, and bladder) were collected for analyses. The tissues were weighed and recorded. The prostate index was estimated by dividing the prostate weight with the total body weight.
2.5. Biochemical analysis
Serum PSA, testosterone, and DHT levels were analyzed using PSA/testosterone ELISA kit (Monobind Inc., Lake Forest, CA 92630, USA) and DHT ELISA kit (ALPCO, 26-G Keewaydin Drive Salem NH 03079, USA) respectively, following the manufacturer’s instructions. The concentration of serum lipid profile was determined using the following methods: cholesterol, Abell et al. (Citation1952); triacylglycerols, Tietz (Citation1990); high-density lipoproteins, Albers et al. (Citation1978); and low-density lipoproteins, Friedewald et al. (Citation1972). The oxidative stress markers were determined after depriving the rat food and water overnight. The prostate tissue was harvested and homogenized for the assays. Superoxide dismutase (SOD) activity was assayed following the method of Fridovich (Citation1989). Catalase (CAT) was assayed by the method of Aebi (Citation1983). Glutathione (GSH) level was analyzed by the method of Goldberg and Spooner (Citation1983.). Lipid peroxidation product, malondialdehyde (MDA) was determined spectrophotometrically by the method of Wallin et al. (Citation1993). Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities were assayed using the method of Reitman and Frankel (Citation1957). Alkaline phosphatase activity was assayed according to the method of Babson et al. (Citation1966).
2.6. Tissue histology
The histological examination of the experimental animal prostate tissues was carried out using Drury et al. (Citation1967). The tissues were fixed in 10% phosphate-buffered formalin for about 48 h. Subsequently, they were prepared for histopathological examination using standard techniques. The slides were examined with a Motic® light microscope and the photomicrographs were taken using the Motic® microscope camera.
2.7. Statistical analysis
Data were analyzed with Statistical Package for Service Solutions (SPSS, version 23) and were presented as means ± standard deviation (SD). This was subjected to a one-way analysis of variance (ANOVA) followed by Duncan multiple tests and differences between means were considered significant when p < .05.
3. Results and discussion
3.1. Quantitative phytochemical constituents
The phytochemical screening of ethanol extract of Cassia sieberiana (CS) leaves showed the distribution of secondary metabolites. The presence of saponins, terpenoids, flavonoids, alkaloids, glycosides, phenolics, steroids, and tannins in substantial quantities (Table ). Further studies on the comparative chemical constituents of some cassia species and their pharmacological importance by Awomukwu et al. (Citation2015) has also reported the presence of alkaloids, tannins, flavonoids, saponins, and phenols in the leaf, stem, root, and pod of the CS. The scavenging of free radical by various constituents of plants may be due to phenolics and flavonoids (Boumerfeg et al. Citation2009). Sakagami et al. (Citation2000) reported that tannins, phenolics, flavonoids, and alkaloids have a cytoprotective effect against human oral tumor cell lines.
Table 1. Quantitative phytochemical compositions of ethanol extract of Cassia sieberiana leaves.
Table 2. Effect of EECSL on body weight gain of the rats.
Table 3. Effect of EECSL on the weight of prostate, bladder, testes, and prostate index.
3.2. Bodyweight gain
A significant increase was recorded in the final bodyweight of the animals in comparison to their initial weight. Subcutaneous injection of testosterone had no significant (p > .05) difference in weight gains in the untreated/treatment groups in comparison to the normal control (Table ). A similar trend was observed by Park et al. (Citation2017) which reported a non-significant elevation in the body weight gain of experimentally induced BPH animals compared to the normal control. This could be attributed to the differences in the initial whole body weights and unknown physiological processes that may have occurred during prostate enlargement and the metabolism of administered substances.
3.3. Effect of EECSL on prostate parameters
Prostate weight was significantly high in the BPH group in comparison to the normal control whereas, EECSL treated group (high dose) significantly lowered the prostate weight in comparison to the untreated control. The standard group gave a 17.24% decrease in comparison to the untreated control while the EECSL administered groups displayed a dose-dependent (100, 300, and 500 mg/kg) decrease of 17.24%, 20.69%, and 27%, respectively (Table ). Changes in the bladder and testes weight of the rats were observed.
Table 4. Effect of EECSL on serum PSA of testosterone-induced BPH.
The prostate index of the untreated control significantly elevated more than half in comparison to the normal control group whereas, the standard and EECSL treated groups were significantly lowered in the prostate index in comparison to the non-treated control. The standard control gave a 14.63% decrease in comparison to the untreated control while the EECSL administered groups gave a dose-dependent (100, 200, and 500 mg/kg) decrease of 20.73%, 26.83%, and 38.41%, respectively.
The bladder weight was significantly high in the untreated group in comparison to the normal control whereas, the testes’ weight was significantly decreased in the BPH group. The oral doses of standard drug and EECSL did not affect the weight of the testes in comparison to the untreated control. This elevated weight of prostate parameters could be attributed to the effect of the exogenous hormone administered to the untreated group. Prostatic weight increase is a vital signal in BPH biomarkers (Veeresh Babu et al. Citation2010). Treatment with different doses of the EECSL has demonstrated to be effective in prostatic tissues haven caused a relative decrease in prostate weight and index (PWI). A similar decline in PWI was recorded in standard control which however displayed comparably less effect compared to the plant extract. The decrease in the prostate weight in extract-treated group could be ascribed to the abundance of antioxidants in the EECSL. Mbaka et al. (Citation2017) reported that effective use of plant extracts could reduce prostate weight and reverses degenerative changes in the structure of the prostate gland. The reduction in the size of the prostate that occurred in the EECSL treated group (high dose) indicates that the prostate size reduction could not have been caused by the spontaneous reversal of hyperplasia exogenously induced. Therefore, the extract may possess the ability to inhibit growth factor that causes cell proliferation or glandular expansion as observed in prostate weight reduction.
Interestingly, Cassia sieberiana leaves extract enhanced antioxidant defense mechanism which could be attributed to its phenolics content such as flavonoids, and tannins that may result in attenuating testosterone-induced prostatic hyperplasia (Donkor et al. Citation2013). The antioxidant properties of the plant may explain its utilization and benefits in traditional medicine to alleviate the pathological condition of the prostate. Also, a significant elevation and decrease in bladder weight and testes weight respectively was noticed in the untreated control in comparison to the normal control. The changes noticed in the bladder may result in an inflamed prostate gland which obstructs bladder outflow thereby causing difficulties in urination and weakens the bladder muscles (Bhavsar and Verma Citation2014; Thu et al. Citation2017).
3.4. Effect of the EECSL on serum PSA level
Subcutaneous induction of testosterone significantly raised serum PSA level in untreated control in comparison to the normal control. The elevation of serum PSA level after injection with testosterone followed the same trend with Li et al. (Citation2018) who reported that rat prostate respond to testosterone-induced BPH and more sensitive model in targeting urinary obstruction problem which is comparable to the human prostate. This elevation of PSA values shows that testosterone injection successfully induced BPH since PSA is secreted by the epithelial cell of the prostate gland. Thus, abnormal serum level of PSA serves as a direct indicator of prostatic disorder (Velonas et al. Citation2013).
The PSA level in the standard control and the EECSL treated groups was significantly (p < .05) lowered after two weeks of administration in comparison to the initial PSA level before treatment (Table ). The finasteride group displayed a 60.78% reduction in PSA level compared to the initial PSA whereas, the PSA level in the EECSL treated groups (100, 300, and 500 mg/kg) showed a dose-dependent decrease of 43.68%, 51.45%, and 57.30%, respectively compared to their initial PSA levels before treatment. The PSA level of the untreated control was not significantly lowered. Finasteride selectively inhibits type II 5α-reductase, which catalyzes the formation of dihydrotestosterone (DHT) from testosterone (Traish and Morgentaler Citation2013). The administration of EECSL blocked the evolution of BPH and reversed the prostatic condition closely to normal, which was observed by a significant reduction in serum PSA level. The result agrees with the report that treatment of BPH-induced rats with ethanol extract of Z. portoricensis stem has a positive outcome by reducing PSA level and prostate weight (Joshua et al. Citation2018). PSA is generally elevated in BPH is a reliable marker of BPH. This is because reductions in PSA level is associated with reduced prostate hyperplasia (Ejike and Ezeanyika Citation2011). The lowered serum PSA levels of EECSL treated rats may be related to its inhibitory effect on the 5α-reductase activity which converses testosterone to its potent form (DHT) and has a high affinity for binding androgen receptor leading to BPH progression and development (Agbo et al. Citation2010; Afriyie et al. Citation2014; Mohamed et al. Citation2016; Liu et al. Citation2019). Although the peak reversal effect on serum PSA levels observed in the standard and extract-treated groups was not the same as the PSA level of the normal control, the difference may be largely due to the short duration of treatment. Therefore, if the treatment period was extended, a maximum effect of the extract may be revealed. The untreated control was used to monitor the reversal rate of the prostatic condition.
3.5. Effect of the EECSL on serum testosterone and DHT level
The subcutaneous injection of testosterone significantly elevated the serum testosterone in comparison to the normal control (Table ). The elevated serum testosterone concentration was significantly reduced by finasteride and different doses of EECSL. The extract-treated group showed a dose-dependent decrease observed at 500 mg/kg (27.30%). Also, the untreated control group showed a significant elevation in serum DHT concentration in comparison to normal control. The EECSL administered groups were significantly lowered the serum DHT level with a maximum decrease at 100 mg/kg. The reduction in testosterone and DHT serum levels in the EECSL treated rats could be attributed to the EECSL activity which enhanced the decrease in the levels of circulating testosterone and DHT that could constitute risk factors for hyperplasia of the prostate gland. This indicates that the EECSL improves the clearing of unbound testosterone in the bloodstream and blocks its translation to active DHT by 5α-reductase (Roehrborn et al. Citation2007).
Table 5. Effect of EECSL on serum testosterone and DHT levels of testosterone-induced BPH.
3.6. Effect of EECSL on lipid profile
The injection of testosterone significantly elevated serum TAG, TC, and LDL compared to normal control. The increase in serum lipid (TAG, TC, and LDL) was reduced by treatment of rats with standard drug and different doses of EECSL with a sharp reduction recorded at a higher dose (500 mg/kg) of the EECSL. The increment supports the reports by Parsons et al. (Citation2008) that hypercholesterolemia may affect the sex steroid axis and result in BPH.
Meanwhile, the serum concentration of LDL was lowered (p < .05) significantly at mid and high doses in comparison to the untreated group. However, the induction of testosterone significantly lowered the serum HDL level in comparison to the normal control (Table ). High-density lipoprotein in contrast to the TAG, TC, and LDL was elevated significantly in the standard and the EECSL administered groups in comparison to the untreated control. Dyslipidemia has a link to metabolic syndrome and invariable exposes one to BPH (Liu et al. Citation2010; Duru et al. Citation2015), which represents potential risk factors in BPH (Tewari et al. Citation2011). Our result suggests that the extract showed some anti-hyperlipidemic and modulatory effects on dyslipidemia induced by BPH.
Table 6. Effect of EECSL on lipid profile of testosterone-induced BPH.
3.7. Effect of EECSL on oxidative stress markers
The activities of superoxide dismutase (SOD) and catalase (CAT) were significantly lowered whereas; glutathione reductase (GSH) showed no significant change in the untreated control compared to the normal control (Table ). The standard and EECSL treated groups showed improved recovery in the SOD and CAT activities compared to the untreated group with the EECSL administered groups showing a dose-dependent elevation in SOD activity. Also, the induction of animals with testosterone gave a significant elevation in malondialdehyde (MDA) concentration (Table ). Lipid peroxidation lowers antioxidant levels in BPH (Aryal et al. Citation2007). Elevation of MDA serves as a signal to lipid peroxidation and tissue damage which is associated with the development of BPH (Aydin et al. Citation2006). The EECSL treated groups significantly lowered MDA at mid/high dose compared to the untreated control. The preventive action of EECSL could be attributed to its secondary metabolites (Donkor et al. Citation2013). In general, the results indicate that EECSL has antioxidant potential that reduced the rate of lipid peroxidation. This antioxidant property might be attributed to the rich phytochemical constituents of the EECSL (Table ) that are involved in scavenging free radicals and reducing lipid peroxidation.
Table 7. Effect of EECSL on oxidative stress markers in testosterone-induced BPH rats.
3.8. Effect of EECSL on liver marker enzymes
There was no significant change in the activities of the liver marker enzymes (AST and ALT) in the treatment group when compared to the untreated control (Table ). However, ALP was significantly lowered in standard control and the high dose of EECSL compared to the untreated group with the treatment groups showing a dose-dependent decrease. The serum activities of these markers are indicators of liver damage and their concentrations may be elevated in the bloodstream (Mimae Citation2017). In our study, no significant increase in these markers was recorded in the administration of standard drug and EECSL in rats, indicating that the EECSL administered at varying doses were are not hepatotoxic. Elevated levels in activities of these enzymes in the bloodstream can be linked to liver damage by hepatotoxins. Liver damage causes permeability of the cell membrane of the hepatocytes which results in injury in the liver and other complications (Moke et al. Citation2015).
Table 8. Effect of EECSL on liver marker enzymes.
3.9. Effect of EECSL on histology of rats
Photomicrograph of prostate tissue in the control rat showed normal histological appearances composed of normal convolutions, usually lined by two layers of the epithelium with the clear basal polarity of the nuclei (Figure ). At the same time, the prostate tissue of albino rats in the untreated group showed severe multiple blood congestions observed in the fibromuscular stroma (Figure ) compared to Figure . Further degeneration in the untreated group such as enlarged acini, mostly distended with secretory materials was observed. Moreover, prostate tissue exhibited back–to–back micro acini with improved intra acini papillary convolutions in the treatment groups (Figures ). The presence of dilation of the prostate characterized by acini distend with secretory materials and papillary infoldings especially in test groups (Figures ) and standard control (Figure ) are suggestive of overall histological improvement when compared with BPH control (Figure ). The histological change observed in Figure (BPH untreated group) confirms the characteristics earlier documented by Mbaka et al. (Citation2017) and Ikeyi et al. (Citation2020). The mode of action may be linked to the binding of DHT to androgen receptors which promote protein synthesis and prostatic cell growth.
Figure 1. Normal control: Photomicrograph of prostate tissue showing normal infoldings (convolutions), corpora amylacea (CA) (red arrow), Luminal secretions (LS) (blue arrow) are adequate and intact seminal vesicles (black arrow). H&E. mag. 400×.
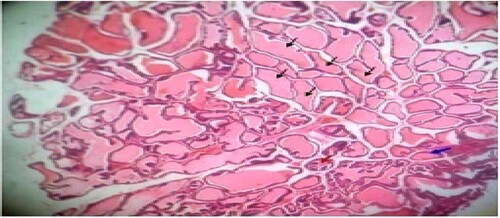
Figure 2. Untreated control: Photomicrograph of prostrate tissue showing slight disruption of architectural appearances (blue arrow) and multiple blood congestions (black star) within the fibromuscular stroma. Enlarged acini (black arrow) also observed. H&E. mag. 400×.
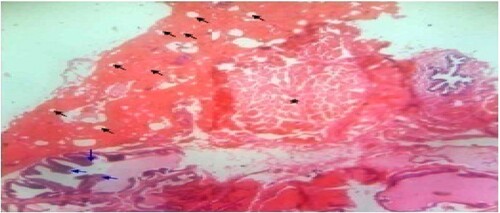
Figure 3. Standard control: Photomicrograph of prostate tissue showed back-to-back micro acini with improved intra acina papillary infoldings/or convolutions (black arrow) of the seminal vesicles duct with fibrous capsules (star). Reduced secretions and focal blood congestions were observed within the fibromuscular stroma of the prostate. H&E. mag. 100×.
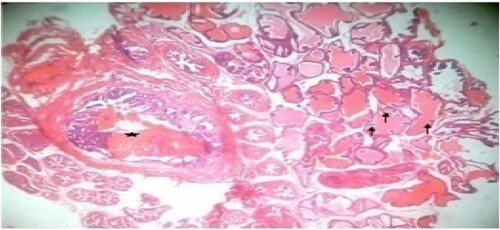
Figure 4. Low dose: Photomicrograph of prostate tissue showed ectasia or dilation of the prostate characterized by acini distended with secretory materials (SM) (star), compression(black arrow) of surrounding unaffected acini with dilation appearing cystic, with fused cuboidal epithelium (blue arrow) lining were present. Infoldings or occasional papillary infoldings was observed. H&E. mag. 400×.
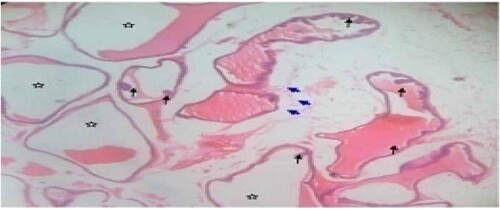
Figure 5. Mid dose: Photomicrograph of dorsal prostate tissue showed extensively enlarged acini with reduced secretory (star) materials in the lumen. The hyperplastic condition (black arrow) was also observed. H&E. mag. 400×.
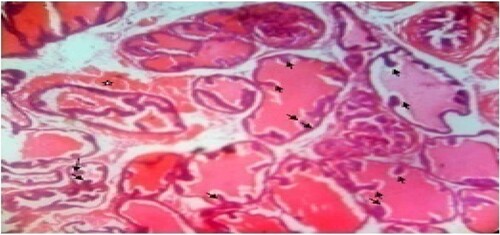
Figure 6. High dose: Photomicrograph of prostate tissue showed glandular hyperplasia predominated and characterized by exaggerated intra-acinar papillary infoldings (convolutions) (black arrow) with fibrovascular cores. Infiltrates mainly lymphocytic cells were observed in the acinar without any admixed tumor cells. Secretions (star) in the acini were observed. H&E. mag. 400×.
4. Conclusion
The administration of EECSL improved and restored serum prostatic epithelial cells, antioxidant, and lipid profile levels. It lowered the serum testosterone which had a direct influence on the DHT concentration. The safety of EECSL is assured, hence, may be considered effective in ameliorating benign prostatic hyperplasia. This study may serve as scientific documentation by the traditional healers in the management of BPH in North Central of Nigeria. Further studies are required to establish the mechanism of action of EECSL which is the major limitation of our work. Also, the extension of treatment days beyond fourteen days for a better potency of the extract is highly recommended.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available in OSFHOME check finder tool at https://mfr.osf.io/render?url=https%3A%2F%2Fosf.io%2F3grd9%2Fdownload
References
- Aakula A, Kohonen P, Leivonen S. 2016. Systematic identification of microRNAs that impact on proliferation of prostate cancer cells and display changed expression in tumor tissue. Eur Urol 69(6):1120–1128.
- Abell LL, Levey BB, Brodie BB, Kendall FE. 1952. A simplified method for the estimation of total cholesterol in serum and demonstration of its specificity. J Biol Chem. 195(1):357–366.
- Aebi HE. 1983. Catalase. In: Bergmeyer H. U., J. Bergmeyer, Grassl M., editor. Methods of Enzymatic Analysis. 3rd ed. Florida: Weinheim Deerfield Beach; p. 273–285.
- Afriyie DK, Asare GA, Bugyei K, Adjei S, Lin JM, Peng J, Hong ZF. 2014. Treatment of benign prostatic hyperplasia with croton membranaceus in an experimental animal model. J Ethnopharmacol. 157:90–98.
- Agbo MO, Okoye FBC, Nwodo JN. 2010. In vivo antiinflammatory effect of Zapoteca portoricensis. Int J Health Res. 3(1):29–35.
- Al-Trad B, Aljabali A, Al Zoubi M, Shehab M, Omari S. 2019. Effect of gold nanoparticles treatment on the testosterone-induced benign prostatic hyperplasia in rats. Int J Nanomed. 14:3145–3154.
- Albers JJ, Warmick GR, Cheng MC. 1978. Quantitation of high density lipoproteins. Lipids. 13:926–932.
- Aliyu Z, Yusha’u M, Aliyu BS. 2013. Anti malarial activity of Cassia sieberiana leaf extracts. Open Conf Proc J. 4:72–76.
- Aryal M, Pandeya A, Gautam N, Baral N, Lamsal M. 2007. Oxidative stress in benign prostatic hyperplasia. Nepal Med Coll J. 9(4):222–224.
- Awomukwu DA, Nyananyo BL, Ikpeama AI, Adieze CU. 2015. Comparative chemical constituents of some Cassia species and their pharmacognistic importance in south eastern Nigeria. Sci. J. Chem. 3(3):40–49.
- Aydin A, Arsova-Sarafinovska Z, Sayal A, Eken A, Erdem O, Erten O, Özgök Y, Dimovski A. 2006. Oxidative stress and antioxidant status in non-metastatic prostate cancer and benign prostatic hyperplasia. Clin Biochem. 39:176–179.
- Babson AL, Greeley SJ, Coleman CM, Philips GE. 1966. Phenolphthalein monophosphate as a substrate for serum alkaline phosphatase. Clin Chem. 12(8):482–490.
- Bhavsar A, Verma S. 2014. Anatomic imaging of the prostate. Biomed Res Int. 1–9.
- Boumerfeg S, Baghiani A, Messaoudi D, Khennouf S, Arrar L. 2009. Antioxidant properties and xanthine oxidase inhibitory effects of Tamus communis L. root extracts. Phytother Res. 23:283–288.
- Braeckman J, Denis L. 2017. Management of BPH then 2000 and now 2016 – from BPH to BPO. Asian J Urol. 4:138–147.
- Bullock TL, Andriole GL. 2006. Emerging drug therapies for benign prostatic hyperplasia. Expert Opin Emerg Drugs. 11:111–123.
- Cartwright R, Mangera A, Tikkinen KAO, Rajan P, Pesonen J, Kirby AC, Thiagamoorthy G, Ambrose C, Gonzalez-Maffe J, Bennett PR, et al. 2014. Systematic review and meta-analysis of candidate gene association studies of lower uri-nary tract symptoms in men. Eur Urol. 66(4):752–768.
- Donkor K, Okine KNL, Abotsi, MKW, Woode E. 2013. Anti-inflammatory and anti-nociceptive effects of ethyl acetate fraction of root bark of Cassia sieberiana D. C. in Murine models. C. in Murine models. Pharmacologia. 4(4):301-310.
- Drury RA, Wallington A, Cameroun SR. 1967. Carleton’s histological techniques. New York: Oxford University Press; p. 1–420.
- Duru RC, Njoku OU, Maduka IC, Ugonabo MC, Ugwueme FO. 2015. Atherogenic lipid markers and testosterone levels in Nigerian men with prostate disorders. Asian Pacific J Health Sci. 2:48–55.
- Duwiejua M, Panyin AB, Weremfo A, Woode E, Ansah C. 2007. Antinociceptive activity of the ethanolic extract of the root bark of Cassia sieberiana (Fam. Caesalpinaceae). J Pharm Biores. 4(2):49–58.
- Ejike CECC, Ezeanyika LUS. 2011. Management of experimental benign prostatic hyperplasia in rats using a feedbased therapy containing Telfairia occidentalis seeds. Afr J Tradit Complement Altern Med. 8(4):398–404.
- Fridovich I. 1989. Superoxide dismutase: An adaptation to a pragmatic gas. J Biol Chem. 264:7761–7764.
- Friedewald WT, Levy RI, Fredricson DS. 1972. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. J Clin Chem. 18:499–502.
- Goldberg DM,Spooner RJ. 1983. Assay of Glutathione Reductase. In: Bergmeyen H. V., editor. Methods of Enzymatic Analysis, 3rd ed. Vol. 3, Deerfiled Beach: Verlog Chemie; p. 258–265.
- Hagberg KW, Divan HA, Persson R, Nickel JC, Jick SS. 2016. Risk of erectile dysfunction associated with use of 5-α reductase inhibitors for benign prostatic hyperplasia or alopecia: population based studies using the clinical practice research datalink. Br Med J. 354:1–15.
- Ikeyi AP, Okagu IU, Ezeanyika LUS, Alumanah EO. 2020. Zapotecaportoricensis root crude methanol extract and its fractions normalizes aberrations associated with benign prostatic hyperplasia in rats. All Life. 13(1):360–372.
- Indian Council of Medical Research. 2001. Guidelines for the use of laboratory animals in medical colleges. New Delhi: Academic Press; p. 3–13.
- Joshua PE, Ezugwu CH, Chilaka FC, Nwodo OFC, Dasofunjo K, Ezugwu MU. 2018. Effect of ethanol extract of Zapoteca portoricensis stem on testosterone-induced benign prostate hyperplasia (BPH) in adult male albino rats. Australian J Basic Appl Sci. 12(12):9–18.
- Li J, Tian Y, Guo S, Gu H, Yuan Q, Xie X. 2018. Testosterone-induced benign prostatic hyperplasia rat and dog as facile models to assess drugs targeting lower urinary tract symptoms. PLoS ONE. 13(1):1–13.
- Liao CH, Li HY, Chung SD, Chiang HS, Yu HJ. 2012. Significant association between serum dihydrotestosterone level and prostate volume among Taiwanese men aged 40–79 years. Aging Male. 15(1):28–33.
- Liu J, Fang T, Li M, Song Y, Li J, Xue Z, Li J, Bu D, Liu W, Zeng Q, et al. 2019. Pao pereira extract attenuates testosteroneinduced benign prostatic hyperplasia in rats by inhibiting 5α-reductase. Sci Rep. 9:19703–19711.
- Liu Y, Zuckier LS, Ghesani NV. 2010. Dominant uptake of fatty acid over glucose by prostate cells: a potential new diagnostic and therapeutic approach. Anticancer Res. 30:369–374.
- Mbaka G, Anunobi C, Ogunsina S, Osiagwu D. 2017. Histomorphological changes in induced benign prostatic hyperplasia with exogenous testosterone and estradiol in adult male rats treated with aqueous ethanol extract of Secamone afzelii. Egypt J Basic Appl Sci. 4:15–21.
- Mimae R. 2017. Liver enzymes as an indicator of hepatic insult. J Healthcare Hyg. 1(1):1–4.
- Mohamed DA, Rashed MM, Shallan M, Fouda K, Hanna LM. 2016. Amelioration of benign prostate hyperplasia in rats through plant foods. Int J Pharmacogn Phytochem Res. 8(12):2063–2070.
- Moke EG, Ilodigwe EE, Okonta JM, Emudainohwo Jot, Ajaghaku DL, Erhirhie OE, Chinwuba P, Ahante E. 2015. Antidiabetic activity and toxicity evaluation of aqueous extracts of Spondias mombin and Costus afer on wistar rats. British J Pharm Res. 6(5):333–342.
- Mustafa M, Salih AF, Illzam EM, Sharifa AM, Suleiman M, Hussain SS. 2016. Prostate cancer: pathophysiology, diagnosis, and prognosis. IOSR J Dent Med Sci. 15(6):4–11.
- Olapade AA, Akinoso R, Oduwaye AO. 2012. Changes in some physicochemical properties of Cassia sieberiana seeds during roasting. Niger. Food J. 30(1):26–34.
- Park KH, Kim KS, Lee WS, Chung J, Lee B, Na WS, Park GC, Kim OY. 2017. A herbal formula, comprising Panax ginseng and bee-pollen, inhibits development of testosteroneinduced benign prostatic hyperplasia in male Wistar rats. Saudi J Biol Sci. 24:1555–1561.
- Parsons JK, Bergstrom J, Barrett-Connor E. 2008. Lipids, lipoproteins and the risk of benign prostatic hyperplasia in community-dwelling men. BJU Int. 101:313–318.
- Rafieian-Kopaei M. 2012. Medicinal plants and the human needs. J Herbmed Pharmacol. 1(1):1–2.
- Reitman S, Frankel S. 1957. A colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 28:56–63.
- Roehrborn CG, Nuckolls JG, Wei JT, Steers W. 2007. The benign prostatic hyperplasia registry and patient survey: study design, methods and patient baseline characteristics. BJU Int. 100:813–819.
- Sakagami H., Jiang Y., Kusama K., Atsumi T., Ueha T., Toguchi M., Iwakura I., Satoh K., Ito H., Hatano T., Yoshida T. 2000. Cytotoxic activity of hydrolyzable tannins against human oral tumor cell lines — a possible mechanism. Phytomedicine. 7:39–47.
- Sam GH, Mensah MLK, Nyakoa-Ofori N. 2011. Pharmacognostic studies and standardization of Cassia sieberiana roots. Pharmacogn J. 3(21):12–17.
- Singh D. 2017. Leaf phenology of Cassia sieberiana L. in Ksusta campus of Kebbi State, Nigeria. Sci Technol Public Policy. l(1):23–28.
- Singh R. 2015. Medicinal plants: A review. J Plant Sci. 3(1):50–55.
- Sliva O, Ferreira E, Vaz-pato M, Gome E. 1997. Guinea Bissau’s plant vitro susceptibility studies on Neisseria gonorrhoeae. Int J Pharm. 35(5):323–328.
- Sy GY, Fall AD, Diatta W, Gueye M, Badji K, Bassène E, Faye B. 2009. Analgesic and anti-inflammatory activity of aqueous root extract of Cassia sieberiana DC (Caesalpiniaceae). Afr J Pharm Pharmacol. 3(12):651–653.
- Tewari R, Prabhat P, Natu SM, Dalela D, Goel A, Goel MM, Tandon P. 2011. Association of benign prostatic hyperplasia (BPH) with the metabolic syndrome (MS) and its components – ‘a growing dilemma’. J Men’s Health. 8(2):66–71.
- Thu HE, Mohamed IN, Hussain Z, Jayusman PA, Shuid AN. 2017. Eurycoma longifolia as a potential adoptogen of male sexual health: a systematic review on clinical studies. Chin J Nat Med. 15(1):71–80.
- Tietz NW. 1990. Clinical Guide to Laboratory tests (ELISA). 2nd ed. Philadelphia: W. B. Saunders, Company. pp. 932–933.
- Traish AM, Morgentaler A. 2013. RE: Effect of finasteride on serum levels of androstenedione, testosterone and their 5α-reduced metabolites in men at risk for prostate cancer. J Steroid Biochem Mol Biol. 138:462–463.
- Trost L, Saitz TR, Hellstrom WJG. 2013. Side effects of 5-alpha reductase inhibitors: a comprehensive review. Sex Med Rev. 1(1):24–41.
- Veeresh Babu SV, Veeresh B, Patill AA, Warke YB. 2010. Lauric acid and myristic acid prevent testosterone induced prostatic hyperplasia in rats. Eur J Pharmacol. 626:262–265.
- Velonas VM, Woo HH, Remedios CG, Assinder SJ. 2013. Current status of biomarkers for prostate cancer. Int J Mol Sci. 14:11034–11060.
- Wallin B, Rosengren B, Shertzer HG, Camejo G. 1993. Lipoprotein oxidation and measurement of thiobarbituric acid reacting substances formation in a single microtiter plate: its use for evaluation of antioxidants. Anal Biochem. 208:10–15.
- Yano M, Yasuda K, Kitahara S, Nakajima C, Iimura Y, Yamanishi T. 2001. The reason why prostatic hyperplasia causes lower urinary track symptoms. Asian Med J. 44(2):91–96.
