Abstract
Chinese mitten crab, Eriocheir sinensis (Varunidae), is one of the most popular and widely cultivated freshwater crab species in the Chinese food industry, with high commercial importance and nutritional value. We analyzed the diversity of culturable fungi in the gut of E. sinensis collected from a rice-crab co-culture system in Tianjin, China. We isolated 41 fungal strains from the gut of analyzed male and female crabs, using the dilution plate method. Morphological and molecular identification based on nuclear ribosomal internal transcribed spacer (ITS) sequencing suggested that these isolates belonged to 16 genera in Ascomycota, Basidiomycota, and Mucoromycota. Aspergillus was the dominant identified genus followed by Penicillium and Talaromyces. Yeasts, including Candida, Clavispora, Meyerozyma, and Trichosporon genera, accounted for a significant portion (12.2%) of the isolated strains. Statistical analysis showed significant differences in gut-associated fungal communities between female and male crabs, with female individuals showing a higher species diversity. Our study represents the first report on intestinal fungal communities of Chinese mitten crab, providing valuable microbiological information that could be essential for supporting the effective management and conservation of this crab species, and for the improvement of the economic performance of the crab industry.
Introduction
Rice-aquatic animal co-culture systems, which combine animal production (e.g. fish, shellfish, crab, shrimp, or duck) with rice plantation, are a sustainable strategy to fulfill the increasing demand for rice yield and aquaculture (Bashir et al. Citation2020). As a novel rice-aquatic animal system, the rice-crab co-culture system has been vigorously promoted by the Chinese government. In this co-culture system, rice and Chinese mitten crab (Eriocheir sinensis) are in a beneficial symbiotic relationship. Rice fields provide a suitable environment for crab growth, with sufficient water level, good light conditions, and enough dissolved oxygen (Zhang et al. Citation2017), while the foraging activities of crabs can reduce weed emergence, avoid pest propagation, and improve soil fertility in rice fields (Fernando Citation1993; Xu et al. Citation2014). Compared to traditional rice monoculture, this efficient rice-aquatic animal co-culture model is characterized by complementary resource use, reduction of chemical fertilizers and pesticides, improvement of rice yield and biodiversity enhancement (Halwart Citation2008; Xu et al. Citation2014; Hu et al. Citation2016).
Chinese mitten crab, Eriocheir sinensis H. Milne-Edwards, is a crustacean belonging to the family Varunidae, order Decapoda. It is one of the most popular freshwater crab species in the Chinese food industry, with high commercial importance and nutritional value, widely cultivated in the Liaoning, Jiangsu, and Anhui provinces (Sui et al. Citation2009; Ding et al. Citation2016). As a crucial component of the Chinese freshwater aquaculture industry, the annual output of Chinese mitten crab has increased rapidly from 200,000 tons in 2000 to over 756,000 tons in 2018 (Yuan Citation2005; People's Republic of China Ministry of Agriculture Citation2019). However, frequent outbreaks of Hepatopancreatic Necrosis Disease (HPND) with a high mortality rate (40–50%) have resulted in a significant loss in production and economic value of Chinese mitten crab. HPND is an infection within the cytoplasm of the epithelial cells of the hepatopancreas by microsporidia, which were considered Protozoa for a long time. Microsporidia were reclassified by Cavalier-Smith (Citation1998) into the kingdom Fungi in 1998, and described as Cellular Organisms, Eukaryote, Fungi/Metazoa group, Fungi, Microsporidia in 2002 by NCBI, although there is no universal agreement on their classification yet (Dong et al. Citation2010). Crabs with HPND exhibit multiple clinical symptoms, including colour change of the hepatopancreas (from golden yellow to almost white), soft shells that are darker than usual, loss of appetite, slow action, muscle atrophy and edema (Shen et al. Citation2017). After the stomachs and intestines become empty, diseased crabs eventually die (Ding et al. Citation2016). In spite of the crucial influence of microbes in Chinese mitten crab health, there is a lack of knowledge on microbial diversity associated with E. sinensis, especially at gut level. The gut microbiome is a diverse and complex ecosystem consisting of bacteria, archaea, viruses, fungi, protists, and (sometimes) helminths (Enaud et al. Citation2018). The gut-associated microbiome plays a crucial role in the host health by supplying essential nutrients, maintaining normal gut functions (Zhang et al. Citation2015), modulating immune response, and contributing to host defense against pathogens (Enaud et al. Citation2018). Recently, the intestine microbial diversity has been studied in a wide range of animals, particularly in mammals (Hermann-Bank et al. Citation2013). Crustaceans, which have received the highest attention among aquatic invertebrates, have been found to harbor various genera of bacteria including Vibrio, Pseudomonas, Flavobacterium, Micrococcus, and Aeromonas in their gut (Harris Citation1993). In addition, the presence of intestinal fungi in aquatic crustaceans, such as shrimps, has been reported. For instance, the intestinal mycobiota of Litopenaeus vannamei (Pacific white shrimp) was dominated by the genera Alternaria, Tuber, Hortaea, Sarocladium, and Stagonospora (Li et al. Citation2019). Nevertheless, up to now, studies of microbial communities in crab gut have mainly focused on bacteria. Four bacterial phyla, Proteobacteria, Firmicutes, Tenericutes, and Bacteroidetes, have been found to be dominant in the gut of mud crab (Scylla paramamosain) and E. sinensis (Li et al. Citation2012; Chen et al. Citation2015). Firmicutes and Tenericutes have been described as the most abundant phyla in the foregut, Tenericutes and Proteobacteria in the midgut, and Proteobacteria, Tenericutes, and Bacteroidetes in the hindgut of E. sinensis (Dong et al. Citation2018). Only a few studies have focused on the composition of fungal communities in crab species. Fonsecaea brasiliensis and Exophiala cancerae, two black yeast-like fungal species, were identified in the mangrove-land crab (Ucides cordatus) infected with Lethargic Crab Disease (LCD) (Vicente et al. Citation2012). Thirteen fungal species have been found from the vent crab Xenograpsus testudinatus including Aspergillus terreus, Hortaea werneckii, Parengyodontium album, and Peroneutypa scoparia, using both culture-based and metabarcoding methods (Pang et al. Citation2019).
Thus far, gut bacteria have been the most common object of gut microbiome studies due to the abundant presence of bacteria in the gut (Chin et al. Citation2020), whereas little attention has been given to gut fungi because of their comparatively much lower presence (Chin et al. Citation2020). While gut bacterial communities of E. sinensis have been previously studied, there is no information related to the gut fungal diversity of Chinese mitten crab. In this study, we provided the first assessment of culturable fungal diversity associated with the gut of E. sinensis growing in a rice-crab co-culture area in Tianjin, China, in order to increase our knowledge of gut microbial communities in this economically important crab species and provide useful information for the management of E. sinensis aquaculture.
Materials and methods
Study site and sampling
This study was conducted in a rice-crab co-culture farm located in Qilihai Town, Ninghe District, Tianjin, China (39°19′19″ N, 117°39′44″ E). The investigated farm included two rice paddy fields that were connected with two adjacent ponds by means of canals located along two opposite sides of the fields. Three female and three male Chinese mitten crab (Eriocheir sinensis) individuals, with a weight of approximately 100 g each, were captured alive in the studied environment. All the sampled individuals appeared healthy. The freshly collected crabs, labeled by sex (F and M) and individual (1–3), were placed in sterilized bags and kept on ice in sampling box. They were immediately transported to the laboratory where they were put at 4°C to slow their activity and induce anesthesia within 15–20 min.
Isolation of crab gut-associated fungi
The collected crab individuals were dissected and their intestines were transferred into 15 ml sterilized centrifuge tubes (Eppendorf, Germany) with 3 ml sterilized water. The tubes were shaken thoroughly on an Analog Vortex Mixer (OHAUS, USA). About 100 μl dilutions from each sample were spread onto Petri dishes with Potato Dextrose Agar (PDA; Solarbio, China) supplemented with Streptomycin Sulfate (100 mg/l) to inhibit bacterial growth. The isolation procedure was repeated three times for each diluted sample. All the plates (9 cm diameter) were incubated at 25°C in darkness and monitored daily to assess the growth of fungal hyphae. Colonies appearing on the agar medium were picked up and isolated onto new PDA plates every two days to obtain pure cultures. All isolated fungal strains were stored at 4°C for further analysis. All strains were deposited in the LP Culture Collection (personal culture collection held in the laboratory of Prof. Lorenzo Pecoraro) at the School of Pharmaceutical Science and Technology, Tianjin University, Tianjin, China.
Molecular and morphological identification of isolated fungi
Total genomic DNA was extracted from fresh fungal mycelia isolated from the gut of Eriocheir sinensis using a modified cetyltrimethyl ammonium bromide (CTAB) method (Doyle Citation1987). The fungal nuclear ribosomal internal transcribed spacer (ITS) region of the rDNA gene were amplified by polymerase chain reaction using the universal primers ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) (White et al. Citation1990). The PCR reactions were performed in 50 μl volume containing 25 μl 2× Rapid Taq Master Mix (Vanzyme, China), 2 μl of each forward and reverse primer (10 mM), 2 μl of template DNA, and 19 μl of double-distilled water. The amplification program consisted of an initial denaturation at 95°C for 3 min, followed by 35 cycles of 95°C for 15 s, 55°C for 15 s, 72°C for 15 s and a final elongation step at 72°C for 5 min using a Mastercycler® thermal cycler (Eppendorf, Germany). PCR products, along with a reference 2000 bp ladder marker (TsingKe, China), were analyzed by gel electrophoresis in 1% agarose gels stained with 3× Gel Red (TsingKe, China) and visualized under Gel imager (TianNeng, China). Sequencing of the PCR products was performed by Tsingke Biological Technology Company (Beijing, China) using the same PCR primers. All obtained sequences were submitted to the National Center for Biotechnology Information (NCBI) for a nucleotide BLAST search (GenBank; http://www.ncbi.nlm.nih.gov/BLAST/index.html) to determine the closest sequence matches that enabled taxonomic identification. DNA sequences from the fungal isolates were deposited in GenBank under the accession numbers MW958029–MW958069. Fungal morphology (branched septate hyphae, pseudohyphae, conidiophores, conidia, poroconidia, arthroconidia and sporangiosphores, etc.) was examined under a Nikon ECLIPSE Ci microscope for identification of isolates following the taxonomic keys for different taxa (Gilman Citation1957; Alexopoulos and Mims Citation1979).
Statistical analysis
Krona Charts illustrating the composition of fungal communities identified from gut of Chinese mitten crab were prepared using Krona Tools (https://github.com/marbl/Krona/wiki/KronaTools) (Ondov et al. Citation2011). In order to quantify differences in the gut fungal communities between female and male crabs, alpha diversity indexes were calculated in Multi-Variate v. 3.22 (MVSP 3.22). The fungal diversity was evaluated using Shannon index (H′), which has two main components: evenness and the number of species (Spellerberg and Fedor Citation2003). The Simpson index estimates the probability that two randomly selected fungal individuals from a community belong to different species (Simpson Citation1949). The Margalef diversity index (Margalef Citation1957) and Pielou index (Pielou Citation1966) were used to assess the species richness and evenness, respectively.
Results
Culturable fungal diversity associated with Eriocheir sinensis gut
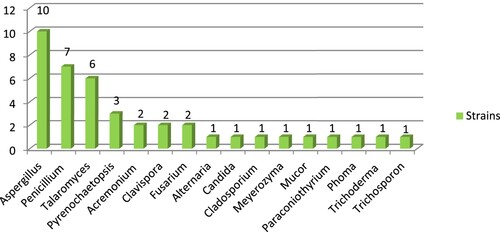
A total of 41 strains were isolated from 6 gut samples of Chinese mitten crab growing naturally in the investigated rice-crab co-culture system. The isolated strains belonged to 3 fungal phyla, 6 classes, 7 orders, 15 families, 16 genera, and 30 species as assessed through BLAST searches and morphological identification (Figure ). Among the identified species, 95.12% (28 species and 39 strains) belonged to 14 genera of Ascomycota, while Basidiomycota and Mucoromycota were represented by a single stain each (2.4%) of Trichosporon coremiiforme and Mucor circinelloides, respectively (Table ). Aspergillus was the dominant genus with 10 strains followed by Penicillium (7 strains), Talaromyces (6 strains), Pyrenochaetopsis (3 strains), Acremonium (2 strains), Clavispora (2 strains), and Fusarium (2 strains), while Alternaria, Candida, Cladosporium, Meyerozyma, Mucor, Paraconiothyrium, Phoma, Trichoderma, and Trichosporon were represented by a single strain each (Figure ). The most common species were Aspergillus niger (6 strains) and Talaromyces verruculosus (4 strains), followed by A. tubingensis, T. funiculosus, and Clavispora lusitaniae, which yielded 2 strains each (Table ).
Figure 1. Krona chart indicating the taxonomic identification and relative abundance of fungi isolated from gut samples of Chinese mitten crab.
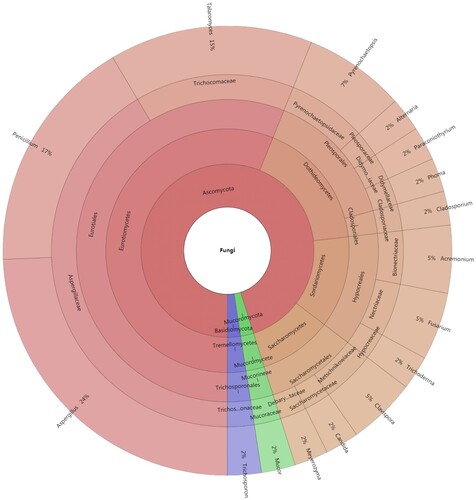
Table 1. Fungal diversity molecularly detected in the gut of Chinese mitten crab.
Comparison of gut fungal communities in female and male crabs
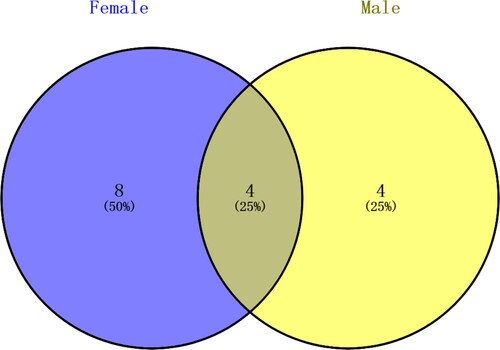
There were significant differences in gut fungal diversity between female and male Chinese mitten crabs. The 22 strains isolated from the gut of male crabs were found to represent 13 species and 8 genera of Ascomycota, whereas the 19 strains isolated from the gut of female crabs belonged to 16 species and 10 genera of Ascomycota (17 strains), 1 species of Mucoromycota (1 strain) and 1 species of Basidiomycota (1 strain) (Figure ). Female gut samples showed a higher genus richness than male: 12 genera from female and 8 genera from male crabs. Four genera including Aspergillus, Fusarium, Penicillium and Pyrenochaetopsis were found in common for both female and male crab individuals (Figure ). The dominant fungi were different in the two analyzed groups of samples, with Aspergillus being the most abundant genus in male gut, with 8 strains, and Penicillium in female gut, with 4 strains. The four genera Candida, Cladosporium, Phoma, and Talaromyces were only present in male crabs, while 8 genera (Acremonium, Alternaria, Clavispora, Meyerozyma, Mucor, Paraconiothyrium, Trichoderma, and Trichosporon) were exclusively found in female crabs. The Shannon (H′) and Simpson (D) indexes for fungal diversity from female crabs were 2.871 and 0.005847, respectively, whereas for male crabs the two measured indexes were 2.347 and 0.07792, respectively. The Shannon index of female crab gut was higher than male, while in contrast, the Simpson index of female crab gut was lower than males. Pielou index and Margalef index of female samples (0.9934 and 5.774, respectively) was significantly higher than that of male samples (0.9150 and 3.882, respectively) (Table ). The results indicated that the taxonomic diversity of the fungal community in the gut of the female crab was higher than in males.
Figure 3. Taxonomic profiles of gut fungal communities at different taxonomic levels. (a) phylum; (b) class; (c) family; and (d) genus.
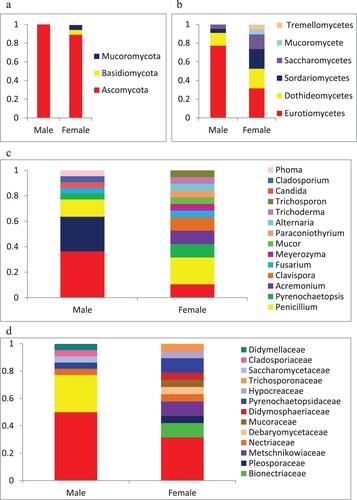
Table 2. Indexes of alpha diversity of fungal communities associated with the gut of Chinese mitten crab.
Discussion
This is the first study exploring the fungal community in the gut of Chinese mitten crab E. sinensis using molecular and morphological identification of isolated strains. The analysis of gut microbial diversity could be essential for understanding the ecological functions of microbes in E. sinensis, and their influence on crab health, given that the interactions between gut microorganisms and hosts have been found to be an important factor influencing aquatic animal health (Chaiyapechara et al. Citation2012; Yan et al. Citation2012; Dong et al. Citation2018). The results obtained in this study indicated the presence of various filamentous fungi and yeasts inhabiting the gut of E. sinensis. These results contribute to filling the gap in our knowledge of gut microorganisms in the studied crab species from a mycological point of view and complement previous information on bacterial diversity associated with E. sinensis, which revealed the dominant presence of the phyla Proteobacteria, Firmicutes, Tenericutes, and Bacteroidetes (Chen et al. Citation2015).
Ascomycota (95.12%), Basidiomycota (2.4%), and Mucoromycota (2.4%) were identified from the intestine of E. sinensis using ITS sequencing. Ascomycota and Basidiomycota have been frequently isolated from various crustaceans, including Xenograpsus testudinatus (shallow water hydrothermal vent crab), Litopenaeus vannamei (Pacific white shrimp), and Cherax quadricarinatus (red claw crayfish) (Li et al. Citation2019; Pang et al. Citation2019; Victor and Pahirulzaman Citation2020). On the contrary, the presence of Mucoromycota represents a peculiar aspect of the Chinese mitten crab gut mycobiota, since previous records of zygomycetous fungi in animals gut have been mainly from mammals, including human and panda (Hamad et al. Citation2012; Yang et al. Citation2018). The sole previous study reporting on the presence of Mucoromycota in the gut of crabs was performed by Pitts and Cowley (Citation1974) on fiddler crab (Uca pugilator) individuals collected from South Carolina. However, in the latter study, Mucor sp. was only found in the midgut of a group of crabs that was maintained in a covered pan at 20°C for 87 days, supplied with sea water, and fed pellets of Purina rabbit chow (Pitts and Cowley Citation1974). It seems likely that the presence of Mucor sp. in this group of Uca pugilator crabs was induced by the experimental procedure used by the authors, given that this fungal taxon was not found in the other two groups of crab individuals that were directly analyzed, without maintenance period, within 3 h after collection (Pitts and Cowley Citation1974).
We found a total of 16 fungal genera colonizing the gut of the studied crab species (Table ). Among them, Aspergillus, Candida, Cladosporium, Fusarium, Penicillium, Phoma, and Trichosporon have been previously isolated from aquatic micro- and macroinvertebrates, and vertebrates from freshwater, estuarine, and marine environments (Rand Citation2004). Aspergillus, a taxon including ubiquitous saprotrophic fungi that play an important role in recycling carbon and nitrogen on Earth (Fang and Latgé Citation2018) was found to have the highest relative abundance (24.39%) among the fungal genera recorded in E. sinensis. The dominant Aspergillus species was A. niger, which was isolated from both female and male gut samples, while A. awamori, A. flavus, and A. tubingensis, were only isolated from male individuals. Previous studies have demonstrated that Aspergillus species are essential components of crab fungal communities. For instance, Aspergillus has been described as the dominant genus retrieved from the crushed dilutions of the vent crab Xenograpsus testudinatus (Pang et al. Citation2019). More specifically, A. niger has been previously isolated from midgut of the fiddler crab (Uca pugilator) (Pitts and Cowley Citation1974), whereas A. flavus has been found from dried powder of fresh water crab (Sesarma boulengeri) living in the Shatt-Al-Arab River in southern Iraq (Al-Duboon and Farhan Citation2007). Although the presence of Aspergillus fungi has been documented in several crab species, the role of these fungi in the crab host is still unclear and further physiological studies are needed to understand the real function of this fungus-crab association. Phoma, a ubiquitous fungal genus reported in various natural habitats, including aquatic environments, water distribution systems, soil and air (Bennett et al. Citation2018), was only recorded once from the gut of the male crab (M3) in the present study. This fungal taxon has been previously described as the dominant genus isolated from cuticle of the invasive crayfish Procambarus clarkii (Dörr et al. Citation2012). In addition, Phoma has been isolated from the exoskeleton of snow crab (Chionoecetes bairdi) collected in the USA by Hyning and Scarborough (Citation1973) hypothesis.
According to our BLAST results, yeasts, represented by Candida intermedia, Clavispora lusitaniae, Meyerozyma caribbica, and Trichosporon coremiiforme, accounted for a high portion (12.2%) of the 41 isolated plates. Trichosporon, a genus of anamorphic basidiomycetous yeasts, is widely distributed in rotting wood, sludge, soil, plants, leaf-cutting ants, birds, and mammals (Obana et al. Citation2010). Trichosporon species, represented by T. caseorum, T. asahii, and T. cutaneum, have been found in gastrointestinal microbiota from a healthy African man using culture-independent molecular identification methods based on various primer sets (Hamad et al. Citation2012), whereas T. coremiiforme has never been described as a gut-associated fungus. Meyerozyma caribbica (anamorph Candida fermentati) recorded with a single strain in this study has been previously isolated from the gut of four different Diptera species, namely Drosophila suzukii, Culex quinquefasciatus, Anopheles stephensi, and Aedes aegypti (De Marco et al. Citation2018). Two strains isolated from two E. sinensis female individuals (F1 and F2) were molecularly found to share a high similarity with Clavispora lusitaniae (Synonymy: Candida lusitaniae) previously isolated from the gastrointestinal tracts of warm-blooded animals (Lodder Citation1970). The Candida strain isolated from male individual M2 highly matched (>99% similarity) with Candida intermedia (accession number DQ665263.1), which was isolated from the marine environment in China by Gao et al. in an unpublished study. Candida species have been frequently documented to colonize the gut of numerous different hosts. For instance, Candida species, such as C. albicans, have been often detected in human stool (Truss Citation1981), and sometimes associated with diseases, such as diabetes and inflammatory bowel disease (IBD) (Sonoyama et al. Citation2011; Gosiewski et al. Citation2014). Other studies have demonstrated that species of the genus Candida could colonize the gut of birds (Larus michahellis) and insects (Dendroctonus adjunctus, D. approximatus, D. pseudotsugae, and Megaplatypus mutatus) (Rivera et al. Citation2009; Al-Yasiri et al. Citation2016; Ceriani-Nakamurakare et al. Citation2020). To our knowledge, there is no previous information about the presence of Candida intermedia in crab intestine. Our culture-dependent approach to assess the gut microbial diversity in E. sinensis showed the presence of a rich variety of yeasts in the studied crab species gut. Further studies based on metabarcoding culture-independent analyses are needed to provide a comprehensive description of the intestinal yeasts associated with E. sinensis, in particular for those species that are difficult or impossible to isolate in axenic culture.
With the prevalence of foodborne diseases, close attention has been paid to the food safety problems originated from microbial infections since 1992 in China (Liu et al. Citation2004; Xu and Zhang Citation2012). A total of 5021 foodborne incidents were reported in China during 2001–2012, where microbial infections were the most common cause, accounting for 40.9% of total outbreaks (Xu and Zhang Citation2012). It has been reported that 260,000 people get sick from contaminated fish every year in the United States (Barrett et al. Citation2017). In China, freshwater food production and consumption have increased rapidly during the past few years, and the yield of freshwater products in 2018 was over 3,1562,000 tons (Republic of China Ministry of Agriculture 2019). However, little is known about the fungal pathogens harbored by freshwater food in China. Our results showed that E. sinensis harbored a variety of human fungal pathogens. Among them, species from the genera Aspergillus and Candida are primarily responsible for the lethal fungal infections (Li et al. Citation2019). White piedra is defined as the superficial infection of hair shafts caused by Trichosporon (Erer et al. Citation2000). Trichoderma species, comprising T. harzianum, T. koningii, T. longibrachiatum, T. pseudokoningii, and T. viride, have been regarded as important human pathogenic fungi (Loeppky et al. Citation1983; Ragnaud et al. Citation1984; Tanis et al. Citation1995; Guiserix et al. Citation1996; Rota et al. Citation2000). People who wear contact lenses are likely to suffer from keratomycosis caused by Acremonium species (Fincher et al. Citation1991). Fusarium has emerged as a pathogenic factor of disseminated infection in patients who are undertaking intensive chemotherapy or bone marrow transplantation (Anaissie et al. Citation1988). Some species of the genus Penicillium are known to cause pneumonia and disseminated disease in cancer patients (Anaissie et al. Citation1989). A variety of fungi previously recognized as plant pathogen such as Fusarium (Burdon and Silk Citation1997) have been recently found to represent potential pathogens for humans (Fleming et al. Citation2002). Therefore, a more comprehensive risk assessment for fungal pathogens derived from Chinese mitten crab is required to effectively protect public health.
It has been shown that human males and females have gender-specific differences in their gut microbial composition (Haro et al. Citation2016). Additionally, a study conducted on the diversity of hand surface bacteria showed that women had significantly higher diversity than men (Fierer et al. Citation2008). In our study, we hypothesized that fungal communities associated with the gut of males and females were different. The results confirmed our hypothesis, showing that there were statistically significant differences in fungal species diversity between analyzed female and male crab individuals. The Shannon and Simpson indexes were 2.871 and 0.005847, respectively, for gut from female crabs, while the two calculated values were 2.347 and 0.07792 for male crabs. This result revealed that the fungal population observed in E. sinensis female individuals was more diverse and species rich than that in males. A previous study investigating the gut mycobiota of a cohort of healthy human subjects revealed that female subjects possessed a higher number of fungal isolates and species compared to male subjects (Strati et al. Citation2016 ). It has been described that sex hormones may play a key role in modulating microbiota composition in mice (Markle et al. Citation2013). Moreover, sex-diet interaction may also influence the composition of vertebrate microbiota (Bolnick et al. Citation2014). Our findings showed, for the first time, gender-related differences in the Chinese mitten crab gut fungi. Due to the limited knowledge on sex-dependent microbiota in crabs, it is difficult to assess the mechanisms that trigger this special phenomenon. Further investigations are necessary to understand which host sexual differences influence the gut fungal diversity in Chinese mitten crabs and how.
Conclusion
Our study represents the first report on intestinal fungal communities of Chinese mitten crab. We observed that the gut of E. sinensis harbored a considerable number of filamentous fungi in combination with ascomycetous and basidiomycetous yeasts. Significant difference of intestinal fungal diversity was found between female and male crabs, with female individuals showing a higher number of fungal species. Our findings provide valuable information on microbes associated with one of the most popular fresh water crab species in the Chinese food industry, and represent a starting point in the analysis of Chinese mitten crab microbiology to support the effective management and conservation of this crab species. Knowledge of gut-associated microbial communities is essential for the improvement of economic performance of the crab industry. Further studies are necessary to describe the interaction mechanisms between intestinal fungal communities and Chinese mitten crab hosts, and to understand the exact role that gut-associated microbes play in the analyzed crab species health.
Data availability statement
The Fungal DNA sequences amplified during this study are available in GenBank (https://blast.ncbi.nlm.nih.gov/Blast.cgi) under accessions MW958029–MW958069.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Al-Duboon AH, Farhan FJ. 2007. Fungi associated with the crab Sesarma boulengeri Calman from Shatt-AI-Arab river, Basrah-Iraq. Marina Mesopotamica. 22:71–80.
- Al-Yasiri MH, Normand AC, Piarroux R, Ranque S, Mauffrey JF. 2016. Gut yeast communities in Larus michahellis from various breeding colonies. Med Mycol. 55:436–444.
- Alexopoulos CJ, Mims CW. 1979. Introductory mycology, 3rd ed. New York: John Wiley & Sons.
- Anaissie E., Bodey G. P., Kantarjian H., Ro J., Vartivarian S. E., Hopfer R., Hoy J., Rolston K. 1989. New spectrum of fungal infections in patients with cancer. Clin Infect Dis. 11:369–378.
- Anaissie E, Kantarjian H, Ro J, Hopfer R, Rolston K, Fainstein V, Bodey G. 1988. The emerging role of Fusarium infections in patients with cancer. Medicine (Baltimore). 67:77–83.
- Barrett KA, Nakao JH, Taylor EV, Eggers C, Gould LH. 2017. Fish-associated foodborne disease outbreaks: United States, 1998–2015. Foodborne Pathog Dis 14:537–543.
- Bashir MA, Liu J, Geng Y, Wang H, Pan J, Zhang D, Rehim A, Aon M, Liu H. 2020. Co-culture of rice and aquatic animals: an integrated system to achieve production and environmental sustainability. J Clean Prod. 249:119310.
- Bennett A, Ponder MM, Garcia-Diaz J. 2018. Phoma infections: classification, potential food sources, and their clinical impact. Microorganisms. 6:58–69.
- Bolnick DI, Snowberg LK, Hirsch PE, Lauber CL, Org E, Parks B, Lusis AJ, Knight R, Caporaso JG, Svanbäck R. 2014. Individual diet has sex-dependent effects on vertebrate gut microbiota. Nat Commun. 5:4500.
- Burdon JJ, Silk J. 1997. Sources and patterns of diversity in plant-pathogenic fungi. Phytopathology. 87:664–669.
- Cavalier-Smith T. 1998. A revised six-kingdom system of life. Biol Rev. 73:203–266.
- Ceriani-Nakamurakare E, Mc Cargo P, Gonzalez-Audino P, Ramos S, Carmarán C. 2020. New insights into fungal diversity associated with Megaplatypus mutatus: gut mycobiota. Symbiosis. 81:127–137.
- Chaiyapechara S, Rungrassamee W, Suriyachay I, Kuncharin Y, Klanchui A, Karoonuthaisiri N, Jiravanichpaisal P. 2012. Bacterial community associated with the intestinal tract of P. monodon in commercial farms. Microb Ecol. 63:938–953.
- Chen X, Di P, Wang H, Li B, Pan Y, Yan S, Wang Y. 2015. Bacterial community associated with the intestinal tract of Chinese mitten crab (Eriocheir sinensis) farmed in Lake Tai, China. PLoS One. 10:e0123990.
- Chin VK, Yong VC, Chong P, Nordin SA, Abdullah M. 2020. Mycobiome in the gut: a multiperspective review. Mediat Inflamm. 2020:1–16.
- De Marco L, Epis S, Capone A, Martin E, Bozic J, Crotti E, Ricci I, Sassera D. 2018. The genomes of four Meyerozyma caribbica isolates and novel insights into the Meyerozyma guilliermondii species complex. G3 Genes|Genomes|Genetics. 8:755–759.
- Ding Z, Meng Q, Liu H, Yuan S, Zhang F, Sun M, Zhao Y, Shen M, Zhou G, Pan J, Xue H, Wang W 2016. First case of hepatopancreatic necrosis disease in pond reared Chinese mitten crab, Eriocheir sinensis, associated with microsporidian. J Fish Dis. 39:1043–1051.
- Dong J, Li X, Zhang R, Zhao Y, Wu G, Liu J, Zhu X, Li L. 2018. Comparative analysis of the intestinal bacterial community and expression of gut immunity genes in the Chinese mitten crab (Eriocheir sinensis). AMB Express. 8:192.
- Dong S, Shen Z, Xu L, Zhu F. 2010. Sequence and phylogenetic analysis of SSU rRNA gene of five microsporidia. Curr Microbiol. 60:30–37.
- Dörr AJM, Rodolfi M, Elia AC, Scalici M, Garzoli L, Picco AM. 2012. Mycoflora on the cuticle of the invasive crayfish Procambarus clarkii. Fundam Appl Limnol. 180:77–84.
- Doyle J. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 19:11–15.
- Enaud R, Vandenborght LE, Coron N, Bazin T, Prevel R, Schaeverbeke T, Berger P, Fayon M, Lamireau T, Delhaes L. 2018. The mycobiome: A neglected component in the microbiota-gut-brain axis. Microorganisms. 6:22–34.
- Erer B, Galimberti M, Lucarelli G, Giardini C, Polchi P, Baronciani D, Gaziev D, Angelucci E, Izzi G 2000. Trichosporon beigelii: A life-threatening pathogen in immunocompromised hosts. Bone Marrow Transplant. 25:745–749.
- Fang W, Latgé JP. 2018. Microbe profile: Aspergillus fumigatus: a saprotrophic and opportunistic fungal pathogen. Microbiology. 164:1009–1011.
- Fernando CH. 1993. Rice field ecology and fish culture - an overview. Hydrobiologia. 259:91–113.
- Fierer N, Hamady M, Lauber CL, Knight R. 2008. The influence of sex, handedness, and washing on the diversity of hand surface bacteria. Proc Natl Acad Sci USA. 105:17994–17999.
- Fincher R-ME, Fisher JF, Lovell RD, Newman CL, Espinel-Ingroff A, Shadomy HJ. 1991. Infection due to the fungus Acremonium (cephalosporium). Medicine (Baltimore). 70:398–409.
- Fleming RV, Walsh TJ, Anaissie EJ. 2002. Emerging and less common fungal pathogens. Infect Dis Clin North Am. 16:915–933.
- Gilman JC. 1957. A manual of soil fungi. Ames: The Iowa State University Press.
- Gosiewski T, Salamon D, Szopa M, Sroka A, Malecki MT, Bulanda M. 2014. Quantitative evaluation of fungi of the genus Candida in the feces of adult patients with type 1 and 2 diabetes – a pilot study. Gut Pathog. 6:43–47.
- Guiserix JE, Ramdane M, Finielz P, Michault A, Rajaonarivelo P. 1996. Trichoderma harzianum peritonitis in peritoneal dialysis. Nephron. 74:473–474.
- Halwart M. 2008. Biodiversity nutrition and livelihoods in aquatic rice-based ecosystems. Biodiversity. 9:36–40.
- Hamad I, Sokhna C, Raoult D, Bittar F, Badger JH. 2012. Molecular detection of eukaryotes in a single human stool sample from Senegal. PLoS One. 7:e40888.
- Haro C, Rangel-Zúñiga OA, Alcalá-Díaz JF, Gómez-Delgado F, Pérez-Martínez P, Delgado-Lista J, Quintana-Navarro GM, Landa BB, Navas-Cortés JA, Tena-Sempere M, et al. 2016. Intestinal microbiota is influenced by gender and body mass index. PLoS One. 11:e0154090.
- Harris JM. 1993. The presence, nature, and role of gut microflora in aquatic invertebrates: a synthesis. Microb Ecol. 25:195–231.
- Hermann-Bank ML, Skovgaard K, Stockmarr A, Larsen N, Mølbak L. 2013. The Gut microbiotassay: a high-throughput qPCR approach combinable with next generation sequencing to study gut microbial diversity. BMC Genom. 14:788.
- Hu L, Zhang J, Ren W, Guo L, Cheng Y, Li J, Li K, Zhu Z, Zhang J, Luo S, et al. 2016. Can the co-cultivation of rice and fish help sustain rice production? Sci Rep. 6:28728.
- Hyning J, Scarborough AM. 1973. Identification of fungal encrustation on the shell of the Snow crab (Chionoecetes bairdi). J Fish Res Board Can. 30:1738–1739.
- Li J, Jiang H, Li L, Zhang X, Chen J. 2019. The effect of disease and season to hepatopancreas and intestinal mycobiota of Litopenaeus vannamei. Front Microbiol. 10:889.
- Li S, Sun L, Wu H, Hu Z, Liu W, Li Y, Wen X. 2012. The intestinal microbial diversity in mud crab (Scylla paramamosain) as determined by PCR-DGGE and clone library analysis. Appl Microbiol. 113:1341–1351.
- Liu X, Yan C, Wang X, Rong J. 2004. Foodborne disease outbreaks in China from 1992 to 2001 national foodborne disease surveillance system. J Hygiene Res. 33:725–727.
- Lodder J. 1970. The yeast, a taxonomic study, 2nd ed. Amsterdam: North-Holland Publishing Co.
- Loeppky CB, Sprouse RF, Carlson JV, Everett ED. 1983. Trichoderma viride peritonitis. South Med J. 76:798–799.
- Margalef R. 1957. Information theory in ecology. Gen Syst. 3:36–71.
- Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, von Bergen M, McCoy KD, Macpherson AJ, Danska JS. 2013. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 339:1084–1088.
- Obana Y, Sano M, Jike T, Homma T, Nemoto N. 2010. Differential diagnosis of trichosporonosis using conventional histopathological stains and electron microscopy. Histopathology. 56:372–383.
- Ondov BD, Bergman NH, Phillippy AM. 2011. Interactive metagenomic visualization in a web browser. BMC Bioinform. 12:385.
- Pang KL, Guo SY, Chen IA, Burgaud G, Luo ZH, Dahms HU, Hwang JS, Lin YL, Huang JS, Ho TW, et al. 2019. Insights into fungal diversity of a shallow-water hydrothermal vent field at Kueishan Island, Taiwan by culture-based and metabarcoding analyses. PLoS One. 14:e0226616.
- People's Republic of China Ministry of Agriculture. 2019. China fishery statistical yearbook 2019. Beijing: China Agriculture Press.
- Pielou EC. 1966. Species-diversity and pattern-diversity in the study of ecological succession. J Theor Biol. 10:370–383.
- Pitts GY, Cowley GT. 1974. Mycoflora of the habitat and midgut of the fiddler crab, Uca pugilator. Mycologia. 66:669–675.
- Ragnaud JM, Marceau C, Roche-Bezian MC, Wone C. 1984. Infection péritonéale à Trichoderma koningii sur dialyse péritonéale continue ambulatoire. Med Mal Infect. 14:402–405.
- Rand TG. 2004. Fungi associated with aquatic animals. In: Mueller GM, Bills GF, Foster MS, editor. Biodiversity of fungi: inventory and monitoring methods. Burlington: Elsevier Academic Press; p. 577–586.
- Rivera FN, González E, Gómez Z, López N, Hernández-Rodríguez C, Berkov A, Zúñiga G. 2009. Gut-associated yeast in bark beetles of the genus Dendroctonus erichson (Coleoptera: Curculionidae: scolytinae). Biol J Linn Soc. 98:325–342.
- Rota S, Marchesi D, Farina C, de Bièvre C. 2000. Trichoderma pseudokoningii peritonitis in automated peritoneal dialysis patient successfully treated by early catheter removal. Perit Dial Int. 20:91–93.
- Shen H, Zang Y, Song K, Ma Y, Dai T, Serwadda A. 2017. A meta-transcriptomics survey reveals changes in the microbiota of the Chinese Mitten Crab Eriocheir sinensis infected with hepatopancreatic necrosis disease. Front Microbiol. 8:732.
- Simpson H. 1949. Measurement of diversity. Nature. 163:688–688.
- Sonoyama Kei, Miki Atsuko, Sugita Ryusuke, Goto Haruka, Nakata Mayumi, Yamaguchi Natsu 2011. Gut colonization by Candida albicans aggravates inflammation in the gut and extra-gut tissues in mice. Med Mycol. 49:237–247.
- Spellerberg IF, Fedor PJ. 2003. A tribute to Claude Shannon (1916–2001) and a plea for more rigorous use of species richness, species diversity and the ‘Shannon–Wiener’ index. Glob Ecol Biogeogr. 12:177–179.
- Strati F, Di Paola M, Stefanini I, Albanese D, Rizzetto L, Lionetti P, Calabrò A, Jousson O, Donati C, Cavalieri D, De Filippo C. 2016. Age and gender affect the composition of fungal population of the human gastrointestinal tract. Front Microbiol. 7:1227.
- Sui LY, Wu XG, Wille M, Cheng YX, Sorgeloos P. 2009. Effect of dietary soybean lecithin on reproductive performance of Chinese mitten crab Eriocheir sinensis (H. Milne-Edwards) Broodstock. Aquac Int. 17:45–56.
- Tanis BC, van der Pijl H, van Ogtrop ML, Kibbelaar RE, Chang PC. 1995. Fatal fungal peritonitis by Trichoderma longibrachiatum complicating peritoneal dialysis. Nephrol Dial Transplant. 10:114–116.
- Truss CO. 1981. The role of Candida albicans in human illness. J Orthomol Med. 10:228–238.
- Vicente VA, Orélis-Ribeiro R, Najafzadeh MJ, Sun J, Guerra RS, Miesch S, Ostrensky A, Meis JF, Klaassen CH, de Hoog GS, Boeger WA. 2012. Black yeast-like fungi associated with lethargic crab disease (LCD) in the mangrove-land crab, Ucides cordatus (Ocypodidae). Vet Microbiol. 158:109–122.
- Victor SS, Pahirulzaman KAK. 2020. Molecular identification of fungi isolated from infected redclaw crayfish, Cherax quadricarinatus. IOP Conf Ser Earth Environ Sci. 596:012092.
- White TJ, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editor. PCR protocols: A guide to methods and applications. San Diego: Academic Press; p. 315–322.
- Xu M, Ma XZ, Wang W. 2014. Effects of different cultivation patterns on rice yield and crab in rice-crab culture system. Scientia Agricultura Sinica. 47:1828–1835.
- Xu JF, Zhang JZ. 2012. Analysis of foodborne disease outbreaks in China from 2001 to 2010. Chin Agric Sci Bull. 28:313–316.
- Yan Q, van der Gast CJ, Yu Y, Bereswill S. 2012. Bacterial community assembly and turnover within the intestines of developing zebrafish. PLoS One. 7:e30603.
- Yang S, Gao X, Meng J, Zhang A, Zhou Y, Long M, Li B, Deng W, Jin L, Zhao S, et al. 2018. Metagenomic analysis of bacteria, fungi, bacteriophages, and helminths in the gut of giant pandas. Front Microbiol. 9:1717.
- Yuan X. 2005. China national fishery statistic and analysis in 2004. Fish Wealth Guide. 13:13–16.
- Zhang YJ, Li S, Gan RY, Zhou T, Xu DP, Li HB. 2015. Impacts of gut bacteria on human health and diseases. Int J Mol Sci. 16:7493–7519.
- Zhang JQ, Zhang L, Wang XC. 2017. Effects of rice culture and pond culture on the quality of Chinese mitten crab (Eriocheir sinensis). Sci Technol Food Ind. 38:229–236.