Abstract
This study aimed to explore the potential molecular mechanisms of FuZheng XiaoJi prescription (FZXJP) on Colorectal Cancer (CRC) cells. Transcriptome sequencing was performed on HT-29 cells treated with FZXJP and not followed by selecting differentially expressed genes (DEGs). Functional enrichment analysis and protein-protein interaction (PPI) analysis were performed for the DEGs. The transcription factors were predicted using TRRUST and miRWalk 3.0. Finally, Real-time PCR (qRT-PCR) was used to verify the results of transcriptome sequencing. HT-29 had a more sensitive response to FZXJP. Transcriptome sequencing revealed 1522 DEGs, and they were enriched in various biological processes and pathways, such as regulation of cellular biosynthetic process and MAPK signaling pathway. Totally 61 proteins in the PPI network with degree > 5 were screened as hub genes, including EGR1, KLHL11, UBA7, IRF1, HERC6, and IFI35. The qRT-PCR confirmed that the expression of those genes was consistent with that of transcriptome analysis. Three transcription factors and 15 miRNAs were predicted. All the three transcription factors were found to interact with EGR1. IRF1 was a target of miR-93-5p. This study provided a theoretical basis for the regulation of FZXJP in HT-29cells, which lays a foundation for the treatment of CRC.
Introduction
Colorectal cancer (CRC) ranks the fourth most deadly diseases in the world with approximately 900,000 deaths every year (Dekker et al. Citation2019). CRC is a multifactorial disorder caused by genetic, environmental factors and lifestyle, and it is of two types (non-hereditary and hereditary). Non-hereditary CRC is most prevalent, which results mainly from somatic mutations in response to environmental factors (Aran et al. Citation2016). Effective cancer screening measures have reduced the incidence and mortality of CRC, but the number of young people diagnosed with colon cancer has increased (Thanikachalam and Khan Citation2019). Metastatic CRC is identified at the initial diagnosis for 20% of cases; about 30–50% of primary CRC cases will relapse and die of metastatic tumor (Mody et al. Citation2018). Recently, the outcomes of CRC patients have been improved due to the advancement in diagnosis and therapies. Nevertheless, the tumors often develop drug resistance due to the heterogeneity (Osumi et al. Citation2019). Therefore, it is needed to develop new medicines with less toxicity and side effect.
Traditional Chinese Medicine (TCM) has more than 2000 years of history. Recently, TCM has gained a wide acceptance and has achieved increasing clinical applications due to its multi-target, multi-level function features and its less toxicity and side effect (Hu and Wang Citation2019; Wang et al. Citation2018). For example, based on a prospective cohort study, Xu et al. showed the outcomes of stage II and III CRC cases were significantly improved after TCM treatment (Xu et al. Citation2017). Lee et al. demonstrated that Danggui-Sayuk-Ga-Osuyu-Saenggang-Tang could repress the invasion and migration of CRC cells (Lee et al. Citation2018), and Chen et al. showed Dahuang-Zhechong pill could inhibit liver metastasis in CRC (Chen et al. Citation2019a). FuZheng XiaoJi prescription (FZXJP) is a herbal prescription that has been used in the treatment of CRC. A previous study indicated that FZXJP coupled with chemotherapy medicine could increase the immune response of patients and decrease the side effects in the treatment of advanced colon cancer (Kai-jun et al. Citation2015). Wang et al. indicated that FZXJP coupled with capecitabine and oxaliplatin could decrease serum CEA levels and adverse reactions and improve clinical outcomes (Jing Citation2019). However, the pharmacological mechanisms remain unclear.
Transcriptome analysis allows the investigation of gene expression pattern changes response to diseases, environmental challenges and so on (Jiang et al. Citation2015). Based on genomic and transcriptomic analysis, Woolston et al. found the expression of immune checkpoints and cytotoxic immune infiltration increased after cetuximab treatment in CRC (Woolston et al. Citation2019). In this study, we found that FZXJP could significantly inhibit the proliferation of CRC cells. To further clarify the underlying molecular mechanisms, transcriptomic sequencing was applied for CRC cells and cells with FZXJP treatment. Expression changes of various genes were found in CRC cells after FZXJP treatment. The genes’ involved functions and the microRNA (miRNAs) and transcription factors that regulate their expression were also analyzed. Our study provides potential targets and pathways to investigate the molecular mechanisms of FZXJP for the CRC therapy.
Methods
Cell culture
CRC cell lines, HT-29 and SW620, were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). HT-29 cells were maintained in 90% McCOY’s 5A medium (catalog no. 16600-082, Gibco) coupled with 10% FBS (catalog no. 15140-122, Gibco) + 1% penicillin-streptomycin (catalog no. 15140-122, Gibco) in an incubator with 5% CO2 at 37°C. But, 90% L-15 medium (catalog no. 11415-064, Gibco) coupled with 10% FBS + 1% penicillin-streptomycin was used to maintain SW620 cells at 37°C.
Preparation of FuZheng XiaoJi prescription (FZXJP)
FZXJP consists of 6 Chinese herbs: 30 g Astragalus membranaceus, 15 g bighead atractylodes rhizome, 15 g Ganodorma lucidum, 30 g Coix Seed, 30 g Hedyotis diffusa and 3 g Scorpio. After soaking and decocting, centrifugation (10,000 rpm) was performed for 30 min to gather the supernatant, and evaporation drying was then performed. Finally, the mother solution was prepared by re-dissolving the dried powder into distilled water.
Counting kit-8 (CCK-8) assay
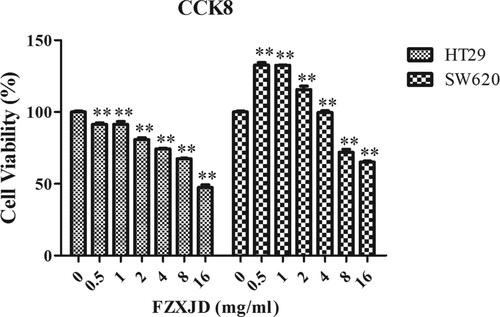
Cells (8000 cells/well) were placed in 96-well plates, and then 0, 0.5, 1, 2, 4, 8, and 16 mg mL−1 mother solutions were supplemented to treat cells for 48 h. The medium containing 10% CCK-8 solution (catalog no. C0039, Beyotime) was then supplemented to each well. After 1 h incubation, cell viability was determined by detecting optical density at 450 nm on a Microplate Reader. The IC50 value was calculated using GraphPad Prism5. Finally, a lower IC 50 value was obtained for HT-29 cells (16.11 mg/mL). Therefore, HT-29 cells treated with 16.11 mg mL−1 mother solution for 48 h were selected in the following analysis.
RNA extraction and sequencing
Total RNA extraction was completed using TRIzol reagent, followed by the determination of purity and concentration of RNA on NanoDrop2000. TruseqTMRNA sample prep Kit (Illumina) was used to reverse-transcribe RNA (1 μg, concentration ≥ 50 ng/μL) to construct a cDNA library. The cDNA quantification was performed using Quantus™ Fluorometer QuantiFluor® dsDNA System (Promega). Then cDNA libraries were collected using Agencourt AMPure XP (Beckman) and were sequenced on an Illumina HiSeq™ 4000 (Illumina).
Quality control and data preprocessing
The quality control of raw data was done using SeqPrep (Sturm et al. Citation2016) and Sickle software (Piel et al. Citation2017). The obtained clean reads was mapped to a reference genome using HISAT2 (Kim et al. Citation2015) (Version 2.1.0). Mapped reads was then assembled using StringTie (Pertea et al. Citation2015) (Version 1.3.3b). DESeq2 (Love et al. Citation2014) (Version 1.24.0) was used to perform differential expression analysis, and the Benjamini/Hochberg method was used to perform FDR correction. The screening for differentially expressed genes (DEGs) was performed with the parameters of P-adjust < 0.001 and |log2FC| ≥ 2.
Functional enrichment analysis and protein-protein interaction (PPI) analysis
The Gene Ontology (GO) function annotations and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways were analyzed using the Fisher’s exact test of Goatools software (Klopfenstein et al. Citation2018), followed by the Bonferroni correction. Adjust-P < 0.05 was used to screen the significant GO terms and KEGG pathways. The interactions among proteins encoded by the DEGs were predicted using STRING database (Szklarczyk et al. Citation2019) (version 11,). The parameters were set as Homo sapiens and highest confidence (PPI score = 0.9). Cytoscape was used to visualize the PPI network. Besides, Degree Centrality was used to calculate the degree of nodes in the PPI network.
Prediction of transcription factor and miRNA
Genes in the PPI network with degree > 5 were screened according to a previous study (Han et al. Citation2004), in which 5 can be used as a criterion to separate the population with high PCC (Pearson correlation coefficient) from the population with low PCC. Transcription factor and miRNA that target these genes were predicted. The miRNA-gene pairs were analyzed using miRWalk 3.0 (Dweep et al. Citation2011). The miRNA-gene pairs with score >0.95 existing in Targetscan, Mirdb, Mirtarbase databases were selected. The transcription factor-gene pairs were predicted using TRRUST (version 2) (Han et al. Citation2018) with species setting as human. The transcription factors with FDR < 0.05 and overlapped genes >5 were selected. Finally, the transcription factor-gene network, miRNA-gene network, and miRNA-gene-transcription factor network were constructed using Cytoscape.
Reverse transcription-quantitative PCR (RT-qPCR)
Total RNA extraction was completed using TRIzol reagent, followed by the determination of purity and concentration of RNA on NanoDrop2000. Then, reversed transcription of total RNA was performed using PrimeScript™RT Master Mix (catalog no. RR036A, Takara), followed by RT-qPCR using Power SYBR Green PCR Master Mix (catalog no. A25742, Thermo). This process was performed as follows: 50°C for 2 min, 95°C for 10 min, followed by 40 cycles at 95°C for 10 s and 60°C for 30 s. Finally, the relative expressions levels of genes were calculated by the 2−ΔΔCt method. The primer sequences are displayed in Supplementary Table 1.
Statistical analysis
Data were presented as the mean ± standard deviation (SD). Graphpad Prism 5 (Graphpad Software, San Diego, CA) was used to complete the data analyses, and one way anova and Newman-Keuls Multiple Comparison Test were applied. P < 0.05 and P < 0.01 were selected to show the results with statistically significant difference and highly significant difference.
Results
FZXJP inhibited proliferation of CRC cells
After treated with FZXJP, cell proliferation of HT-29 and SW620 cells was detected by the CCK-8 assay (Figure ). The results indicated that the cell viability of HT29 cells decreased in a time- and dose- dependent manner in comparison with that of control (P < 0.01). The cell viability of SW620 cells increased with 0.5, 1, 2, 4 mg mL−1 FZXJP treatment, but decreased with 8 and 16 mg mL−1 FZXJP treatment (P < 0.01). We further calculated the IC50 value for HT-29 cells (16.11 mg mL−1) and SW620 cells (20.13 mg mL−1), a lower IC 50 value was obtained for HT-29 cells. Therefore, HT-29 cells that treated with 16.11 mg mL−1 mother solution for 48 h were selected in the following analysis.
Sequencing data statistics and data preprocessing
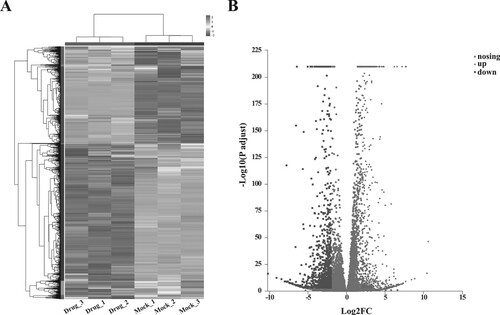
The raw data and the obtained clean reads are shown in Supplementary Table 2. High-throughput sequencing generated 151,124,406 clean reads from three samples with FZXJP treatment, and 139,927,996 clean reads from three control samples. After transcription assembly and expression analysis, 12,711 genes were screened from the six samples (Supplementary Figure 1A). Principal component analysis showed there were significant differences of samples between the two groups (Supplementary Figure 1B). A total of 1522 DEGs were screened with |logFC| > 2 and P-adjust < 0.001, including 585 up-regulated genes and 937 down-regulated genes (Figure , Supplementary Table 3).
Functional enrichment analysis
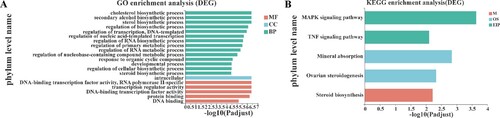
Functional enrichment was analyzed to explore the functions of these DEGs. A total of 230 significant GO annotation terms were enriched, including 194 terms of biological process, 8 terms of cellular component, and 30 terms of molecular function. Figure (A) displayed the top 20 GO terms (ranked by P value), and 14 biological processes were included, such as regulation of the primary metabolic process, cholesterol/sterol/secondary alcohol/steroid biosynthetic processes, regulation of cellular biosynthetic process. In addition, a total of 5 KEGG pathways were enriched, including MAPK signaling pathway, TNF signaling pathway, mineral absorption, ovarian steroidogenesis, and steroid biosynthesis (Figure (B)).
Figure 3. Results of functional enrichment analysis. (A) The top 20 GO function terms for DEGs. BP, biological process; CC, cellular component; MF, molecular function; (B) KEGG pathways for DEGs; M, metabolize; OS, organisms system; EIP, environmental information processing. Vertical axis represents the enriched GO terms and KEGG pathways, respectively; horizontal axis represents the significant value; the smaller PDR represent more significant value of the GO terms and KEGG pathways.
PPI network analysis
The PPI analysis was conducted to investigate the interactions among DEGs. The PPI network contained 307 genes and 530 edges (Figure ). Early growth response 1 (EGR1, degree = 11), Rho-related BTB domain containing 2 (RHOBTB2, degree = 11), Ras homolog family member V (RHOV, degree = 11), gigaxonin (GAN, degree = 10), Kelch like family member 11 (KLHL11, degree = 10) and KLHL21 (degree = 10) had a high degree. Table lists the hub genes with degree > 5 in the PPI network, including 61 genes (35 down-regulated and 26 up-regulated).
Figure 4. Protein-protein interaction network. Light gray nodes represent up-regulated genes, and dark gray nodes represent down-regulated genes; node size represents the degree of each node.
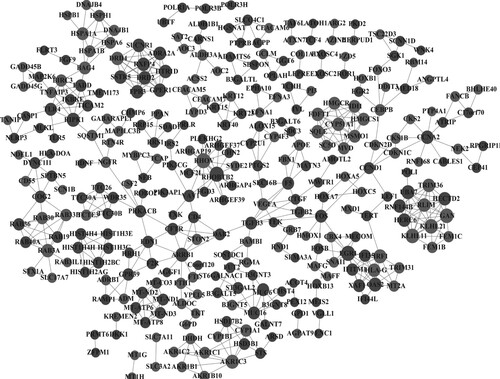
Table 1. The 61 genes with degree > 5 in the PPI network.
miRNA-gene-transcription factor network
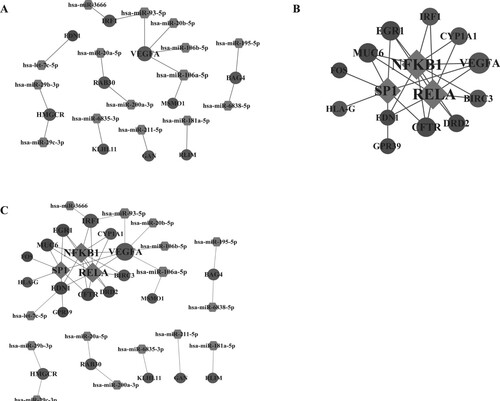
Transcription factors and miRNAs were predicted for the 61 hub genes with degree >5. A total of 17 miRNA-gene pairs and 25 transcription factor-gene pairs were obtained (Figure (A)). The constructed transcription factor-gene network included 15 nodes and 25 pairs (Figure (B)). Then, the miRNA-gene-transcription factor network was constructed based on the obtained miRNA-gene pairs and transcription factor-gene pairs, including 37 nodes and 42 interaction pairs (Figure (C)). Among the 37 nodes, there were 3 transcription factors (SP1, RELA, NFKB1), 15 miRNAs and 19 genes (EGR1, KLHL11, VEGFA, IRF1, etc.). All the three transcription factors interacted with EGR1. KLHL11 was a target of miR-6835-3P. IRF1 was a target of miR-93-5P.
Figure 5. The regulatory network. (A) The miRNA-gene regulatory network; (B) The transcription factor-gene regulatory network; (C) The miRNA-gene-transcription factor regulatory network. Light grey nodes represent up-regulated genes, and dark grey nodes represent down-regulated genes; rhombus represents transcription factors; hexagon represents miRNAs.
Expression of key DEGs determined by RT-qPCR
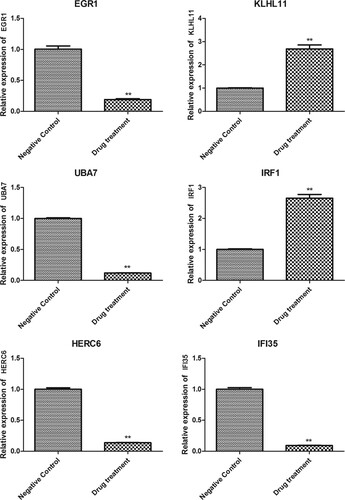
To verify the results of transcriptome sequencing and further analyze the expression pattern of genes with important anti-cancer roles, the genes with degree >5 in the PPI network and related to interferon or ubiquitination were verified using RT-qPCR, including EGR1, KLHL11, UBA7, IRF1, HERC6 and IFI35 (Figure ). The mRNA levels of EGR1, UBA7, HERC6, and IFI35 were obviously decreased in HT-29 cells with FZXJP treatment, while the mRNA levels of KLHL11 and IRF1 were obviously increased (P < 0.01). The expression level determined by RT-qPCR was consistent with the results in bioinformatics analysis.
Discussion
FZXJP was a TCM prescription used in the treatment of CRC. However, the underlying mechanism remains unclear. In this study, we investigated the mechanism of FZXJP for the treatment of colorectal cancer in vitro. We found that FZXJP could significantly inhibit the proliferation of HT-29 cells. Further transcriptome sequencing showed that large-scale changes in gene expression patterns in HT-29 cells following FZXJP treatment. A total of 1522 DEGs were screened, and these genes were significantly enriched in MAPK signaling pathway, regulation of cellular biosynthetic process, and TNF signaling pathway. EGR1 interacted with transcription factor SP1, and IRF1 acted as a target of miR-93-5P.
MAPK signaling pathway involves 4 different cascades: ERK1/2, JNK1/2/3, p38-MAPK and ERK5, and has been implicated in a variety of cellular processes, containing the proliferation, migration, apoptosis, etc. (Sun et al. Citation2015). Studies have demonstrated the important roles of MAPK signaling pathway in CRC. For example, Tang et al. suggested that up-regulated PEA15 in CRC could accelerate the progression and liver metastasis of CRC by increasing MAPK/ERK signals (Tang et al. Citation2019). Huang et al. indicated that the activation of p38-MAPK signals play the decisive role in osteopontin-mediated invasion, autophagy and apoptosis in CRC (Huang et al. Citation2017). Moreover, p38-MAPK signal mediated the resistance of multiple drugs and chemoresistance in CRC patients (Grossi et al. Citation2014). In the current study, MAPK signaling pathway was a significant one enriched for DEGs. Hereby, we speculated that MAPK signaling pathway was implicated in the role of FZXJP in inhibiting the proliferation in HT-29 cells.
EGR1, early growth response 1, up-regulated in resistant CRC cells, and high expression of EGR1 was correlated with a worse outcome for patients that underwent anti-EGFR therapy (Kumar et al. Citation2017). Kim et al. reported ERG1 was directly activated in response to the role of Krüppel-like factor 12 in promoting CRC tumor growth (Kim et al. Citation2016). The inhibition of EGR1 could induce tumor cell death and inhibit tumor growth in CRC (Zhao et al. Citation2015). Consistently, EGR1 was down-regulated in CRC cells after FZXJP treatment in our study. In addition, EGR1 interacted with transcription factor SP1. SP1 is a member of specificity protein family that has been implicated in cell proliferation, apoptosis, metastasis and carcinogenesis in different cancers (Vizcaíno et al. Citation2015). Studies had revealed the important role of SP1 in CRC (Chen et al. Citation2018; Yu et al. Citation2019). Yu et al. suggested that SP1 contributed the tumor progression of CRC through the up-regulation of forkhead box O3A (Yu et al. Citation2018). Snyder et al. indicated that 13-cis retinoic acid-mediated ERK1/2 activating could disrupt the expression of SP1 by EGR1 (Snyder and Thekkumkara Citation2013). Therefore, we concluded that FZXJP inhibited HT-29 cell proliferation by regulating the expression of EGR1 which further disrupts SP1 expression.
IRF1, interferon regulatory factor 1, is a tumor suppressor that involves DNA damage and repair, cellular necrosis and immune response (Ohsugi and Yamaguchi Citation2019). IRF1 could repress CRC metastasis and tumor growth by up-regulating the expression of ras association of domain-containing protein 5 (Hong et al. Citation2019). In addition, IRF1 was related to tumor immune microenvironment in CRC, and had been considered as a biomarker for the diagnosis of CRC recurrence (Wu et al. Citation2020). IRF1 was up-regulated in HT-29 cells after FZXJP treatment in our study, and IRF1 acted as a target of miR-93-5p. Tang et al. suggested that miR-93 showed tumor inhibitor role in the development of HT-29 cells by mediating Wnt/β-catenin signaling (Tang et al. Citation2015). Chen et al. indicated that miR-93-5p could attenuate radiation-trigged CRC cell apoptosis through the decrease of FOXA1 expression and the increase of TGFB3 expression (Chen et al. Citation2020). Koelzer et al. showed that the up-regulation of miR-93 and miR-34a in CRC could reduce the expression of IRF1 and STAT1 by 2 to 2.5-fold (Koelzer et al. Citation2017). We speculated that the miR-93-5p might be one of the targets to investigate the mechanism of FZXJP in the treatment of HT-29 cells.
Ubiquitination refers to dynamic modulation of proteins which are important for biological processes, , including cell differentiation, immune repsonse and cell survival (Mansour Citation2018). Ubiquitination has been involved in tumorigenesis and progression of different cancers (Faktor et al. Citation2019). For example, ubiquitination of TLE3 was involved in RNF6-mediated tumor progression via activating Wnt/β-Catenin signaling in CRC (Liu et al. Citation2018). Ubiquitin ligases act crucial roles in a variety of biological processes in CRC, containing facilitating CRC metastasis (Chen et al. Citation2019b), inhibiting CRC cell apoptosis (Schneider et al. Citation2019), regulating CRC cell growth (Xu et al. Citation2019). In this study, ubiquitin ligase HERC6 and ubiquitin like modifier activating enzyme UBA7 were down-regulated in HT-29 cells after FZXJP treatment. We suggested that ubiquitination was implicated in the effect of FZXJP in HT-29 cells.
In spite of those findings, there remain some limitations in the current study. More experimental groups, including normal control group, normal mice treated with FZXJP group and positive control group, should be set up in the future study. In addition, western blotting, immunohistochemical staining, shRNA and other approaches are also needed to study the molecular mechanism of FZXJP in the treatment of CRC. Moreover, considering the heterogeneity of tumor, only one cell line HT-29 was used in this study. We plan to use multiple cell lines to study the mechanism of FZXJP in the treatment of CRC.
In sum, we suggested that FZXJP could inhibit HT-29 cells’ proliferation and change the gene expression pattern of HT-29 cells. TNF and MAPK signaling pathway might be involved in the effect of FZXJP in HT-29 cells.
Supplemental Material
Download Zip (1.2 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available in NCBI BioProject repository [https://www.ncbi.nlm.nih.gov/bioproject/PRJNA746620] with accession number PRJNA746620.
Additional information
Funding
References
- Aran V, Victorino AP, Thuler LC, Ferreira CG. 2016. Colorectal cancer: epidemiology, disease mechanisms and interventions to reduce onset and mortality. Clin Colorectal Cancer. 15:195–203. doi:10.1016/j.clcc.2016.02.008
- Chen D, Li Y, Zhang X, Wu H, Wang Q, Cai J, Cui Y, Liu H, Lan P, Wang J, et al. 2019b. Ubiquitin ligase TRIM65 promotes colorectal cancer metastasis by targeting ARHGAP35 for protein degradation. Oncogene. 38:6429–6444. doi:10.1038/s41388-019-0891-6
- Chen X, Liu J, Zhang Q, Liu B, Cheng Y, Zhang Y, Sun Y, Ge H, Liu Y. 2020. Exosome-mediated transfer of miR-93-5p from cancer-associated fibroblasts confer radioresistance in colorectal cancer cells by downregulating FOXA1 and upregulating TGFB3. J Exp Clin Cancer Res. 39:65. doi:10.1186/s13046-019-1507-2
- Chen C, Yao X, Xu Y, Zhang Q, Wang H, Zhao L, Wen G, Liu Y, Jing L, Sun X. 2019a. Dahuang Zhechong pill suppresses colorectal cancer liver metastasis via ameliorating exosomal CCL2 primed pre-metastatic niche. J Ethnopharmacol. 238:111878. doi:10.1016/j.jep.2019.111878
- Chen X, Zeng K, Xu M, Hu X, Liu X, Xu T, He B, Pan Y, Sun H, Wang S. 2018. SP1-induced lncRNA-ZFAS1 contributes to colorectal cancer progression via the miR-150-5p/VEGFA axis. Cell Death Dis. 9:982. doi:10.1038/s41419-018-0962-6
- Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. 2019. Colorectal cancer. Lancet. 394:1467–1480. doi:10.1016/s0140-6736(19)32319-0
- Dweep H, Sticht C, Pandey P, Gretz N. 2011. miRWalk – database: prediction of possible miRNA binding sites by “walking” the genes of three genomes. J Biomed Inform. 44:839–847. doi:10.1016/j.jbi.2011.05.002
- Faktor J, Pjechová M, Hernychová L, Vojtěšek B. 2019. Protein ubiquitination research in oncology. Klin Onkol Casopis Ceske Slovenske Onkologicke Spolecnosti. 32:56–64. doi:10.14735/amko20193S
- Grossi V, Peserico A, Tezil T, Simone C. 2014. P38α MAPK pathway: a key factor in colorectal cancer therapy and chemoresistance. World J Gastroenterol. 20:9744–9758. doi:10.3748/wjg.v20.i29.9744
- Han JD, Bertin N, Hao T, Goldberg DS, Berriz GF, Zhang LV, Dupuy D, Walhout AJ, Cusick ME, Roth FP, Vidal M. 2004. Evidence for dynamically organized modularity in the yeast protein-protein interaction network. Nature. 430:88–93. doi:10.1038/nature02555
- Han H, Cho JW, Lee S, Yun A, Kim H, Bae D, Yang S, Kim CY, Lee M, Kim E, et al. 2018. TRRUST v2: an expanded reference database of human and mouse transcriptional regulatory interactions. Nucleic Acids Res. 46:D380–d386. doi:10.1093/nar/gkx1013
- Hong M, Zhang Z, Chen Q, Lu Y, Zhang J, Lin C, Zhang F, Zhang W, Li X, Zhang W, Li X. 2019. IRF1 inhibits the proliferation and metastasis of colorectal cancer by suppressing the RAS-RAC1 pathway. Cancer Manag Res. 11:369–378. doi:10.2147/cmar.s186236
- Hu Y, Wang J. 2019. Interactions between clopidogrel and traditional Chinese medicine. J Thromb Thrombolysis. 48:491–499. doi:10.1007/s11239-019-01945-3
- Huang RH, Quan YJ, Chen JH, Wang TF, Xu M, Ye M, Yuan H, Zhang CJ, Liu XJ, Min ZJ. 2017. Osteopontin promotes cell migration and invasion, and inhibits apoptosis and autophagy in colorectal cancer by activating the p38 MAPK signaling pathway. Cell Physiol Biochem. 41:1851–1864. doi:10.1159/000471933
- Jiang Z, Zhou X, Li R, Michal JJ, Zhang S, Dodson MV, Zhang Z, Harland RM. 2015. Whole transcriptome analysis with sequencing: methods, challenges and potential solutions. Cell Mol Life Sci. 72:3425–3439. doi:10.1007/s00018-015-1934-y
- Jing W. 2019. Clinical study of Fuzheng Xiaoji decoction combined with capecitabine and oxaliplatin in the treatment of advanced colon cancer. J Chang Med Coll. 33:467–430.
- Kai-jun C, Huiming W, Jiangping Q, Liping L, Caili L. 2015. The application value of Fuzheng Xiaoji decoction combined with chemotherapy medicine in treating advanced colon cancer. CJGMCM. 30:2638–2640.
- Kim D, Langmead B, Salzberg SL. 2015. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 12:357–360. doi:10.1038/nmeth.3317
- Kim SH, Park YY, Cho SN, Margalit O, Wang D, DuBois RN. 2016. Krüppel-like factor 12 promotes colorectal cancer growth through early growth response protein 1. PLoS One. 11:e0159899. doi:10.1371/journal.pone.0159899
- Klopfenstein DV, Zhang L, Pedersen BS, Ramírez F, Warwick Vesztrocy A, Naldi A, Mungall CJ, Yunes JM. 2018. GOATOOLS: a python library for gene ontology analyses. Sci Rep. 8:10872. doi:10.1038/s41598-018-28948-z
- Koelzer VH, Sokol L, Zahnd S, Christe L, Dawson H, Berger MD, Inderbitzin D, Zlobec I, Lugli A. 2017. Digital analysis and epigenetic regulation of the signature of rejection in colorectal cancer. Oncoimmunology. 6:e1288330. doi:10.1080/2162402x.2017.1288330
- Kumar SS, Tomita Y, Wrin J, Bruhn M, Swalling A, Mohammed M, Price TJ, Hardingham JE. 2017. High early growth response 1 (EGR1) expression correlates with resistance to anti-EGFR treatment in vitro and with poorer outcome in metastatic colorectal cancer patients treated with cetuximab. Clin Transl Oncol. 19:718–726. doi:10.1007/s12094-016-1596-8
- Lee K, Cho SG, Choi YK, Choi YJ, Lee GR, Jeon CY, Ko SG. 2018. Herbal prescription, Danggui-Sayuk-Ga-Osuyu-Senggang-Tang, inhibits TNF-α-induced epithelial-mesenchymal transition in HCT116 colorectal cancer cells. Int J Mol Med. 41:373–380. doi:10.3892/ijmm.2017.3241
- Liu L, Zhang Y, Wong CC, Zhang J, Dong Y, Li X, Kang W, Chan FKL, Sung JJY, Yu J. 2018. RNF6 promotes colorectal cancer by activating the Wnt/β-catenin pathway via ubiquitination of TLE3. Cancer Res. 78:1958–1971. doi:10.1158/0008-5472.can-17-2683
- Love MI, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15:550. doi:10.1186/s13059-014-0550-8
- Mansour MA. 2018. Ubiquitination: friend and foe in cancer. Int J Biochem Cell Biol. 101:80–93. doi:10.1016/j.biocel.2018.06.001
- Mody K, Baldeo C, Bekaii-Saab T. 2018. Antiangiogenic therapy in colorectal cancer. Cancer J. 24:165–170. doi:10.1097/ppo.0000000000000328
- Ohsugi T, Yamaguchi K. 2019. Anti-apoptotic effect by the suppression of IRF1 as a downstream of Wnt/β-catenin signaling in colorectal cancer cells. Oncogene. 38:6051–6064. doi:10.1038/s41388-019-0856-9
- Osumi H, Shinozaki E, Yamaguchi K, Zembutsu H. 2019. Clinical utility of circulating tumor DNA for colorectal cancer. Cancer Sci. 110:1148–1155. doi:10.1111/cas.13972
- Pertea M, Pertea GM, Antonescu CM, Chang TC, Mendell JT, Salzberg SL. 2015. Stringtie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat Biotechnol. 33:290–295. doi:10.1038/nbt.3122
- Piel FB, Steinberg MH, Rees DC. 2017. Sickle cell disease. N Engl J Med. 376:1561–1573. doi:10.1056/NEJMra1510865
- Schneider D, Chua RL, Molitor N, Hamdan FH, Rettenmeier EM, Prokakis E, Mishra VK, Kari V, Wegwitz F, Johnsen SA, Kosinsky RL. 2019. The E3 ubiquitin ligase RNF40 suppresses apoptosis in colorectal cancer cells. Clin Epigenet. 11:98. doi:10.1186/s13148-019-0698-x
- Snyder R, Thekkumkara T. 2013. Interplay between EGR1 and SP1 is critical for 13-cis retinoic acid-mediated transcriptional repression of angiotensin type 1A receptor. J Mol Endocrinol. 50:361–374. doi:10.1530/jme-12-0154
- Sturm M, Schroeder C, Bauer P. 2016. Seqpurge: highly-sensitive adapter trimming for paired-end NGS data. BMC Bioinform. 17:208. doi:10.1186/s12859-016-1069-7
- Sun Y, Liu WZ, Liu T, Feng X, Yang N, Zhou HF. 2015. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct Res 35:600–604. doi:10.3109/10799893.2015.1030412
- Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork P, et al. 2019. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 47:D607–d613. doi:10.1093/nar/gky1131
- Tang B, Liang W, Liao Y, Li Z, Wang Y, Yan C. 2019. PEA15 promotes liver metastasis of colorectal cancer by upregulating the ERK/MAPK signaling pathway. Oncol Rep 41:43–56. doi:10.3892/or.2018.6825
- Tang Q, Zou Z, Zou C, Zhang Q, Huang R, Guan X, Li Q, Han Z, Wang D, Wei H, et al. 2015. MicroRNA-93 suppress colorectal cancer development via Wnt/β-catenin pathway downregulating. Oncol ReportsTumour Biol. 36:1701–1710. doi:10.1007/s13277-014-2771-6
- Thanikachalam K, Khan G. 2019. Colorectal cancer and nutrition. Nutrients. 11:164. doi:10.3390/nu11010164
- Vizcaíno C, Mansilla S, Portugal J. 2015. Sp1 transcription factor: a long-standing target in cancer chemotherapy. Pharmacol Ther. 152:111–124. doi:10.1016/j.pharmthera.2015.05.008
- Wang J, Wong YK, Liao F. 2018. What has traditional Chinese medicine delivered for modern medicine? Expert Rev Mol Med. 20:e4. doi:10.1017/erm.2018.3
- Woolston A, Khan K, Spain G, Barber LJ, Griffiths B, Gonzalez-Exposito R, Hornsteiner L, Punta M, Patil Y, Newey A, et al. 2019. Genomic and transcriptomic determinants of therapy resistance and immune landscape evolution during anti-EGFR treatment in colorectal cancer. Cancer Cell. 36:35–50. doi:10.1016/j.ccell.2019.05.013
- Wu Y, Zhang S, Yan J. 2020. IRF1 association with tumor immune microenvironment and use as a diagnostic biomarker for colorectal cancer recurrence. Oncol Lett. 19:1759–1770. doi:10.3892/ol.2020.11289
- Xu X, Huang A, Cui X, Han K, Hou X, Wang Q, Cui L, Yang Y. 2019. Ubiquitin specific peptidase 5 regulates colorectal cancer cell growth by stabilizing Tu translation elongation factor. Theranostics. 9:4208–4220. doi:10.7150/thno.33803
- Xu Y, Mao JJ, Sun L, Yang L, Li J, Hao Y, Li H, Hou W, Chu Y, Bai Y, et al. 2017. Association between use of traditional Chinese medicine herbal therapy and survival outcomes in patients with stage II and III colorectal cancer: a multicenter prospective cohort study. J Natl Cancer Inst Monogr. 2017. doi:10.1093/jncimonographs/lgx015
- Yu Y, Peng K, Li H, Zhuang R, Wang Y, Li W, Yu S, Liang L, Xu X, Liu T. 2018. SP1 upregulated FoxO3à promotes tumor progression in colorectal cancer. Oncol Rep. 39:2235–2242. doi:10.3892/or.2018.6323
- Yu S, Wang D, Shao Y, Zhang T, Xie H, Jiang X, Deng Q, Jiao Y, Yang J, Cai C, Sun L. 2019. SP1-induced lncRNA TINCR overexpression contributes to colorectal cancer progression by sponging miR-7-5p. Aging. 11:1389–1403. doi:10.18632/aging.101839
- Zhao DY, Jacobs KM, Hallahan DE, Thotala D. 2015. Silencing Egr1 attenuates radiation-induced apoptosis in normal tissues while killing cancer cells and delaying tumor growth. Mol Cancer Ther. 14:2343–2352. doi:10.1158/1535-7163.mct-14-1051