Abstract
The liver, an organ with robust regenerative capacity, is involved in many physiological functions, such as detoxification and modulation of optimal brain function. Exosomes are secreted by almost all cell types and mediate intercellular communication, as well as, they are involved in neuroinflammatory process. In this study, we found that fetal liver cell-derived exosomes (FLC-EXOs) administration to lipopolysaccharide (LPS)-induced microglia, which are brain resident immune cells responsible for neuroinflammation, decreased the expression of pro-inflammatory cytokines, such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α), in the LPS-activated microglia. We also examined the different signaling pathways that may be involved in this process to determine the anti-inflammatory mechanism of FLC-EXOs. The results showed that activation of the p38 pathway was attenuated after FLC-EXOs administration, which may be related to the anti-inflammatory effects of FLC-EXOs.
KEYWORDS:
Introduction
Neuroinflammation plays a critical role in neurodegenerative diseases. A growing number of studies have shown that neuroinflammation is a key pathological event in a variety of neurodegenerative diseases and acts as a trigger and modulator of neurodegenerative processes. Microglia are the dominant type of immune cells in the brain and function as the first line of defense in the brain in response to any potential insult (Dokalis and Prinz Citation2019; Song and Colonna Citation2018). When activated, microglia secrete cytokines and chemokines to modulate neuroinflammation (von Bernhardi et al. Citation2015). The effect of microglia is context-dependent, and chronic activation of microglia is usually detrimental, whereas acute activation is beneficial (Dwyer and Ross Citation2016). Therefore, functional regulation that targeted microglia may be a potential therapeutic strategy for neurodegenerative diseases.
In recent years, stem cells, especially mesenchymal stem cells (MSCs), have attracted a lot of attention as a source of cell-based transplantation in neurodegenerative diseases. MSCs are able to improve cognitive functions through modulation of neuroinflammation and microglia activation in some neurodegenerative diseases(Lee et al. Citation2012; Lee et al. Citation2009; Yun et al. Citation2013). However, accumulating researches suggest that the biodistribution of MSCs in the target organs is rare, and its therapeutic effect appears to be a consequence of the paracrine action(Deng et al. Citation2015; Witwer et al. Citation2019). Exosomes as the nanoparticles that are secreted by MSC are important constitute pathway of paracrine action of MSCs.
Exosomes are phospholipid-bilayer nanoparticles that derived from endosome. The limiting membrane of endosome could bud inwards and lead to the vesicle-containing multivesicular bodies (MVB). After fuzing with plasma membrane, MVB release the intraluminal vesicles into extracellular space and finally form the exosomes(Gould and Raposo Citation2013). Because they contain various cargoes such as proteins, lipids, and micro RNAs, exosomes can mediate intercellular communication and participate in several different functions as heterogeneous biologically active vesicles (Colombo et al. Citation2014; De Toro et al. Citation2015). Many studies have focused on the potential application of exosomes as biomarkers for diagnosis (Vella et al. Citation2016) or as nanocarriers to deliver medicine in some diseases (Alvarez-Erviti et al. Citation2011), especially in neurodegenerative disorders. However, most of research has focused on therapeutic effects of stem cell-derived exosomes, few studies have examined exosomes that derived from liver cells.
Liver is an organ with a strong capacity of regeneration (Michalopoulos Citation2007). To some extent, liver cells are similar with stem cells. Hepatocytes constitute the vast majority of liver cells and are responsible for the major specific hepatic functions, and under physiological conditions hepatocytes possess high proliferative capacity (Michalopoulos and DeFrances Citation1997) which is an important characteristic of stem cell. Hepatocytes have also been shown to contribute to liver progenitor cells in vitro and in vivo using animal models of chronic liver injury(Chen et al. Citation2012; Tarlow et al. Citation2014). Recently one research found that mature hepatocytes could be dedifferentiated into cells bearing progenitor characteristics, and these hepatocyte-derived cells demonstrate self-renewal and engraftment capacity at the single cell level(Yimlamai et al. Citation2014). These researches demonstrated that hepatocytes possess some differentiation potency to generate particular cell types, which is to some extent similar with stem cells. Therefore, liver cells resemble stem cells in some aspects. Owing to the mechanism of their biogenesis, exosomes possess partial molecular constituents and functions of their cells of origin to a certain extent (Yanez-Mo et al. Citation2015). Therefore, we hypothesized that liver exosomes share functions similar to those of liver or stem cells, and may play a role in the modulation of inflammation.
In this study, we isolated exosomes from fetal liver cells and studied their effects on lipopolysaccharide (LPS)-induced inflammation in microglia. We found that fetal liver cell-derived exosomes inhibited LPS-induced inflammation in microglia. We also found that the activation of the p38 pathway was attenuated after exosome administration, which could be related to the anti-inflammatory effect of FLC-EXOs.
Materials and methods
Chemicals and reagents
Mouse fetal liver cells (FL-83B) were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA; ATCC® CRL-2390TM). All cell culture reagents were purchased from Gibco (Waltham, MA USA), unless otherwise stated. Exosome antibody kit, including antibodies against CD9, CD63, CD81 and HSP70 (EXOAB-KIT-1, 1:1000) and exosome-depleted fetal bovine serum (FBS) (EXO-FBS-250A-1) were obtained from System Biosciences (Palo Alto, CA, USA). Antibodies against mitogen-activated protein kinase (MAPKs) (CST #9926 and # 9910, 1:1000), and glycogen synthase kinase-3β (GSK3β) (CST#8213, 1:1000) signaling pathways were purchased from Cell Signaling Technology Inc. (Danvers, MA, USA). Mouse IL-1β and TNF-α enzyme-linked immunosorbent assay (ELISA) kits were purchased from eBioscience Inc. Pharmingen (San Diego, CA, USA). Amicon® Ultra 15 mL filters (100 kDa, UFC910096) were purchased from Millipore (Merck, Burlington, MA, USA). All other chemicals and reagents were purchased from Sigma-Aldrich (Burlington, MA, USA) unless otherwise stated.
Cell culture
FL-83B cells were cultured as it previously described before(Sun et al. Citation2014). Briefly, they were cultured in Kaighn's modification of Ham's F-12 medium (F-12 K) with 10% fetal bovine serum and 100 U/mL penicillin–streptomycin and maintained in 5% CO2 at 37°C. For exosome isolation, the F-12 K culture medium of the FL-83B cells was replaced with F-12 K medium containing exosome-depleted FBS for 48 h, and then the medium was collected for exosome isolation. Mouse embryonic fibroblasts (MEFs) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% FBS and 100 U/mL penicillin–streptomycin. BV2 microglia (ATCC, Manassas, VA, USA) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% FBS and 100 U/mL penicillin–streptomycin to determine the effect of exosomes on inflammation. They were treated with different concentrations of exosomes for 4 h, followed by treatment with LPS (100 ng/mL) for 2 h. After treatment, the cell culture media were collected and pro-inflammatory cytokines were detected by ELISA.
Isolation of exosomes from FL-83B cells and MEFs
The exosomes were isolated by ultracentrifugation using the following steps: First, 200 mL of culture medium was collected from FL-83B cells and centrifuged at 500 × g for 10 min to eliminate floating cells. The supernatant was centrifuged at 2000 × g for 20 min to remove dead cells, and the supernatant was further centrifuged at 10000 × g for 60 min to remove cell debris. Next, the supernatant was concentrated to 4 mL using Amicon® Ultra 15 mL filter tubes. To isolate the exosomes, the concentrated supernatant was further centrifuged at 100,000 × g for 90 min using a Beckman Optima MAX-TL ultracentrifuge and a TLA110 rotor. The pellet was resuspended in phosphate buffer solution (PBS) and centrifuged at 100,000 × g for 90 min to wash the exosomes. Finally, the pellet was resuspended in PBS and stored at −80 °C. Exosomes derived from MEFs were isolated the same as FL-83B cells.
Western blot (WB) analysis
5 µg exosomal proteins were loaded in each lane for exosome characterization, and during signaling pathway detection, proteins that extracted from 1 × 105 cells were loaded in each lane for WB. The brief process was described as follow. Proteins were mixed with sodium dodecyl sulfate (SDS) loading buffer and denatured at 100 °C for 10 min. Then, they were rapidly transferred into an ice bath before being individually loaded onto an SDS gel. After electrophoresis, the proteins were transferred to nitrocellulose membranes (Bio-Rad), washed, and blocked with 5% nonfat dry milk dissolved in Tris-buffered saline (TBS) for 1 h at room temperature. After blocking, the membranes were hybridized with various primary antibodies overnight. The next day, the membrane was washed thrice with sterilized water for 5 min and incubated for 1 h with the appropriate HRP-conjugated secondary antibody in 5% nonfat dry milk. After washing thrice for 5 min each with sterilized water, the protein bands in the blots were visualized using the Super Signal Pico Chemiluminescent Substrate (Thermo) and Odyssey Fc Imaging System (LI-COR).
Transmission electron microscope (TEM) analysis
Exosomes (3 µL) were mixed with 2% paraformaldehyde solution (PFA) in equal volumes. The suspended droplets were loaded into the special copper grids of the electron microscope. The excess liquid was sucked at the edge of the copper mesh using filter paper, and the grids were left to dry at room temperature. After drying, the grids were negatively dyed with tungstophosphoric acid for approximately 15 s, and then quickly dried with filter paper. After the copper mesh was dried, 3 µL of ultrapure water was added to wash off the salt components contained in the PBS buffer. This step was repeated four times. When the grids were dried, the size and shape of the exosomes were observed under TEM.
Nanoparticle tracking analysis
When FLC-EXOs were isolated with ultracentrifugation, the distribution of particle diameter and concentration of FLC-EXOs were analyzed using the nanoparticle tracking analysis system of qNano (Izon, Christchurch, New Zealand) according to user manual. The process was described briefly. First, nanopore np100 was selected and calibrated before it was wetted with 75 µL of wetting solution. After wetting, the baseline current was established until the current remains stable and does not jump or drift. Then the calibration particles were used to optimize signal-to-noise ratio. When the current was stable, 45 µL of FLC-EXOs that diluted 2 folds with PBS were used to measure their diameter distribution and concentration, and the data was analyzed with the Izon Control Suite Software. The measurement details are as follow. Electrolyte: PBS, pressure: 8, nanopore ID: A65122, nanopore stretch: 45.5 mm.
Fluorescent labeling of exosomes
Exosome labeling was performed as described previously (Long et al. Citation2017). Exosomes were transferred from PBS solution to diluent C solution (Sigma) by centrifugation at 100,000 × g for 70 min. PKH26 dye was diluted to 4 mM. PKH26 and exosomes (200 µg/mL) were filtered separately through small 0.2 µm filters, mixed with at a ratio of 1:1, and incubated for 5 min. Then, 5% BSA was added to wash the mixture. After centrifugation at 100,000 × g for 70 min, the exosome pellet was suspended in PBS solution (0.5 mL). To avoid dye-stained aggregates, exosomes were filtered through a 0.2 µm filter before use.
ELISA
The expression of inflammation-associated cytokines was determined using Ready-SET-GoTM ELISA kit (eBioscience) as follows: Capture antibody (100 µL/well) was used to coat a 96-well plate by incubating at 42 °C overnight. After washing with 250 µL/well wash buffer thrice, 200 µL/well of 1× Assay Diluent was added and incubated for 1 h at room temperature (RT). After washing thrice, 100 µL/well of cell medium was added and incubated for 2 h at RT. After washing five times, 100 µL/well of avidin-HRP was added and incubated for 30 min at RT. Finally, after washing the plate seven times, 100 µL/well of substrate solution was added and incubated for 15 min at RT. Then, 100 µL/well of stop solution was added and the signals were measured at 450 nm.
Statistical analysis
All experiments were repeated at least three times. Gray values were calculated using Image J, and graphs are generated with GraphPad 5.0. For data analyzes, SPSS (version 16.0, USA) was used to test for data normality and homogeneity of variance. Comparisons between two sample means were made using t-test, whereas comparisons between multiple means were made using one-way analysis of variance (ANOVA). A value of P < .05 was considered statistically significant.
Results
Characterization of FLC-EXOs isolated from mouse fetal liver cell
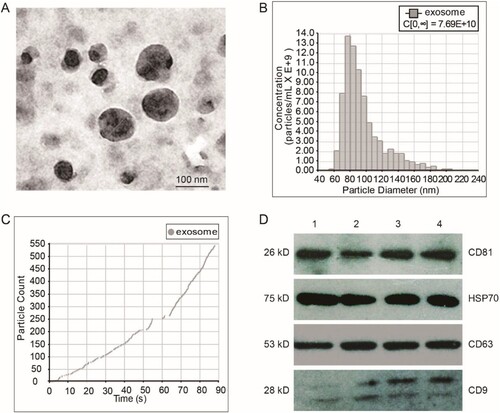
We successfully isolated exosomes from fetal liver cells by ultracentrifugation combined with ultrafiltration. TEM was used to examine the morphology of the isolated exosomes. The diameter of the exosomes ranged from 50 to 150 nm and all exosomes exhibited a typical cup-like structure (Figure (a)). Nanoparticle tracking analysis (NTA) was used to determine the size distribution and concentration of exosomes. The results showed that the mean diameter of exosomes was 96 nm, and the concentration was 7.69E+10 (Figure (b, c)), we also analyzed the expression of several exosomal markers including CD63, CD81, HSP70, and CD9 by WB. The results showed that the samples were positive for all the markers (Figure (d)). These results confirmed that the vesicles that we isolated were exosomes.
Figure 1. Characterization of fetal liver cell-derived exosomes (FLC-EXOs). Exosomes were isolated by ultracentrifugation combined with ultrafiltration. After isolation, FLC-EXOs were observed using TEM. The isolated vesicles had a cup-like shape (a). After ultracentrifugation, the pellets of FLC-EXOs were dissolved in 500 µL PBS, and then the distribution of particle diameter and concentration of FLC-EXOs were analyzed using the nanoparticle tracking analysis system of qNano (Izon, Christchurch, New Zealand) (b), and 550 particles were analyzed to make the data statistically representative (c). To further confirm the identity of FLC-EXOs, 5 µg of FLC-EXOs were loaded in each lane and probed with exosome-specific antibodies against CD81, HSP70, CD63, and CD9. Number 1–4 represents four samples of exosomes that isolated in parallel (d).
FLC-EXOs were colocalized with microglia
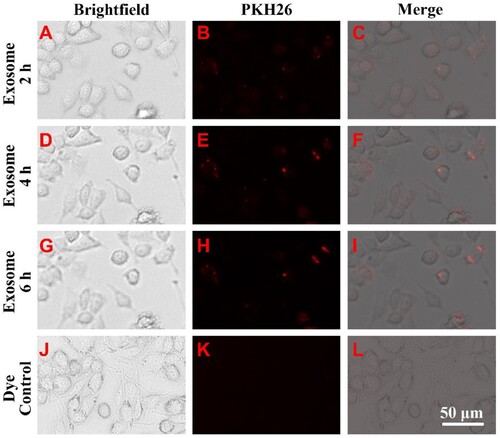
Exosomes mediate intercellular communication by fuzing with the cell membrane of recipient cells or by endocytosis by recipient cells. To determine whether FLC-EXOs were endocytosed by microglia, PKH26-labeled FLC-EXOs were incubated with microglia and observed at different time points. The results showed that FLC-EXOs began to appear on or in microglia after 2 h and the positive signal of FLC-EXOs increased with time. By calculating the ratio of the fluorescent spot diameter to the length of the reference bar, we found that the diameter of endocytosed exosomes was less than 250 nm (Figure ). These results demonstrated the colocalization of FLC-EXOs and microglia.
Figure 2. FLC-EXOs and BV2 microglia showed colocalization. To determine whether FLC-EXOs were colocalized with microglia, after ultracentrifugation, exosomes were dissolved in a diluent solution and labeled with the red fluorescent membrane dye, PKH26. After filtering through a 0.2 µm filter, PKH26-labeled FLC-EXOs were incubated with BV2 microglia and observed at 2 h (a–c), and 4 h (d–f), and 6 h (g–i) using a living cell imaging system after incubation. To avoid dye aggregates, the PKH26-only group was treated with the same exosome group and used as the control (j–l).
FLC-EXOs inhibit LPS-induced inflammation in microglia and the inhibition is FLC-EXOs dose-dependent
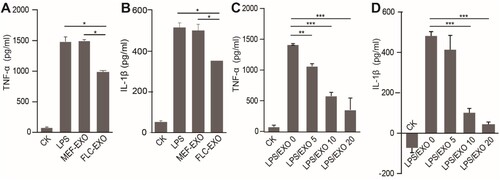
LPS-induced activation of microglia is widely used as a cell model to study neuroinflammation and its mechanism. The liver has robust regenerative capacity and possesses many physiological functions, such as detoxification and modulation of optimal brain function. Therefore, we wanted to investigate whether fetal liver cell-derived exosomes (FLC-EXOs) modulate inflammation. To test whether FLC-EXOs can modulate the activation of microglial cells, we treated microglia cells with exosomes before LPS stimulation. We found that exogenous exosomes were taken up by microglia cells after 4 h (Figure ). To ensure that most of the exosomes were taken up by microglia, the exosomes were incubated with microglia for 4 h before LPS addition. After 2 h of LPS stimulation, the levels of the pro-inflammatory factors TNF-α and IL-1β in the supernatant were detected by ELISA. We found that the expression levels of TNF-α and IL-1βdecreased compared with the group of cells treated with LPS only. after exosome treatment (Figure (a, b)). To further confirm that the reduction of pro-inflammatory cytokine expression was due to the addition of exosomes, we treated BV2 cells with increasing concentrations of exosomes and analyzed the expression of TNF-α and IL-1β in the supernatant. The results showed that the expression of TNF-α and IL-1β markedly decreased with an increase in the amount of exosomes, indicating that the inhibition of LPS-induced inflammation by exosomes is FLC-EXOs dose-dependent (Figure (c, d)).
Figure 3. Administration of FLC-EXOs decreased the expression of pro-inflammatory cytokines in BV2 microglia. BV microglia were seeded in a 96-well plate overnight, 5 × 104 per well. Then BV2 cells were treated with 5 µg of FLC-EXOs/well. After treatment for 4 h with FLC-EXOs, BV2 microglia were treated with LPS at a concentration of 100 ng/mL for 2 h. The culture media were collected the expression levels of TNF-α and IL-1β were analyzed using ELISA. It showed that administration of FLC-EXOs decreased the levels of TNF-α and IL-1β compared with the group that treated with LPS only or treated with MEF exosomes (MEF-EXO). The group that without any treatment or that treated with LPS only was labeled with CK and LPS respectively in the figure. The group that first treated with LPS and then with exosomes was labeled with LPS/EXO (a, b). Then, the BV2 cells were treated with FLC-EXOs at concentrations of 0, 5, 10, and 20 µg/well in the same way. The expression levels of TNF-α and IL-1β were measured using ELISA. The results indicated that all treatment with different concentrations decreased TNF-α and IL-1β expression and the decrease was dose-dependent (c, d). (Data are means ± standard deviation of 3 replicates. *P < .05, **P < .01, *** P < .001)
P38/MAPK signal pathway is involved in the inhibition of LPS-induced inflammation by FLC-EXOs
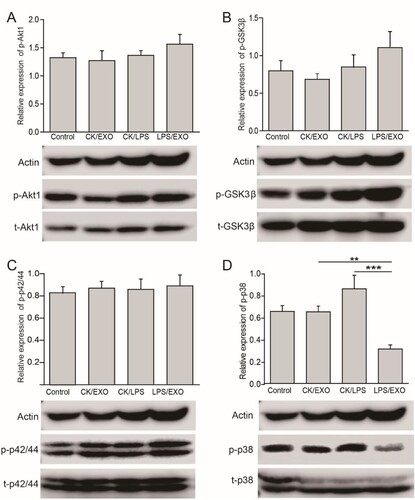
Akt/NFκB, Erk1/2-MAPK, p38/MAPK, and GSK3β signaling pathways have been shown to be involved in LPS-induced inflammation. To understand the molecular mechanism by which exosomes inhibit the expression of pro-inflammatory cytokines, we analyzed the Akt/NFκB, Erk1/2-MAPK, p38/MAPK, and GSK3β signaling pathways using WB. The results showed that exosome treatment did not affect the levels of total or phosphorylated Akt/NFκB and Erk1/2 (Figure (a, b)), but the level of phosphorylated GSK3β level was increased slightly (Figure (c)), indicating that exosomes may reduce the inflammatory response by mildly activating the GSK3β signaling pathway. Whereas exosome administration significantly decreased the level of phosphorylated p38 (p, 0.000078), thereby decreasing the activation of the p38 signaling pathway. (Figure (d)). This indicated that the inhibition of LPS-induced inflammation by FLC-EXOs may involve inactivation of the p38/MAPK signaling pathway.
Figure 4. Administration of FLC-EXOs inhibited the activation of p38/MAPK and slightly enhanced GSK3β pathway in BV2 microglia. BV2 cells were treated with 5 µg of FLC-EXOs for 4 h, and then treated with LPS at a concentration of 100 ng/mL for 2 h. Cell lysates were prepared and subjected to WB analysis with antibodies against phosphorylated Akt1 (p-Akt1), total Akt1 (t-Akt1), phosphorylated GSK3β (p-GSK3β), total GSK3β (t-GSK3β), phosphorylated p42/44(p-p42/44), total p42/44 (t-p42/44), phosphorylated p38 (p-p38), and total p38 (t-p38) (a). The ratios of p-Akt1 to t-Akt1, p-GSK3β to t-GSK3β, p-p42/44 to p42/44, and p-p38 to p38 were determined by densitometry analysis. Administration of FLC-EXOs did not affect the Akt1 and p42/44 MAPK signaling pathways (a, b), slightly activated the GSK3β pathway (c), and inhibited the activation of the p38 pathway (d). (Data are means ± standard deviation of 3 replicates.**P < .01)
Discussion
In this study, we successfully isolated FLC-EXOs using ultracentrifugation combined with ultrafiltration, and observed that FLC-EXOs administration decreased the expression of pro-inflammatory cytokines, such as IL-1β and TNF-α, in LPS-activated microglia. We further examined the different signaling pathways that may be involved in this process and found that activation of the p38 pathway was attenuated after FLC-EXOs administration, which may be related to the anti-inflammatory effects of FLC-EXOs.
LPS-induced activation of microglia is widely used as a cell model to investigate neuroinflammation and the underlying mechanism (Batista et al. Citation2019). Many studies have found that LPS induction leads to increased IL-1β and TNF-α production in murine microglia (Francois et al. Citation2013; Karababa et al. Citation2017). The elevation of cytokines is considered to cause neuroinflammation and promote the progression of neurodegenerative disorders (Ransohoff Citation2016). Thus, these cytokines may serve as potential therapeutic targets to actively regulate the pathological progression of these diseases by regulating microglial pro-inflammatory activation (Bachiller et al. Citation2018).
Another popular approach used to explore the therapeutic options for neurodegenerative disorders is based on stem cells, including embryonic stem cells (ESCs) and mesenchymal stem cells (MSCs). In addition to the replacement of impaired neurons by differentiation, most effects are exerted through the paracrine expression of neurotrophic factors and cytokines (Lo Furno et al. Citation2018). Exosomes are essential for paracrine signaling in stem cells, and they can modulate inflammation and play a beneficial role in CNS disorders. However, most studies have focused on exosomes from MSCs, and few studies have examined liver exosomes (Lou et al. Citation2017; Sung et al. Citation2018). The liver is an organ with a robust capacity for regeneration (Michalopoulos Citation2007), and hepatocytes, the major type liver cells, are highly capable of proliferation and detoxification under physiological conditions (Grant Citation1991; Michalopoulos and DeFrances Citation1997). Thus, exosomes derived from hepatocytes may function as an extension of hepatocytes and modulate the inflammatory response. Therefore, we explored whether FLC-EXOs regulate inflammation. Our results suggest that after activation by LPS, FLC-EXOs administration obviously down-regulated the expression of TNF-α and IL-1B in microglia.
In this study, we explored the molecular mechanisms by which exosomes inhibit LPS-induced microglial activation. Multiple signaling pathways are involved in LPS-induced activation of microglia (Ransohoff Citation2016). Fiebich et al. found that LPS induced p42/44 MAP kinase activation in rat primary microglial cells (Fiebich et al. Citation2002). However, our results showed that the p42/44 pathway was not activated by LPS, and FLC-EXOs did not affect the p42/44 pathway. We also found that FLC-EXOs did not affect the Akt1 signaling pathway, although Akt1 activation was observed in some stimulus-induced neuroinflammation (An et al. Citation2020). These controversial results may be associated with the differences in LPS and the cells between the groups. GSK3β acts on multiple cellular signaling pathways and various aspects of brain function. Castaño et al. found that GSK3β is required for axon growth (Castano et al. Citation2010). Kimura et al. showed that GSK-3β is required for memory reconsolidation in the adult brain(Kimura et al. Citation2008). In our results, we found that FLC-EXOs slightly increased phosphorylated GSK3β levels. This mild increase may be related to the anti-inflammatory effect of FLC-EXOs.
In our study, we observed that FLC-EXOs administration inhibited the activation of the p38 pathway. This is consistent with the results of other studies. Bachstetter et al found that increased IL-1β and TNF-α production in BV-2 microglial cells was inhibited in a concentration-dependent manner by a p38 MAPK-targeted inhibitor (Bachstetter et al. Citation2011). Gee et al. also found that inhibition of p38 MAPK had similar effects in LPS-induced BV2 microglia (Gee et al. Citation2018). Combined with these findings, we hypothesized that the reduced production of IL-1β and TNF-α in our study may be associated with the attenuated activation of p38/MAPK pathway. However, the exact cargo of exosomes mediating this anti-inflammatory response are of great interest and will be investigated in future research. In addition, the BV2 microglia that we used in the research are immortalized cell line, so the biological characteristics and their functions may differ with microglia that reside in vivo. Therefore, whether FLC-EXOs have the same effects on microglia in vivo is also worth to explore.
Regarding neurodegenerative diseases, the endotoxin hypothesis proposes that endotoxin causes or contributes to neurodegeneration(Brown Citation2019). Many studies support this hypothesis. An intraperitoneal injection of LPS caused microglial activation and inflammation in the brains of rodents (Qin et al. Citation2007). Chronic LPS administration are used to establish models of Alzheimer’s (AD) and Parkinson’s disease (PD) (Tufekci et al. Citation2011; Zakaria et al., Citation2017). AD and PD are associated with endotoxin-producing microbes in the gut or gum, and elevated blood endotoxin levels were found in AD, PD, or sepsis (Brown Citation2019). Whether hepatocyte exosomes can reach the brain to regulate endotoxin levels and/or inflammation remains unclear, and there is little research on this subject. However, the liver is essential for the optimal function of the brain. Loss of liver function leads to chronic ‘‘hepatic encephalopathy’’ and eventually coma (Michalopoulos Citation2007). A Japanese study reported that the oral administration of porcine liver decomposition product (PLDP) improved cognitive function in older adults in a randomized, double-blind, placebo-controlled study, and this may be contributed to that PLDP could regulate the neuroinflammation of microglia i and of (Tsukahara et al. Citation2020). The role of exosomal regulation in these processes is very interesting, and the possibility exists because paracrine pathways are important for the liver to perform its physiological functions. Whether the mechanism of action occurs via the regulation of endotoxin levels or inflammation by circular liver exosomes is also worth exploring.
Conclusion
In this study, we isolated exosomes from fetal liver cells and found that they attenuate LPS-induced activation of microglia, possibly by inhibiting the activation of the P38 pathway. This study sheds light on a new source of exosomes that may be used to modulate inflammation in CNS disorders in the future.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available in “figshare” at https://doi.org/10.6084/m9.figshare.14191052.v2.
Additional information
Funding
References
- Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. 2011. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 29:341–345.
- An J, Chen B, Kang X, Zhang R, Guo Y, Zhao J, Yang H. 2020. Neuroprotective effects of natural compounds on LPS-induced inflammatory responses in microglia. Am J Transl Res. 12:2353–2378.
- Bachiller S, Jimenez-Ferrer I, Paulus A, Yang Y, Swanberg M, Deierborg T, Boza-Serrano A. 2018. Microglia in neurological diseases: a road map to brain-disease dependent-inflammatory response. Front Cell Neurosci. 12:488.
- Bachstetter AD, Xing B, de Almeida L, Dimayuga ER, Watterson DM, Van Eldik LJ. 2011. Microglial p38alpha MAPK is a key regulator of proinflammatory cytokine up-regulation induced by toll-like receptor (TLR) ligands or beta-amyloid (Abeta). J Neuroinflammation. 8:79.
- Batista CRA, Gomes GF, Candelario-Jalil E, Fiebich BL, de Oliveira ACP. 2019. Lipopolysaccharide-induced neuroinflammation as a bridge to understand neurodegeneration. Int J Mol Sci. 20(9):2293.
- Brown GC. 2019. The endotoxin hypothesis of neurodegeneration. J Neuroinflammation. 16:180.
- Castano Z, Gordon-Weeks PR, Kypta RM. 2010. The neuron-specific isoform of glycogen synthase kinase-3beta is required for axon growth. J Neurochem. 113:117–130.
- Chen Y, Wong PP, Sjeklocha L, Steer CJ, Sahin MB. 2012. Mature hepatocytes exhibit unexpected plasticity by direct dedifferentiation into liver progenitor cells in culture. Hepatology. 55:563–574.
- Colombo M, Raposo G, Thery C. 2014. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 30:255–289.
- Deng K, Lin DL, Hanzlicek B, Balog B, Penn MS, Kiedrowski MJ, Hu Z, Ye Z, Zhu H, Damaser MS. 2015. Mesenchymal stem cells and their secretome partially restore nerve and urethral function in a dual muscle and nerve injury stress urinary incontinence model. Am J Physiol Renal Physiol. 308:F92–F100.
- De Toro J, Herschlik L, Waldner C, Mongini C. 2015. Emerging roles of exosomes in normal and pathological conditions: new insights for diagnosis and therapeutic applications. Front Immunol. 6:203.
- Dokalis N, Prinz M. 2019. Resolution of neuroinflammation: mechanisms and potential therapeutic option. Semin Immunopathol. 41:699–709.
- Dwyer JB, Ross DA. 2016. Modern microglia: novel targets in psychiatric neuroscience. Biol Psychiatry. 80:e47–e49.
- Fiebich BL, Lieb K, Engels S, Heinrich M. 2002. Inhibition of LPS-induced p42/44 MAP kinase activation and iNOS/NO synthesis by parthenolide in rat primary microglial cells. J Neuroimmunol. 132:18–24.
- Francois A, Terro F, Janet T, Rioux Bilan A, Paccalin M, Page G. 2013. Involvement of interleukin-1beta in the autophagic process of microglia: relevance to Alzheimer's disease. J Neuroinflammation. 10:151.
- Gee MS, Kim SW, Kim N, Lee SJ, Oh MS, Jin HK, Bae JS, Inn KS, Kim NJ, Lee JK. 2018. A novel and selective p38 mitogen-activated protein kinase inhibitor attenuates LPS-induced neuroinflammation in BV2 microglia and a mouse model. Neurochem Res. 43:2362–2371.
- Gould SJ, Raposo G. 2013. As we wait: coping with an imperfect nomenclature for extracellular vesicles. J Extracell Vesicles. 2:20389.
- Grant DM. 1991. Detoxification pathways in the liver. J Inherit Metab Dis. 14:421–430.
- Karababa A, Groos-Sahr K, Albrecht U, Keitel V, Shafigullina A, Gorg B, Haussinger D. 2017. Ammonia attenuates LPS-induced upregulation of pro-inflammatory cytokine mRNA in co-cultured astrocytes and microglia. Neurochem Res. 42:737–749.
- Kimura T, Yamashita S, Nakao S, Park JM, Murayama M, Mizoroki T, Yoshiike Y, Sahara N, Takashima A. 2008. GSK-3beta is required for memory reconsolidation in adult brain. PLoS One. 3:e3540.
- Lee JK, Jin HK, Bae JS. 2009. Bone marrow-derived mesenchymal stem cells reduce brain amyloid-beta deposition and accelerate the activation of microglia in an acutely induced Alzheimer's disease mouse model. Neurosci Lett. 450:136–141.
- Lee HJ, Lee JK, Lee H, Carter JE, Chang JW, Oh W, Yang YS, Suh JG, Lee BH, Jin HK, et al. 2012. Human umbilical cord blood-derived mesenchymal stem cells improve neuropathology and cognitive impairment in an Alzheimer's disease mouse model through modulation of neuroinflammation. Neurobiol Aging. 33:588–602.
- Lo Furno D, Mannino G, and Giuffrida R. 2018. Functional role of mesenchymal stem cells in the treatment of chronic neurodegenerative diseases. J Cell Physiol. 233:3982–3999.
- Long Q, Upadhya D, Hattiangady B, Kim DK, An SY, Shuai B, Prockop DJ, Shetty AK. 2017. Intranasal MSC-derived A1-exosomes ease inflammation, and prevent abnormal neurogenesis and memory dysfunction after status epilepticus. Proc Natl Acad Sci U S A. 114:E3536–E3545.
- Lou G, Chen Z, Zheng M, Liu Y. 2017. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp Mol Med. 49:e346.
- Michalopoulos GK. 2007. Liver regeneration. J Cell Physiol. 213:286–300.
- Michalopoulos GK, DeFrances MC. 1997. Liver regeneration. Science. 276:60–66.
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. 2007. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 55:453–462.
- Ransohoff RM. 2016. How neuroinflammation contributes to neurodegeneration. Science. 353:777–783.
- Song WM, Colonna M. 2018. The identity and function of microglia in neurodegeneration. Nat Immunol. 19:1048–1058.
- Sun Q, Li Q, Zhong W, Zhang J, Sun X, Tan X, Yin X, Zhang X, Zhou Z. 2014. Dysregulation of hepatic zinc transporters in a mouse model of alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 307:G313–G322.
- Sung S, Kim J, Jung Y. 2018. Liver-derived exosomes and their implications in liver pathobiology. Int J Mol Sci. 19(12):3715.
- Tarlow BD, Pelz C, Naugler WE, Wakefield L, Wilson EM, Finegold MJ, Grompe M. 2014. Bipotential adult liver progenitors are derived from chronically injured mature hepatocytes. Cell Stem Cell. 15:605–618.
- Tsukahara T, Haniu H, Uemura T, Matsuda Y. 2020. Therapeutic potential of porcine liver decomposition product: new insights and perspectives for microglia-mediated neuroinflammation in neurodegenerative diseases. Biomedicines. 8:446.
- Tufekci KU, Genc S, Genc K. 2011. The endotoxin-induced neuroinflammation model of Parkinson's disease. Parkinsons Dis. 2011:487450.
- Vella LJ, Hill AF, Cheng L. 2016. Focus on extracellular vesicles: exosomes and their role in protein trafficking and biomarker potential in Alzheimer's and Parkinson's disease. Int J Mol Sci. 17:173.
- von Bernhardi R, Eugenin-von Bernhardi L, Eugenin J. 2015. Microglial cell dysregulation in brain aging and neurodegeneration. Front Aging Neurosci. 7:124.
- Witwer KW, Van Balkom BWM, Bruno S, Choo A, Dominici M, Gimona M, Hill AF, De Kleijn D, Koh M, Lai RC, et al. 2019. Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J Extracell Vesicles. 8:1609206.
- Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, et al. 2015. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 4:27066.
- Yimlamai D, Christodoulou C, Galli GG, Yanger K, Pepe-Mooney B, Gurung B, Shrestha K, Cahan P, Stanger BZ, Camargo FD. 2014. Hippo pathway activity influences liver cell fate. Cell. 157:1324–1338.
- Yun HM, Kim HS, Park KR, Shin JM, Kang AR, il Lee K, Song S, Kim YB, Han SB, Chung HM, et al. 2013. Placenta-derived mesenchymal stem cells improve memory dysfunction in an Abeta1-42-infused mouse model of Alzheimer's disease. Cell Death Dis. 4:e958.
- Zakaria R, Wan Yaacob WM, Othman Z, Long I, Ahmad AH, Al-Rahbi B. 2017. Lipopolysaccharide-induced memory impairment in rats: a model of Alzheimer's disease. Physiol Res. 66:553–565.
