Abstract
Coleus aromaticus is a herbal medicine that has been referred for its medicinal properties in traditional, ayurvedic, and folklore medicines. Therefore, various research in the area for biochemical characterization of its biochemical compounds are extensively carried out using various techniques including spectroscopy and chromatography analysis. The list of bioactivities reported for this plant includes antioxidant, antiepileptic, antiurolithic, antidiabetic, anticancer, anthelmintic, antiprotozoal, and antiviral activity. The plant is effectively used against cardiovascular disorders, respiratory disorders, digestive diseases and is used for its larvacidal property, allelopathic property and finds its use even in the food and beverage industry. Along with various healing properties, C. aromaticus possess antimycobacterial property i.e. the plant is effective in inhibiting both bacterial and fungal pathogens. Even though this herb is not cultivated on a larger scale in comparison to other plants of the same genus or family due to their phytochemical dominance but still C. aromaticus finds its use as herbal medicine. With an increasing number of reports on the various bioactivity and medicinal properties of C. aromaticus, this review is an effort to update our current knowledge on C. aromaticus.
Introduction
Herbal medicine is widely used throughout the world due to their safe, cost-effective and their inhibitory nature against various deadly pathogen thus help in maintaining good health. According to the World Health Organization, 80% of the world population is dependent on the traditional use of medicinal plants (Mathur et al. Citation2011; Sandhya et al. Citation2011; Swamy and Sinniah Citation2015). Ayurveda, Unani, Sidda, traditional and folk healthcare management includes the use of herbal medicine. It can be used to benefit the total population thus it is important to explore these plants based on their bioactivity and their bioactive compounds. Pharmaceutical industries involve the use of these bioactive compounds because they are the major source of medicaments and provide a source for the treatment of a wide range of diseases (Arumugam et al. Citation2016). The modern era of natural medicine has increased the demand for new compounds from medicinal plants (Kumara et al. Citation2012; Mohanty et al. Citation2014; Swamy and Sinniah Citation2015). Several approaches to exploit the herbal wealth of the world are explored to extract these bioactive compounds from a variety of plant species. Even with the limited number of reports on the mechanism of the activity it has become obvious that numerous mechanisms are involved in various activities of a given herbal medicine.
Coleus aromaticus Benth. is used to treat various ailments and is studied for its various bioactivities (Retief Citation2000). The common names of the plant include Indian borage, Country borage (English), Indian borage, Parna-Yavaani, Peterechur, Panikkurukka, Kapparillaku (India), Da Shou Xiang (China), Indian Mint, Soup Mint (South Africa, US), Indian Borage, Country borage, Spanish thyme, Mexican mint, French thyme, Indian mint (US), Orégano; Orégano de Cartagena (Cuba), Big thyme (St. Vincent, Grenada & other English speaking Caribbean Islands), Broadleaf Thyme (Barbados), Latai, Suganda, Oregan, Toronjil de limón (Philippines), Orielle (France) and Jamaika thymian (Germany). C. aromaticus possess a variety of bioactive compounds. Most of these compounds are volatile in nature and responsible to cure a variety of ailments. These volatile constituents of the plant majorly contribute to the various therapeutic properties. Thymol (Singh et al. Citation2002) and carvacrol (Castillo and Gonzalez Citation1999) have discrepancies in being named the active constituent in C. aromaticus. Other bioactive compounds include α-humulene, β-caryophyllene, p-cymene, α-terpineol, β-selinene, and γ-terpinene (Murthy et al. Citation2009; Senthilkumar and Venkatesalu Citation2010). These bioactive compounds exhibit many biological properties.
The leaves of C. aromaticus are used to treat conditions like fever, cold, cough, headache, asthma, constipation, and skin diseases (Arumugam et al. Citation2016). C. aromaticus leaves are highly aromatic thus used to enhance the taste and aroma as a flavouring agent or are consumed raw or included as an ingredient in the preparations of traditional food. C. aromaticus juice was confirmed to reduce leptin levels and have an appetite-enhancing effect (Wadikar and Premavalli Citation2014). C. aromaticus leaves are used as a substitute for sage or oregano. The leaves are found as pungent in taste by some consumers due to their volatile content (Khare et al. Citation2011) and are used as meat stuffing or on seafood cuisines such as fish and shellfish (Morton Citation1992). It was added as a secret ingredient in tomato sauce. In India, the leaves are consumed raw with bread or roti’s and mixed with butter or added to fritters or pakoras. The flesh soft leaves are cooked in batter thus have a crunchy astringent snack. The leaves are sometimes added in beer or wine to add flavour (Morton Citation1992).
Based on the recent research reports on the various bioactive constituents and their potential of C. aromaticus, a plethora of experimental procedures were used to isolate a suitable drug compound. Many bioactive constituents isolated from C. aromaticus are reported for effective bioactivity, but the mechanism of certain bioactive compounds is to be explored. In this current review, the bioactive constituents, and the potential of these bioactive compounds of C. aromaticus are discussed.
Botanical description
C. aromaticus (synonym: Plectranthus amboinicus) belongs to the family of lamiacaea. It is a perennial, aromatic, large succulent herb, profusely branched and characteristic smelling leaves. The stem is fleshy, about 30–90 cm, densely covered with many short and erect hairs. The roots are thick, tuberous, fasciculate up to 20 cm long, 0.5–2.5 cm thick, conical, fusiform, straight, and strongly aromatic. Leaves are thick, simple, broad, and ovate with a tapering tip studded with hair. The lower surface possesses numerous glandular hairs that give it a frosted appearance. The taste of the leaf is pleasantly aromatic with an agreeable and refreshing odour. Flowers have short pedicel, pale purplish in dense whorls at distant intervals on a long slender raceme. The pale blue corolla is bilabiate, the lower lobes are elongated and concave. The inflorescence grows to a height of 30–60 cm. Racemes are perfect, the calyx is fine-toothed and deflexed in the front. The placentation is axile i.e. the flowers possess four parted ovaries. Fruits are smooth nutlets, pale brown in colour, 0.7 mm long, and 0.5 mm wide. The plant rarely flowers and seeds are difficult to collect (Soni et al. Citation2012).
Cultivation and propagation
C. aromaticus is a must-have plant of any herb garden. It grows faster and stem cuttings of matured plants are used for propagation because it rarely seeds or sets seed. The plant requires spares watering. It is grown in a semi-shaded and moist location of tropical and subtropical regions of the world. The herb can also adapt to a cooler climate and can be grown as an indoor plant (Dutta Citation1959). The herb grows well in a well-drained, rich, composite soil of neutral pH and high humidity. In case of the presence of excessive water in the soil, the roots are found to rot. The leaves of C. aromaticus turn yellow with exposure to excessive sunlight or may turn dark when not provided with ample sunlight. The herb grows in a well-drained area. It grows well in tropical and sub-tropical locations (Dutta Citation1959).
Bioactive constituents
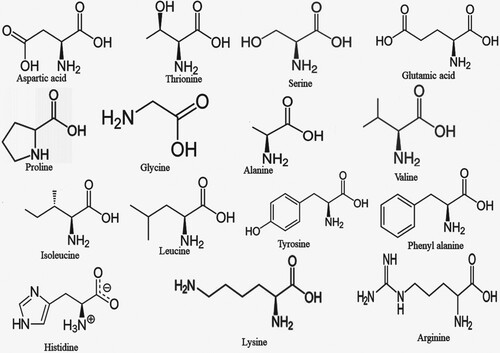
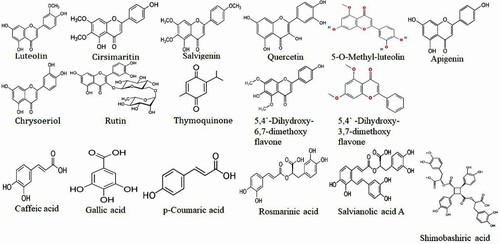
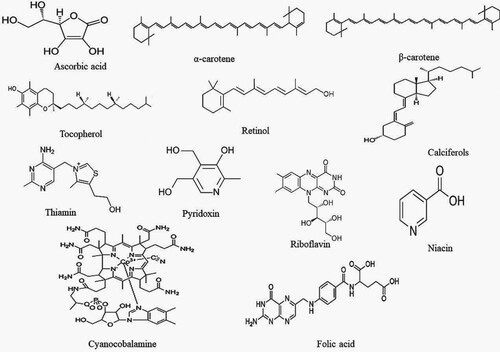
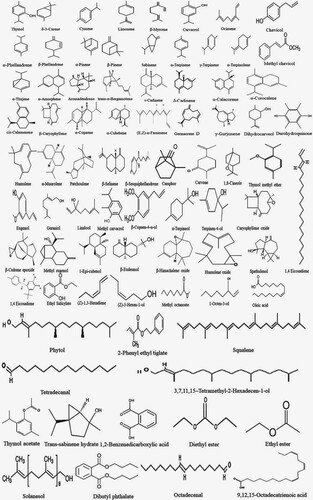
C. aromaticus plant contains many bioactive compounds and therefore has tremendous potential as herbal medicine. The bioactive compounds that contribute to the medicinal potential of this herb involve enzymes, fibre, vitamins, minerals, trace elements, volatile and non-volatile compounds. The bioactive compounds in both essential oil and plant extracts vary in concentration. Table summarizes the bioactive compounds reported in C. aromaticus essential oil and extracts, while Figures depict structural details of these compounds.
Table 1. Bioactive constituents of Coleus aromaticus.
Phytochemical screening of different parts of C. aromaticus like leaves, stems, and roots revealed the presence of sterols, terpenes, terpenoids, alkaloids, phenol, flavonoids, glycosides, lactones, catechol, and tannins (Dutta Citation1959; El-hawary et al. Citation2012) but saponins, steroids were found to be absent (El-hawary et al. Citation2012). Chemical constituents isolated from C. aromaticus include 76 volatile and 30 non-volatile compounds (Asiimwe et al. Citation2014). Volatile compounds are majorly contributed by essential oil present in the stunted glandular hair. These glandular hairs are found on the epidermis of various plant parts. Essential oil of C. aromaticus contains major constituents such as thymol, carvacrol, 1,8-cineole, eugenol, α-humulene, p-cymene, α-terpineol, β-selinene, β-caryophyllene, γ-terpinolene, pinene, methyl eugenol, and phellandrene (Dutta Citation1959; Baslas and Kumar Citation1981; Murthy et al. Citation2009; Senthilkumar and Venkatesalu Citation2010; Girish Citation2016; Saraswati et al. Citation2016). Thymol was reported as a major constituent of essential oil extracted from C. aromaticus. Thymol concentration increased in plants propagated via tissue culture than plant propagated using stem cuttings. Carvacrol was reported as a major constituent in leaf extract (Roja et al. Citation2006; Rout et al. Citation2012). Volatile compounds such as 1-octen-3-ol, terpine-4-ol, eugenol, trans-caryophyllene, caryophyllene oxide, and α-cadinol were present as minor constituents (Rout et al. Citation2012). Thymol and cis-caryophyllene were reported to be present in parent ex-plants, tissue culture regenerated plants as well as in the root cultures of C. aromaticus (Rout et al. Citation2012). The hexane extraction of volatile compounds yields the highest concentration of these compounds when compared to other conventional methods (Khare et al. Citation2011) followed by GC-MS analysis often used to identify the compound isolated. Non-volatile compounds are majorly contributed by phenols, flavonoids, terpenes, and esters (Ragasa et al. Citation1999). These non-volatile compounds were extracted by subjecting the chloroform extract using silica gel chromatography (Ragasa et al. Citation1999) and later identified using UV, HPLC, NMR, and 2-D-NMR (Chiu et al. Citation2012).
Carvacrol was found as a major constituent of the essential oil extracted from C. aromaticus found in the Western Ghats regions of North West Karnataka, India. Other constituents found were p-cymene, β-caryophyllene and trans-α-bergamotene. methyl chavicol, α-calacorene, and α-corocalene were reported as three new chemotypes of carvacrol found in the essential oil extracted from this C. aromaticus (Chen et al. Citation2014). The essential oil extracted from C. aromaticus growing in the mid-hill and foothill regions of northern India when investigated yielded a higher concentration of thymol along with γ-terpinene, p-cymene, (e)-caryophyllene, caryophyllene oxide, and 1-octen-3-ol (MeghaRani et al. Citation2016). This implies that based on the geographic location and climatic conditions, the concentration of the phytoconstituents of C. aromaticus changes. A study conducted by Llarena (Llarena Citation2016) to identify active principle from C. aromaticus leaf extract using ionic liquids through the spectroscopic determination of rosmarinic acid, caffeic acid, and chlorogenic acid with its Functional groups (Llarena Citation2016). The functional groups: p-hydroxyl group, double bonds, and keto group (carbonyl group) are mainly found in anti-inflammatory, antioxidant, and anticancer (Mallavarapu et al. Citation1999; Hassani et al. Citation2012). C. aromaticus extract when analysed using GC-MS resulted in 1,2-benzene dicarboxylic acid, 2-hexadecane-1-ol, 3,7,11,15-tetramethyl, 9,12,15-octadecatrienoic acid, dibutyl phthalate, diethyl ester, ethyl ester hexadecanoic acid, methyl ester, octadecenal, oleic acid, phytol, and solanesol (Mamani and Alhaji Citation2019). The essential oil extracted from dried C. aromaticus leaves revealed to have carvacrol, camphor, δ-3-carene, λ-terpinene, o-cymene, and α-terpinene were the major constituents of the oil (Mallavarapu et al. Citation1999; Hassani et al. Citation2012). Uma et al. (Citation2011) estimated the presence of 3-methyl-4-isopropyl phenol, squalene, caryophyllene, and phytol and correlated their presence to the use of C. aromaticus as herbal medicine. The essential oil extracted from C. aromaticus in the month of September was found to contain higher concentrations of carvacrol, β-caryophyllene, and oxygenated constituents than the oil produced in May (Joshi et al. Citation2011). Crystalized aromatic compounds characterized using HPTLC and XRD (X-ray Powder Diffraction) helped identified 35 types of compounds with immunomodulatory properties yielded rosmarinic acid, chlorogenic acid, coumaric acid, and caffeic acid (Thilagavathi and Hariram Citation2016). Phytochemical analysis of C. aromaticus leaves revealed the presence of flavonoids like apigenin, genkwanin luteolin, quercetin, and salvigenin (Kaliappan and Viswanathan Citation2008).
Non-volatile compounds such as phenols and flavonoids have been identified from C. aromaticus (Khare et al. Citation2011), however, existing reports about the isolation of these compounds are minimum. Ethyl acetate extracts of C. aromaticus were exceptionally used to isolate quercetin, luteolin, and eriodyctiol. This extract also contained caffeic acid, chrysoeriol, p-coumaric acid, and rosmarinic acid were identified using UPLC-MS analysis (Ultra Performance Liquid Chromatography-Mass Spectroscopy) (Dutta Citation1959). Compounds like gallic acid, tannic acid, rutin were isolated from the methanolic stem extract of C. aromaticus. When analysed using HPLC, this extract resulted in the identification of caffeic acid, gallic acid, p-coumaric acid, quercetin, rosmaric acid, and rutin (Joshi et al. Citation2011). Aqueous extract of C. aromaticus resulted in the identification of major components reported as tannins, flavonoids, saponins, polyuronides, and steroid glycosides such as linalool, carvacrol, geranyl acetate, and nerol acetate (Baslas and Kumar Citation1981). Four bioactive compounds with transcription factor inhibitor activity isolated from aqueous stem and leaf extracts of C. aromaticus and analysed using HPLC column-based fractionization along with mass spectrometry and NMR identified thymoquinone, shimobashiric acid, salvianolic acid, and rosmarinic acid (Chiu et al.Citation2012).
Bioactivity and medicinal properties
C. aromaticus is widely used as a herbal medicine due to its various bioactivity and medicinal properties. The leaves and leaf juice are used to treat asthma, bilious affections, bladder stones, bronchitis, cholera, colic, common cold, convulsions, cough, diarrhoea, dysentery, dyspepsia, epilepsy, fever, headache, indicated in kidney indigestion, poisonous bites, renal and vesicle calculi, stimulates the functions of the liver, urinary diseases, vaginal discharges, and vitiated conditions of Kapha and Vata (Girish Citation2016). Whereas, in folk medicine, C. aromaticus is used to expel kidneys and therefore is known as Paashanbhedi (Khare et al. Citation2011). Pharmacologically the herb is used as antiepileptic, antifungal, antimutagenic, anti-tumorogenic, antiviral, neuropharmacological properties, radioprotective effect, and urolithiasis (Verma et al. Citation2012). Traditionally in India, it was used to cure diarrhoea (Uma et al. Citation2011) whereas, a leaf decoction was used to treat asthma, cough, congestive heart failure, dyspepsia, epilepsy, fever, headache, inflammation, kidney troubles, nervous disorders, nasal congestion, spasms, skin ulcerations, stomach-ache, throat infection, and urinary diseases (Morton Citation1992; Verma et al. Citation2012; Thilagavathi and Hariram Citation2016). The various properties exhibited by C. aromaticus are caused by the combinatory action of various bioactive compounds. The explanation of various bioactivity exhibited by C. aromaticus can only be attained by multi-analysis of combinatorial bioactive compounds in animal models. Some of these bioactivity and pharmacological properties are discussed further indetail.
Antioxidant activity
Antioxidants derived from plants are used as dietary supplements in the hope to maintain health and preventing diseases. Antioxidants help in preventing food spoilage. Issues related to the use of artificial antioxidants as food additives causing health risks and toxicity made it unsafe. The replacement of such toxic synthetic antioxidants can prove to be beneficial if antioxidants from natural sources are utilized. The antioxidant activity of C. aromaticus was due to the presence of its bioactive compounds (Gurgel et al. Citation2009). These bioactive compounds that are mainly responsible for antioxidant activity include carvacrol, flavonoids, rosmarinic acid, caffeic acid, and chlorogenic acid were isolated from leaf extracts (Cheryl Citation2007). These compounds are reported to possess anticancer and bronchodilator activity which forms the chemical basis of the health benefits of C. aromaticus in folk medicine. Phenolic compounds mainly contribute significantly to the antioxidant potential due to their unique structure of aromatic rings bearing single or multiple hydroxyl groups. The antioxidant property of C. aromaticus leaves strongly inhibits lipid peroxidation, which could reduce the susceptibility of tissues to alloxan-induced oxidative stress (Khattak et al. Citation2013). Hydroalcoholic extract of C. aromaticus leaves was used to investigate antioxidant, anticlastogenic, and radioprotective effects on Chinese hamster fibroblast cells (V79) exposed to gamma radiations (Rasineni et al. Citation2008). Effective antioxidant activity of C. aromaticus was reported using the DPPH method (Kumaran and Karunakaran Citation2007; Thilagavathi and Hariram Citation2016). In vitro analysis used to evaluate bioactive compounds in the stem of C. aromaticus estimated the presence of major phenols such as rosmarinic acid, caffeic acid, rutin, gallic acid, quercetin, and p-coumaric acid. The extract from the stem of C. aromaticus exhibited properties of antiplatelet aggregation, antibacterial activity, and antiproliferative effect against cancer cell lines: Caco-2, HCT-15, and MCF-7. These appreciable biological activities along with the presence of biomolecules in the methanolic extract of stem thus indicate C. aromaticus posse the potential application as functional food ingredients and nutraceuticals (Candrappa et al. Citation2009).
The presence of carvacrol and thymol was reported to be responsible for antioxidant properties tested in vivo and in vitro in cells that induced lung cancer and gastric adenocarcinoma (Günes-Bayir et al. Citation2018; Günes-Bayir et al. Citation2020). C. aromaticus essential oil is cheaper than possesses no side effects in most of the tested animal models. The presence of these antioxidant components in C. aromaticus makes it an essential component in pharmaceutical drug formulations (Rao et al. Citation2006). Boiling water extracts of C. aromaticus act as an effective antioxidant that could prevent peroxide-mediated oxidative DNA damage (Krithiga and Jayachitra Citation2012). Ethanolic leaf extract of C. aromaticus revealed lowered content of total flavonoids and total phenolics and antioxidant activity (Bhatt et al. Citation2013). C. aromaticus is a rich source of various biological active products. The leaves growing in UV-B radiation were analysed and were reported to have an increased level of thymol (Manjamalai and Grace Citation2012; Ramadas et al. Citation2014). Due to anti-inflammatory properties, crushed leaves are used to heal burns and the leaves are reported to have bronchodilator activity and used to treat tuberculosis (Khare et al. Citation2011).
Antiepileptic activity
C. aromaticus leaves were reported to be used as an antiepileptic and anticonvulsive drug (Castillo and Gonzalez Citation1999). This was predicted based on the presence of enormous bioactive components such as alkaloids, flavonoids, and saponins in alcoholic extract favoured anticonvulsive activity. C. aromaticus was reported to treat nervous disorders including convulsions and epilepsy (Khanum et al. Citation2011). Different extracts of C. aromaticus plant parts such as leaf, stem, and root alcoholic extract were studied for their anticonvulsive activity on swiss mouse models that were already subjected to pentylenetetrazole-induced seizures and maximum electric shock-induced seizures (Kumari and Prasad Citation2014).
Antiurolithic effects
During the past few decades, C. aromaticus extract was tested for a positive effect against the increasing amount of calcium oxalate crystals getting deposited in the kidney. The extracts of C. aromaticus leaves are used to treat urinary diseases and relieve kidney troubles (Morton Citation1992). This suggested that different bioactive compounds present in the leaf extract are responsible for the antiurolithic effect. C. aromaticus extract attenuates the urinary excretion of calcium oxalate without affecting the phosphate as tested in rat models (Wadikar and Patki Citation2016). A study of the antiurolithiatic activity of C. aromaticus in ethylene glycol induced urolithic rats revealed that hydroalcoholic extract of C. aromaticus leaves significantly reduced cholesterol levels and triglyceride levels in urolithic rats. The hydroalcoholic extract of C. aromaticus leaves revealed potential antioxidant in vitro activity (Jaya et al. Citation2014). Leaf extract of the plant efficiently enhances the amount of urine secreted as well as helps in electrolyte excretion (sodium, potassium, calcium) (Bhattacharjee and Majumder Citation2013). Bioactive compounds present in the plant extract efficiently help the liver to function normally by neutralizing the toxic effects of naphthalene. Therefore, proving the hepatoprotective effect of C. aromaticus (Ghosh et al. Citation2000). Jose and Janardhanan (Citation2005) reported fresh juice extracted from C. aromaticus leaves to have antiurolithic activity. Histopathological studies helped to report antiurolithic activity of C. aromaticus on ethylene glycol induced nephrolithiasis in rats thereby proving that the bioactive compounds in C. aromaticus extract contain one or more bioactive compound responsible for antiurolithic activity (Sur et al. Citation2003; Venkatesh et al. Citation2010). C. aromaticus leaf juice was reported to be used as a natural remedy to cure Urolithiasis (Vijayavel et al. Citation2013), a condition where stony concretions form in the bladder or the urinary tract. C. aromaticus leaf juice caused a significant reduction of calcium oxalates and total protein content in urine while histopathological studies revealed the absence of crystal in renal tubules of tested animal models.
Antidiabetic effects
C. aromaticus exhibited antihyperglycemic effects and therefore possesses the ability to heal diabetes. C. aromaticus has a hypoglycemic effect on blood glucose profile and liver antioxidant enzyme activities tested on alloxan-induced diabetic rats (Yoganarasimhan Citation2000). Suryowati et al. (Citation2013) analysed the effect of the extract of C. aromaticus and proved that the treatment of STZ-treated rats with C. aromaticus restored the balance of lipid and carbohydrate metabolism. The extract exhibited significant antihyperglycemic as well as antihyperlipidemic effects. Antihyperlipidemic activity of C. aromaticus extract on diabetic rats induced by streptozotocin. C. aromaticus has the property to act against diabetes and acts towards α-amylase enzyme inhibition (Warrier et al.Citation1996). The essential oil extracted from C. aromaticus leaves and the bioactive constituent Carvacrol positively inhibited diabetes-linked α-amylase and α-glucosidase (Bole and Jayashree Citation2014).
Activity against cardiovascular disorders
C. aromaticus was reported to be used as a treatment of congestive heart failure (Morton Citation1992). Aqueous extracts of the fresh leaves of C. aromaticus were used as an exhibited dose-dependent positive inotropic activity on the isolated frog heart without affecting the heart rate. Aqueous extract of C. aromaticus leaf was reported to increase sodium reflux and caused increased levels of intracellular availability of calcium (Bole and Jayashree Citation2014). The extracts from tissue cultured C. aromaticus, when compared with extracts prepared from C. aromaticus leaves, revealed a significant effect in maintaining good heart health due to the presence of biochemical production (Bole and Jayashree Citation2014). Fresh leaf aqueous extract of C. aromaticus and tissue cultured plants revealed a positive inotropic effect on isolated frog heart possibly by increasing sodium influx, that led to higher intracellular availability of calcium (Hole et al. Citation2009).
Anticancer activity
Koba et al. (Citation2011) reported that the hydroalcoholic extracts of C. aromaticus had both anti-inflammatory and antitumor activities. Govindaraju and Arulselvi (Govindaraju and Arulselvi Citation2018) reported that essential oil from C. aromaticus leaves and its purified constituent, carvacrol, were studied for antiproliferative activity and cytotoxicity on human melanoma cancer (A375) cells. The effect on cell cycle arrest; DNA fragmentation and apoptosis by the cleavage of poly-(ADP ribose)-polymerase (PARP) and Bcl-2 gene expression were examined (Govindaraju and Arulselvi Citation2018). Carvacrol was reported to induce apoptosis by direct activation of the mitochondrial pathway, which plays a vital role in the anti-cancer effect (Günes-Bayir et al. Citation2017). Gurgel et al. (Citation2009) reported significant inhibition of the growth of Sarcoma-180 tumour in mice treated with C. aromaticus hexane extracts. The hexane extract of C. aromaticus was compared with methotrexate, a cancer-treating drug that may cause profoundly serious, life-threatening side effects but completely reduced the tumour growth, revealed that tumour size was reduced and did not reveal any mortality in tested mice. Thymoquinone suppressed the expression of lipopolysaccharide-induced TNF-α (tumour necrosis factor-alpha). Synthesized 2-alkylidenyl-4-cyclopentene-1,3-diones designed based on the structure of thymoquinone had the potential to act as TNF-α inhibitors (Tewari et al. Citation2012). Ramalakshmi et al. (Citation2014) reported that ethanolic extract of C. aromaticus revealed significant anticancer activity through inducing apoptosis in the A549 (human lung cancer) cell line. The severity of the side effects was greatly reduced with the tumour growth destroyed with the use of hexane extracts of C. aromaticus (Ramalakshmi et al. Citation2014). Copper oxide nanoparticles (CuO NPs) are produced using C. aromaticus leaf extract was developed to deliver miRNA-29b to A549 cells. This delivery system was an effective delivery method for miRNAs to cancer cells, with superior performance compared to traditionally available transfection agents, therefore has proved to be an efficient platform for intracellular miRNA delivery and improving therapeutic outcomes for lung cancer (Wu et al. Citation2019). It was reported to have cytotoxic activity, antitumor properties, and was used for the treatment of cancer (Dash and Sachidananda Citation2006).
Anthelmintic effects
Glycosides, tannins, and flavonoids extracted from C. aromaticus are reported to exhibit anthelmintic property by several investigators. C. aromaticus was proved to have these compounds by various investigators through phytochemical tests. Chloroform and methanolic extracts of C. aromaticus revealed significant anthelmintic activity (Prameela and Oommen Citation2011). Methanolic extract of C. aromaticus roots has maximum anthelmintic activity when compared to other herbal and synthetic drugs (Hussain et al. Citation2012). C. aromaticus extract exhibits maximum anthelmintic activity due to the presence of secondary metabolites like glycoside and flavonoids that interfere with the energy generation in helminth parasites by either uncoupling oxidative phosphorylation or, binding to the glycoprotein on the cuticle of the parasite, therefore causes its death (Choudhary Citation2013). Whereas Chaluvaraju et al. (Citation2015) reported that various leaf extracts revealed significant anthelmintic activity. The overall bioactive compounds including tannins present in the leaves are considered responsible for anthelmintic activity. Herbal preparations containing C. aromaticus can be used as an alternative to synthetic drugs. C. aromaticus extracts has no side effects while synthetic anthelmintics such as benzimidazoles, piperazine, diethylcarbamazine citrate, ivermectin, levamisole, etc., exhibited adverse effects like anorexia, nausea, vomiting, dizziness, diarrhoea, occasional fever, rashes (Gilman et al. Citation2001).
Activity against respiratory disorders
Arumugam et al. (Citation2016) compiled all the reports on the use of C. aromaticus in the treatment of respiratory disorder. C. aromaticus was reported to treat used for the treatment of chronic coughs, asthma, bronchitis, and sore throat (Morton Citation1992; Jain and Lata Citation1996; Ruiz et al. Citation1996). Similarly, C. aromaticus leaves were reported to have a positive bronchodilator activity (Ruiz et al. Citation1996). The essential oil extracted from C. aromaticus was reported to be used to treat asthma (Carbajal et al. Citation1991). C. aromaticus leaf juice or decoction or juice was administered orally to treat asthma. catarrhal infections are treated using C. aromaticus leaf extract as it helps to clear the build-up of thick phlegm in an airway (Castillo and Gonzalez Citation1999). Carvacrol and thymol are the main reason for the use of C. aromaticus to treat respiratory diseases. Carvacrol and thymol are excellent expectorants and therefore a drink or a bath of C. aromaticus juice/decoction was recommended to treat bronchitis, cough, influenza, and throat problems (Cano and Volpato Citation2004).
Activity against digestive diseases
The leaves of C. aromaticus are used as carminative and to treat indigestion and diarrhoea (Morton Citation1992; Gurib-Fakim et al. Citation1995; Jain and Lata Citation1996; Ong and Nordiana Citation1999; Cartaxoa et al. Citation2010). C. aromaticus was reported to have a prebiotic effect and stimulated the growth of probiotic bacteria Lactobacillus plantarum that utilizes bioactive compounds to produce necessary metabolic enzymes (Ong and Nordiana Citation1999). Lactobacillus plantarum incubated in C. aromaticus hot water extract was reported to utilize sugars present in the extract and reported β-galactosidase activity. Therefore when C. aromaticus leaves are used in cases of diarrhoea, the microbial gut balance was reported to be balanced (Arumugam et al. Citation2016). Fresh C. aromaticus leaf juice was used to treat constipation (Shubha and Bhatt Citation2015).
Antiprotozoal activity
C. aromaticus tested in vivo against Plasmodium berghei infections in mice models revealed significant antimalarial activity by reduction of parasitemia, therefore C. aromaticus extract was effective against P. berghei infection (Ramli et al. Citation2014). Similarly, an aqueous extract of C. aromaticus leaves was found to be effective against P. berghei (Periyanayagam et al. Citation2008). The essential oil of C. aromaticus reduced the viability of Leishmania braziliensis promastigotes (De Lima et al. Citation2014) while methanolic extract of C. aromaticus revealed significant activity against L. chagasi and L. amazonensis (Tempone et al. Citation2008).
Inhibitory activity against microorganisms
C. aromaticus possess both antibacterial as well as antifungal action, therefore one can rightly say it to have an ‘Antimycobacterial activity’. Essential oil, as well as different solvent extracts from leaves of C. aromaticus, possess great anti-microbial activity on both bacteria and fungi. It was tested against some drug-resistant and phytopathogenic microbes by disc diffusion, agar diffusion method using aqueous, chloroform, ethanol, petroleum ether, acetone extracts of C. aromaticus. C. aromaticus leaves are used as an active ingredient in natural antibiotic formulations (Lukhoba et al. Citation2006). Prudent et al. (Citation1995) studied the bacteriostatic and fungistatic of C. aromaticus extract. Girish (Citation2016) compiled that C. aromaticus had antibacterial property against 28 genera of bacteria, while antifungal activity against 14 genera of fungi. C. aromaticus exhibited inhibitory effects against both gram-negative and gram-positive human pathogenic bacterial strains (Chandrappa et al. Citation2010). Velasco et al. (Citation2009) characterized carvacrol as found to be the major constituent and revealed antibacterial activity against important enteric pathogens such as Salmonella sp., Shigella sp., diarrheagenic Escherichia coli, and Vibrio sp. Whereas Da Costa et al. (Citation2010) revealed that phytochemical analysis revealed thymol, p-cymene, γ-terpinene, and β-caryophyllene as major constituents, and the essential oil extracted from leaves demonstrated antibacterial activity against Staphylococcus aureus, Proteus vulgaris, and Aeromonas caviae as well as moderate fungicidal activity against Aspergillus niger.
Leaf extract of C. aromaticus was proved to be more efficient than standard Gentamycin and Bavistin against Bacillus firmus and Aspergillus niger (Roja et al. Citation2006). The flavonoids extracted from the C. aromaticus leaf extracts salvigenin and cirsimaritin were revealed to have antimicrobial properties and inhibited the growth of Pseudomonas aeruginosa, B. subtills, E. coli, Staphylococcus aureus, Candida albicans, Tricophyton mentagrophytes, and Aspergillus niger (Ragasa et al. Citation1999). Inhibitory activity of ethanolic extract of C. aromaticus via both well and disc diffusion methods (Kumaran and Karunakaran Citation2007). The acetone extract was reported with the presence of high polyphenol content and exhibited high appreciable antibacterial activity with the least MIC values against the pathogens S. aureus, B. cereus, E. coli, and Yersinia enterocolitica (Thilagavathi and HariramCitation2016).
Leaf discs from the leaves of C. aromaticus were tested against reproductive tract infecting microorganisms and exhibited an inhibitory effect in the order Candida krusei, C. albicans, Proteus mirablis, E. coli, S. aureus, Enterococcus faecalis, Klebsiella pneumoniae, and Neisseria gonohorreae (Pritima and Pandian Citation2007). The leaf extracts of C. aromaticus helped improve seed germination percentage, seedling vigour, and yield of okra and helped to deal with the fungal pathogens infecting the plant (Begum et al. Citation2009). C. aromaticus revealed inhibitory effect against S. epidermis, S. aureus, Serratia marcencens, P. aeruginosa, Proteus Vulgaris, methicillin-resistant S. aureus (MRSA), E. coli, Bacillus subtilis, C. albicans, and C. tropicalis. All these bacteria were inhibited by essential oil extracted from C. aromaticus except P. aeruginosa. Essential oil effectively inhibited with a bigger zone of inhibition against E. coli, S. aureus, and C. tropicalis tested and compared against commercial antibiotics: streptomycin and nystatin (Sabrina et al. Citation2014).
Essential oil and hydroalcoholic extract of C. aromaticus revealed significant antimicrobial activity against methicillin-resistant Staphylococcus aureus (MRSA) strains and decreased the ocharatoxin production of Aspergillus ochraceus (Murthy et al. Citation2009). Antimicrobial activity of ethanolic and aqueous extracts of C. aromaticus was tested against wastewater pathogens such as E. coli, Pseudomonas sp., Klebsiella sp., Bacillus sp., Staphylococcus sp. and the inhibitory effect was found highest against E. coli and Bacillus sp. (Malini et al. Citation2013). C. aromaticus leaf extract prepared in petroleum ether, alcohol, and water was tested against four respiratory pathogens: Sporothrix fragilis, Nocardia asteroides, Bacteroides vulgatis, and Bacteroides fragilis (Uma et al. Citation2012). The extract revealed as a positive inhibiter to all the above pathogens except B. fragilis.
The essential oil extracted from C. aromaticus leaves effectively inhibited the growth of C. albicans and A. niger (Revathi et al. Citation2011). Similarly, C. aromaticus leaf extracts prepared using water, crude alcohol, Soxhlet water, and Soxhlet alcoholic revealed significant antibacterial activity against five human pathogens found in sputum (Themozhi et al. Citation2011). The hydroalcoholic extract of C. aromaticus revealed mild to moderate inhibition for both bacteria and fungi using the cup plate method. The inhibitory action of C. aromaticus against bacteria and fungi can prove to be an additional advantage in the therapeutic role that the plant plays in the current pharmaceutic industry (Majee et al. Citation2013). Paul et al. (Citation2014) revealed that multi-drug resistant bacteria such as Bacillus sp., S. aureus, Serratia sp., E. coli, Serratia sp., Streptococcus sp., Klebsiella sp., Vibrio sp., S. typhi, S. paratyphi A, Enterobacter sp., and Pseudomonas were found to be inhibited by crude extracts of fresh and oven-dried leaves of C. aromaticus. This indicated C. aromaticus extracts could be used as an antimicrobial agent against various multi-drug-resistant pathogenic microorganisms. The essential oil extracted from C. aromaticus had small interference on the anti-yeast effect of some clinically used antifungals agents (Oliveira et al. Citation2007). This revealed that the combined effect of certain medicinal plants and/or derivatives with industrial drugs particularly antimicrobials may interfere with the expected therapeutic effects of these industrial drugs.
A comparative study of Ocimum tenuiflorum and C. aromaticus leaves against selected human gram-positive and gram-negative bacteria suggested that C. aromaticus possessed higher antibacterial activity towards all selected common clinical pathogen and had a higher inhibitory effect towards gram-positive bacteria compared to gram-negative bacteria (Jiyauddin et al. Citation2015). In vitro antimicrobial activity of the essential oils of C. aromaticus against seven bacteria: B. megaterium, B. subtilis, E. coli, S. aureus, Proteus vulgaris, P. aeruginosa, and X. campestris, and eight fungi viz., A. niger, A. parasiticus, Rhizoctonia oryzae, Colletotrichum musae, Fusarium solani, Candida albicans and Alternaria brassicicola (Deena et al. Citation2002). Jayachitra and Chitra (Citation2015) reported an aqueous ethanolic extract of C. aromaticus leaves was screened for their antibacterial potential against Klebsiella, Pseudomonas, and Staphylococcus. The essential oil extracted from dried leaves of C. aromaticus revealed higher inhibition against positive Gram S. aureus than negative Gram E. coli (Joshi et al. Citation2011). This was reported by Ramalakshmi et al. (Citation2014) for E. coli, Salmonella typhi, and Proteus spp. Antimycobacterial bioactive compounds identified in C. aromaticus are β-caryophyllene, α-terpinolene, p-cymene, and carvacrol. All the above reports prove that C. aromaticus has effective antimycobacterial properties.
Antiviral activity
C. aromaticus leaf extracts prepared in different solvents: hexane, ethanol, and chloroform have revealed significant antiviral activity against Bombyx mori Nuclear Polyhedrosis Virus (Manimegalai et al. Citation2010) (BmNPV: affects a wide range of silkworm breeds); Herpes Simplex Virus-1 (HSV1); Vesicular Stomatitis viruses (VSV) (Ali et al. Citation1996) and Herpes Simplex Virus-2 (HSV2) (Rasmi Citation1998). Similarly, leaf juice from C. aromaticus exhibited anti-HIV inhibition activity (Rasmi Citation1998). Ethanolic extract of C. aromaticus revealed to have antiviral activity on Vero cell lines when tested against Herpes Simplex Virus-1 (HSV1) and Vesicular Stomatitis (VSV) viruses (Kusumoto et al. Citation1995). C. aromaticus leaf extracts thus are reported to exhibit antiviral activity.
Other beneficial properties
C. aromaticus possess anti-influenza properties, whereas the leaf juice help in wound healing (Vera et al. Citation1992). Studies conducted on animal models revealed therapeutic efficacy in treating rheumatoid arthritis (Chang et al. Citation2010) along with antiepileptic effect (Buznego and Perez Saad Citation1999). C. aromaticus leaves and leaf extract are consumed as breast milk stimulants (Santosa Citation2002; Koba et al. Citation2011; Bhatt and Negi Citation2012). The leaves are consumed to treat reproductive issues such as childbirth and to treat male and female infertility (Koba et al. Citation2011; Bhatt and Negi Citation2012). The polysaccharides extracted from dried plant parts of C. aromaticus revealed anticoagulant activity (Yoon et al. Citation2002). The leaf extracts of C. aromaticus, Aegle marmelos, and Vitex negundo can be used as an alternative to chemical pesticides and revealed significant larvicidal activity against instars and pupa of Culex quinquefasciatus (Dass and Mariappan Citation2014).
The essential oil of C. aromaticus and the isolated constituents are used as natural larvicides to control larvae of Culex tritaeniorhynchus, Aedes albopictus, and Anopheles subpictus (Govindarajan et al. Citation2013). The essential oil extracted from C. aromaticus revealed significant larvicidal property against An. gambiae and thus can be used against the African malaria vector mosquito (Kweka et al. Citation2012). Similarly, Jaya et al. (Citation2014) demonstrated that the essential oils of C. aromaticus along with Ageratum conyzoides and Hyptis suaveolens had significant in vivo and in vitro insecticidal efficacy and was recommended as a non-phytotoxic herbal insecticide against Tribolium castaneum contamination. Similarly, a study conducted using essential oil of C. aromaticus against white termites (Odontotermes obesus Rhamb.), resulting in 100% mortality. Essential oil of C. aromaticus was reported to be more effective than commonly used synthetic insecticides Primoban-20 and Thiodan (Singh et al. Citation2002).
The C. aromaticus hydroalcoholic extract (CAE) revealed radioprotective potential that was essential for chemoprevention and in combating various free-radical mediated human pathological conditions (Rasineni et al. Citation2008). Boomi et al. (Citation2019) demonstrated that AuNPs synthesized using C. aromaticus leaf extracts could be an alternative and effective source of UV protection, antibacterial and anticancer agents.
The allelopathic property of C. aromaticus was studied by testing water suspension of dried powder of C. aromaticus leaves against a troublesome aquatic weed, water hyacinth (Eichhornia crassipes Mart.). This experiment resulted in 100% mortality of the tested water hyacinth (Kathiresan Citation2000). The effect of manuring, drying method, and soaking time on the allelopathic property of C. aromaticus on water hyacinth was carried out by Gnanavel and Kathiresan (Citation2007). This proved that both drying and soaking treatments of C. aromaticus were significantly effective in reducing fresh weight and chlorophyll content of E. crassipes. The allelopathic property of C. aromaticus indicates its ability in effective weedkilling.
Kumar et al. (Citation2007) reported that C. aromaticus leaf extract exhibited mast cell stabilization property in rat peritoneal mast cells. Aqueous extract of leaves and roots of C. aromaticus possessed wound healing activity tested on excisional wound model in albino rats. This was confirmed using histopathological examinations of healing tissues (Jain et al. Citation2012). Poultice prepared using C. aromaticus leaves are used against centipede and scorpion bites (Hussain et al. Citation2012). C. aromaticus was reported to be an excellent treatment for dental cavities and to avoid oral bacterial growth. But when used along with mouthwash, Carvacrol present in C. aromaticus revealed antagonistic effects (Santos et al. Citation2015).
Along with the use of C. aromaticus for its above-described properties, C. aromaticus also finds its application in the food and beverage industry. Other than the use of C. aromaticus leaves as a flavouring agent, stuffing, or fritters, it is also used as an appetiser, in soup mix or to prepare chutney. Wadikar and Premavalli (Citation2011) reported the appetising potential of C. aromaticus. A ready-to-drink C. aromaticus beverage (or karpurvalli beverage) had a significant appetising effect when compared to ginger or ajowan beverages and increased food intake and weight gain. A similar appetising effect was reported for a ready-to-eat C. aromaticus munch (or karpurvalli munch) with a shelf life of 8 months. Furia and Bellanca (Citation1975) reported that Carvacrol, isolated from C. aromaticus may be added to baked goods (120 ppm) and in non-alcoholic beverages (26 ppm), whereas thymol can be added in chewing gums (100 ppm) and non-alcoholic beverages (2.5–11 ppm) (Pawan et al. Citation2014). Pawan et al. (Citation2014) reported that C. aromaticus leaves can be used to prepare chutney that can be served with hot rice and fritters. C. aromaticus was reported to prepare a ready to reconstitute soup mix with a shelf life of 6 months (Wadikar and Premavalli Citation2013).
Conclusion
Coleus aromaticus is packed with many bioactive compounds and nutrients. These bioactive compounds are responsible for their bioactivities and medicinal properties. C. aromaticus has proved to be effective in curing multiple diseases. Thus, it can be stated that this herb is a suitable drug candidate that can be further explored and exploited to meet the global demand for natural, cost-effective, and safer bioactive compounds. The abundant availability of C. aromaticus in India as a must-have plant at home and in herbal gardens helps understand the use as a medicinal drug over its other relatives. This abundant availability of C. aromaticus and the presence of various bioactive compounds further opens new research vistas to strengthen its claim as an effective medicinal drug candidate. Additional research is highly recommended to authenticate the effectiveness of each bioactive compound extracted from C. aromaticus.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing does not apply to this article as no new data were created or analysed in this study.
References
- Ali AM, Mackeen MM, Sharkawy ESH, Hamid JA, Ismail NH, Ahmad FBH, Lajis NH. 1996. Antiviral and cytotoxic activities of some plants used in Malaysian indigenous medicine. Pertanika J Trop Agric Sci. 19(2/3):129–136.
- Arumugam G, Swamy MK, Sinniah UR. 2016. Plectranthus amboinicus (Lour.) Spreng: botanical, phytochemical, pharmacological and nutritional significance. Molecules. 21(4):369. DOI:10.3390/molecules21040369.
- Asiimwe S, Borg-Karlsson AK, Azeem M, Mugisha KM, Namutebi A, Gakunga NJ. 2014. Chemical composition and toxicological evaluation of the aqueous leaf extracts of Plectranthus amboinicus (Lour.) Spreng. Int J Pharm Sci Invent. 3(2):19–27.
- Baslas RK, Kumar P. 1981. Chemical examination of essential oil of Coleus aromaticus Benth. J Indian Chem Soc. 58:103–104.
- Begum M, Lokesh S, Raghavendra VB. 2009. Role of leaf extracts of some medicinal plants in the management of seed-borne fungal diseases of Okra (Abelmoschus esculentus L.) Moench. Arch Phytopathol Pflanzenschutz 42(10):950–955.
- Bhatt P, Joseph GS, Negi PS, Varadaraj MC. 2013. Chemical composition and nutraceutical potential of Indian borage (Plectranthus amboinicus) stem extract. J Chem. 2013:1–7. DOI:10.1155/2013/320329.
- Bhatt P, Negi P. 2012. Antioxidant and antibacterial activities in the leaf extracts of Indian Borage (Plectranthus amboinicus). Food Nutr Sci. 3(2):146–152. DOI:10.4236/fns.2012.32022.
- Bhattacharjee P, Majumder P. 2013. Investigation of phytochemicals and anti-convulsant activity of the plant Coleus amboinicus (Lour.). Int J Green Pharm. 7(13):211–215. DOI:10.4103/0973-8258.120223.
- Bole S, Jayashree J. 2014. Phytochemical screening and biological activities of medicinal plant Coleus aromaticus. World J Pharm Pharm Sci. 3(6):974–986.
- Boomi P, Ganesan RM, Poorani G, Halliah GP, Ravikumar S, Jeyakanthan J. 2019. Biological synergy of greener gold nanoparticles by using Coleus aromaticus leaf extract. Mater Sci Eng C Mater Biol Appl. 99:202–210. DOI:10.1016/j.msec.2019.01.105.
- Buznego MT, Perez Saad H. 1999. Antiepileptic effect of Plectranthus amboinicus (Lour.) Spreng. (French marjoram). Rev Neurol. 29(4):388–389.
- Candrappa SM, Hugar S, Itgappa M, Nagarajappa K. 2009. Antidiabetic acid antioxidant potential of Coleus aromaticus leaf extracts in alloxan-induced diabetic rats. Pharmacol Online. 1(4):316–322.
- Cano JH, Volpato G. 2004. Herbal mixtures in the traditional medicine of Eastern Cuba. J. Ethnopharmacol. 90(2–3):293–316. DOI:10.1016/j.jep.2003.10.012.
- Carbajal D, Casaco A, Arruzazabala L, Gonzalez R, Fuentes V. 1991. Pharmacological screening of plant decoctions commonly used in Cuban folk medicine. J Ethnopharmacol. 33:21–24. DOI:10.1016/0378-8741(91)90155-7.
- Cartaxoa SL, Souzaa MMA, Albuquerque UP. 2010. Medicinal plants with bioprospecting potential used in semi-arid northeastern Brazil. J. Ethnopharmacol. 131(2):326–342. DOI:10.1016/j.jep.2010.07.003.
- Castillo RAM, Gonzalez VP. 1999. Plectranthus amboinicus (Lour.) Spreng. Rev Cuba Plantas Med. 4:110–115.
- Chaluvaraju KC, Makrabbi K, Zaranappa, Naveenkumar KL. 2015. Anthelmintic potentials of leaf extracts of medicinal plants: a brief review. World J Pharm Pharm Sci. 4(12):436–452.
- Chandrappa SM, Harsha R, Dinesha R, Gowda T. 2010.Hydrogen peroxide-induced DNA damage: protection by antioxidant Coleus aromaticus. Int J Pharm. 2(3):63–66.
- Chang JM, Cheng CM, Hung LM, Chung YS, Wu RY. 2010. Potential Use of Plectranthus amboinicus in the treatment of rheumatoid arthritis. Evid Based Complement Alternat Med. 7(1):115–120.
- Chen YS, Yu HM, Shie JJ, Cheng TJR, Wu CY, Fang JM, Wong CH. 2014. Chemical constituents of Plectranthus amboinicus and the synthetic analogs possessing anti-inflammatory activity. Bioorg Med Chem. 22(5):1766–1772. DOI:10.1016/j.bmc.2014.01.009.
- Cheryl L. 2007. Ethnomedicines are used in Trinidad and Tobago for reproductive problems. J Ethnobiol. Ethnomed. 3:13. DOI:10.1186/1746-4269-3-13.
- Chiu YJ, Huang TH, Chiu CS, Lu TC, Chen YW, Peng WH, Chen CY. 2012. Analgesic and anti-inflammatory activities of the aqueous extract from Plectranthus amboinicus (Lour.) Spreng. both in vitro and in vivo. Evid Based complement Altern Med. 2012:1–11.
- Choudhary GP. 2013. Anthelmintic activity of leaves of Coleus aromaticus Benth. IJAPBC. 2(4):609–610.
- Da Costa JG, Pereira CK, Rodrigues FF, De Lima SG. 2010. Chemical composition, antibacterial and fungicidal activities of leaf oil of Plectranthus amboinicus (Lour.) Spreng. J Essent Oil Res. 22(2):183–185. DOI:10.1080/10412905.2010.9700298.
- Dash SK, Sachidananda P. 2006. Review on ethnomedicines for diarrhea diseases from Orissa: prevalence versus culture. J Human Ecol. 20(1):59–64. DOI:10.1080/09709274.2006.11905903.
- Dass K, Mariappan P. 2014. Larvicidal activity of Aegle marmelos, Coleus aromaticus and Vitex negundo leaf extract against filarial vector Culex quinquefasciatus. TURKJANS. 1(Special Issue):858–862.
- Deena MJ, Sreeranjini K, Thoppil JE. 2002. Antimicrobial screening of essential oils of Coleus aromaticus and Coleus zeylanicus. Int J Aromather. 12(2):105–107.
- De Lima SCG, Teixeira MJ, Lopes JEG Jr, De Morais SM, Torres AF, Braga MA, Rodrigues RO, Santiago GMP, Martins AC, Nagao-Dias AT. 2014. In vitro and in vivo leishmanicidal activity of Astronium fraxinifolium (schott) and Plectranthus amboinicus (Lour.) Spreng against Leishmania (Viannia) braziliensis. BioMed Res Int 2014:848293. DOI:10.1155/2014/848293.
- Dutta S. 1959. Essential oil of Coleus aromaticus of Indian origin. In: Indian Oil Soap J. Vol. 25. p. 120.
- El-hawary SS, El-sofany RH, Abdel-Monem AR, Ashour RS. 2012. Phytochemical screening, DNA fingerprinting, and nutritional value of Plectranthus amboinicus (Lour.) Spreng. Pharmacogn J. 4(30):10–13. DOI:10.5530/pj.2012.30.2.
- Furia TE, Bellanca N. 1975. Fenaroli’s handbook of flavor ingredients. In: Adapted from the Italian language works of prof. Dr. Giovanni Fenaroil. 2nd ed., Vol. 2. Cleveland: CRC Press.
- Ghosh RB, Sur TK, Maity LN, Chakraborty SC. 2000. Antiurolithiatic activity of Coleus aromaticus Benth. In rats. Anc Sci Life. 20(1–2):44–47.
- Gilman JG, Hardman JG, Limbird LE. 2001. The pharmacological basis of therapeutics, 10th ed. USA: McGraw-Hill Medical Publishing Division; p. 450–455.
- Girish K. 2016. Antimicrobial activities of Coleus aromaticus Benth. J Pharm Res. 10(10):635–646.
- Gnanavel I, Kathiresan RM. 2007. Effect of manuring, drying methods, and soaking time on the allelopathic potential of Coleus amboinicus/aromaticus on Eichhornia crassipes. Res J Agric Biol Sci. 3(6):723–726.
- Govindarajan M, Sivakumar R, Rajeswary M, Veerakumar K. 2013. Mosquito larvicidal activity of thymol from essential oil of Coleus aromaticus Benth. against Culex tritaeniorhynchus, Aedes albopictus, and Anopheles subpictus (Diptera: Culicidae). Parasitol Res. 112(11):3713–3721. DOI:10.1007/s00436-013-3557-2.
- Govindaraju S, Arulselvi PI. 2018. Characterization of Coleus aromaticus essential oil and its major constituent carvacrol for in vitro antidiabetic and antiproliferative activities. J Herbs Spice Med Plants. 24(1):37–51. DOI:10.1080/10496475.2017.1369483.
- Günes-Bayir A, Kiziltan HS, Kocyigit A, Güler EM, Karataş E, Toprak A. 2017. Effects of natural phenolic compound carvacrol on the human gastric adenocarcinoma (AGS) cells in vitro. Anticancer Drugs. 28(5):522–530.
- Günes-Bayir A, Kocyigit A, Güler EM, Bilgin MG, Ergün İS, Dadak A. 2018. Effects of carvacrol on human fibroblast (WS-1) and gastric adenocarcinoma (AGS) cells in vitro and on Wistar rats in vivo. Mol Cell Biochem. 448(1):237–249. DOI:10.1007/s11010-018-3329-5.
- Günes-Bayir A, Kocyigit A, Guler EM, Dadak A. 2020. In vitro hormetic effect investigation of thymol on human fibroblast and gastric adenocarcinoma cells. Molecules. 25(14):3270. DOI:10.3390/molecules25143270.
- Gurgel APAD, Da Silva JG, Grangeiro ARS, Xavier HS, Oliveira RAG, Pereira MSV, De Souza IA. 2009. Antibacterial effects of Plectranthus amboinicus (Lour.) Spreng (Lamiaceae) in methicillin-resistant Staphylococcus aureus (MRSA). Lat Am J Pharm. 28(3):460–464.
- Gurib-Fakim A, Sewraj MD, Narod F, Menut C. 1995. Aromatic plants of Mauritius: volatile constituents of the essential oils of Coleus aromaticus Benth., Triphasia trifolia (Burm. f.), and Eucalyptus kirtoniana (F. Muell.). J Essent Oil Res. 7(2):215–218. DOI:10.1080/10412905.1995.9698504.
- Hassani MS, Zainati I, Zrira S, Mahdi S, Oukessou M. 2012. Chemical composition and antimicrobial activity of Plectranthus amboinicus (Lour) spring. essential oil from the Archipelago of Comoros. J Essent Oil Bear Pl. 15(2):637–644. DOI:10.1080/0972060X.2012.10644098.
- Hole RC, Juvekar AR, Roja G, Eapen S, D’Souza SF. 2009. Positive inotropic effect of the leaf extracts of parent and tissue culture plants of Coleus amboinicus on an isolated perfused frog heart preparation. Food Chem. 114(1):139–141. DOI:10.1016/j.foodchem.2008.09.047.
- Hussain A, Sonkar AK, Ahmad P, Wahab S. 2012. In-vitro anthelmintic activity of Coleus aromaticus root in Indian adult earthworm. Asian Pac J Trop Dis. 2(1):425–427. DOI:10.1016/S2222-1808(12)60196-0.
- Jain AK, Dixit A, Mehta SC. 2012. Wound healing activity of aqueous extracts of leaves and roots of Coleus aromaticus in rats. Acta Pol Pharm. 69(6):1119–1123.
- Jain SK, Lata S. 1996. Amazonian uses of some plants growing in India. Indigenous Knowl Dev Monit. 4(3):21–23.
- Jayachitra J, Chitra M. 2015. Antibacterial activity of Coleus aromaticus L. World J Pharm Pharm Sci. 4(05):1026–1030.
- Jaya, Singh P, Prakash B, Dubey NK. 2014. Insecticidal activity of Ageratum conyzoides L., Coleus aromaticus Benth. and Hyptis suaveolens (L.) poit essential oils as fumigant against storage grain insect Tribolium castaneum Herbst. J Food Sci Technol. 51(9):2210–2215. DOI:10.1007/s13197-012-0698-8.
- Jiyauddin K, Samer AD, Darashhni T, Rasha S, Jawad A, Kaleemullah M, Budiasih S, Rasny MR, Qamar M, Hamid K, et al. 2015. Comparison of antibacterial activity of Ocimum tenuiflorum and Plectranthus amboinicus (Lour.) spreng against the clinical pathogens Staphylococcus aureus, Pseudomonas aeruginosa and Escherichia coli. World J Pharm. Res. 4(3):1887–1901.
- Jose MA, Janardhanan S. 2005. Modulatory effect of Plectranthus amboinicus Lour. on ethylene glycol-induced nephrolithiasis in rats. Indian J Pharmacol. 37(1):37–45.
- Joshi RK, Badakar V, Kholkute SD. 2011. Carvacrol rich essential oils of Coleus aromaticus (Benth.) from Western Ghats region of North West Karnataka, India. Adv Environ Biol. 5(6):1307–1310.
- Kaliappan ND, Viswanathan PK. 2008. Pharmacognostical studies on the leaves of Plectranthus amboinicus (Lour) Spreng. Int J Green Pharm. 2(3):182–184.
- Kathiresan RM. 2000. Allelopathic potential of native plants against water hyacinth. Crop Prot. 19(8–10):705–708. DOI:10.1016/S0261-2194(00)00119-8.
- Khanum H, Ramalakshmi K, Srinivas P, Borse BB. 2011. Synergistic antioxidant action of Oregano, Ajowan and Borage extracts. Food Nutr Sci. 2:387–392. DOI:10.4236/fns.2011.25054.
- Khare RS, Banerjee S, Kundu KK. 2011. Coleus aromaticus Benth. A nutritive medicinal plant of potential therapeutic value. Int J Pharma Bio Sci 2(3):488–500.
- Khattak MMAK, Taher M, Abdulrahman S, Abu Bakar I, Damanik R, Yahaya A. 2013. Anti-bacterial and anti-fungal activity of Coleus leaves consumed as breast-milk stimulant. Nutr Food Sci. 43(6):582–590. DOI:10.1108/NFS-11-2011-0131.
- Koba K, Nénonéné AY, Sanda K, Garde D, Millet J, Chaumont JP, Raynaud C. 2011. Antibacterial activities of Coleus aromaticus Benth. (Lamiaceae) essential oil against oral pathogens. J Essen Oil Res. 23(1):13–17. DOI:10.1080/10412905.2011.9700424.
- Krithiga N, Jayachitra A. 2012. Antioxidant and antibacterial study on Coleus aromaticus and Lawsonia inermis. Int J Pharm Life Sci. 3(9):1958–1964.
- Kumar A, Elango K, Markanday S, Undhad CV, Kotadiya AV, Savaliya BM, Vyas DN, Datta D. 2007. Mast cell stabilization property of Coleus aromaticus leaf extract in rat peritoneal mast cells. Indian J Pharmacol. 39(2):119–120. DOI:10.4103/0253-7613.32533.
- Kumara SM, Sudipta KM, Lokesh P, Neeki A, Rashmi W, Bhaumik H, Darshil H, Vijay R, Kashyap SSN. 2012. Phytochemical screening and in vitro antimicrobial activity of Bougainvillea spectabilis flower extracts. Int J Phytomed. 4:375–379.
- Kumaran A, Karunakaran RJ. 2007. Activity-guided isolation and identification of free radical-scavenging components from an aqueous extract of Coleus aromaticus. Food Chem. 100(1):356–361. DOI:10.1016/j.foodchem.2005.09.051.
- Kumari R, Prasad MNV. 2014. Effect of UV-B pretreatment on essential oil components, health sensory secondary metabolites and antioxidant potential of Coleus aromaticus. Int J Bio Pharma Res. 5(8):675–688.
- Kusumoto IT, Nakabayashi T, Kida H, Miyashiro H, Hattori M, Namba T, Shimotohno K. 1995. Screening of various plant extracts used in ayurvedic medicine for inhibitory effects on human immunodeficiency virus-type 1 (HIV-1) protease. Phytother Res. 9(3):180–184. DOI:10.1002/ptr.2650090305.
- Kweka EJ, Senthilkumar A, Venkatesalu V. 2012. Toxicity of essential oil from Indian borage on the larvae of the African malaria vector mosquito, Anopheles gambiae. Parasit Vectors. 5:277. DOI:10.1186/1756-3305-5-277.
- Llarena ZM. 2016. Spectroscopic determination of functionalized active principles from Coleus Aromaticus Benth leaf extract using ionic liquids. Proceedings of the 18th International Conference on Analytical Chemistry and Bioanalytical Chemistry 3(1). p. 1169.
- Lukhoba CW, Simmonds MSJ, Paton AJ. 2006. Plectranthus: A review of ethnobotanical uses. J Ethnopharmacol. 103(1):1–24. DOI:10.1016/j.jep.2005.09.011.
- Majee C, Das GK, Mazumder R, Chakraborthy GS. 2013. Evaluation of analgesic and antimicrobial potential of hydroalcoholic extract of leaves of Coleus aromaticus in albino mice. J Phytopharmacol. 2(3):18–25.
- Malini M, Abirami G, Hemalatha V, Annadurai G. 2013. Antimicrobial activity of ethanolic and aqueous extracts of medicinal plants against wastewater pathogens. Int J Pure Appl Microbiol. 3(2):40–42.
- Mallavarapu GR, Rao L, Ramesh S. 1999. Essential oil of Coleus aromaticus Benth. from India. Int J Essent Oil Res. 11(6):742–744. DOI:10.1080/10412905.1999.9712009.
- Mamani R, Alhaji NM. 2019. GC-MS analysis of phytocomponents in methanolic extract of Coleus aromaticus. J Pharmacogn Phytochem. 8(4):106–109.
- Manimegalai S, Rajeswari T, Shanmugam R, Rajalakshmi G. 2010. Botanicals against Nuclear Polyhedrosis Virus infecting three breeds of mulberry silkworm. Bombyx mori L. J Bio Pest. 3(1):242–245.
- Manjamalai A, Grace DVB. 2012. Volatile constituents and antioxidant property of essential oil from Plectranthus amboinicus (Lour). Int J Pharm Biol Sci. 3(4):445–458.
- Mathur A, Verma SK, Singh SK, Prasad GBKS, Dua VK. 2011. Investigation of the antimicrobial, antioxidant, and anti-inflammatory activity of compound isolated from Murraya koenigii. IJABPT. 1(2):545–548.
- MeghaRani N, Sridevi K, Rao SN. 2016. Preliminary phytochemical analysis of fresh juice and aqueous extract of Coleus amboinicus Linn leaves. Int J Appl. 7(1):216–221.
- Mohanty SK, Malappa K, Godavarthi K, Subbanarasiman B, Maniyam A. 2014. Evaluation of antioxidant, in vitro cytotoxicity of micropropagated and naturally grown plants of Leptadenia reticulata (Retz.) Wight & Arn.: An endangered medicinal plant. Asian Pac J Trop Med. 7:267–271. DOI:10.1016/S1995-7645(14)60244-3.
- Morton JF. 1992. Country borage (Coleus amboinicus Lour.): a potent flavoring and medicinal plant. J Herbs Spices Med Plants. 1(1–2):77–90. DOI:10.1300/J044v01n01_09.
- Murthy PS, Ramalakshmi K, Srinivas P. 2009. Fungitoxic activity of Indian borage (Plectranthus amboinicus) volatiles. Food Chem. 114:1014–1018. DOI:10.1016/j.foodchem.2008.10.064.
- Oliveira RAG, Lima EO, De Souza EL, Vieira WL, Freire KRL, Trajano VN, Lima IO, Silva-Filho RN. 2007. Interference of Plectranthus amboinicus (Lour.) Spreng essential oil on the anti-Candida activity of some clinically used antifungals. Rev Bras Farmacogn. 17(2):186–190. DOI:10.1590/S0102-695X2007000200009.
- Ong HC, Nordiana M. 1999. Malay ethno-medico botany in Machang, Kelantan, Malaysia. Fitoterapia. 70(1):502–513. DOI:10.1016/S0367-326X(99)00077-5.
- Paul NM, Anjali M, Elza J, Nayomi J, Ajitha AR, Shanti AA. 2014. Phytochemical screening and evaluation of the antimicrobial potential of Coleus amboinicus. World J Pharm Res. 3(2):2811–2826.
- Pawan K, Wadikar DD, Patki PE. 2014. A ready-to-eat antioxidant-rich appetizer based on Coleus aromaticus: its development and shelf-life evaluation. J Food Process. 1. DOI:10.4314/ajid.v1i1.42081.
- Periyanayagam K, Devi NK, Suseela L, Uma A, Ismail M. 2008. In vivo antimalarial activity of leaves of Plectranthus amboinicus (Lour) Spreng on Plasmodium berghei yoelii. J Commun Dis. 40(2):121–125.
- Prameela TS, Oommen PS. 2011. Phytochemical screening, antimicrobial and anthelminthic studies on Coleus aromaticus Benth. Int J Pharm Res. & Dev. 3(4):93–103.
- Pritima RA, Pandian RS. 2007. Antimicrobial activity of Coleus aromaticus (Benth) against microbes of reproductive tract infections among women. Afr J Infect Dis. 1(1):18–24. DOI:10.4314/ajid.v1i1.42081.
- Prudent D, Perineau F, Bessiere JM, Michel GM,Baccou JC. 1995. Analysis of the essential oil of wild oregano from Martinique (Coleus aromaticus Benth.): evaluation of its bacteriostatic and fungistatic properties. J Essent Oil Res. 7(2):165–173. DOI:10.1080/10412905.1995.9698492.
- Ragasa CY, Sangalang V, Pendon Z, Rideout JA. 1999. Antimicrobial flavones from Coleus amboinicus. Philippine J Sci. 128(4):347–351.
- Ramadas D, Gurumahadevaiah S, Ramakrishna H, Mundasada SC. 2014. Hydrogen peroxide-induced DNA damage: protection by antioxidant Coleus aromaticus . World J Pharm Res. 3(5):743–752.
- Ramalakshmi P, Subramanian N, Saravanan R, Mohanakrishnan H, Muthu M. 2014. Anticancer effect of Coleus amboinicus (Karpooravalli) on human lung cancer cell line (A549). Int J Dev Res. 4(11):2442–2449.
- Ramli N, Syed Ahamed PO, Elhady HM, Taher M. 2014. Antimalarial activity of Malaysian Plectranthus amboinicus against Plasmodium berghei. Pharmacogn Res. 6(4):280–284. DOI:10.4103/0974-8490.138248.
- Rao BSS, Shanbhoge R, Upadhya D, Jagetia GC, Adiga SK, Kumar P, Guruprasad K, Gayathri P. 2006. Antioxidant, anticlastogenic and radioprotective effect of Coleus aromaticus on Chinese hamster fibroblast cells (V79) exposed to gamma radiation. Mutagenesis. 21(4):237–242. DOI:10.1093/mutage/gel023.
- Rasineni GK, Siddavattam D, Reddy AR. 2008. Free radical quenching activity and polyphenols in three species of Coleus. J Med Plant Res. 2(10):285–291. DOI:10.5897/JMPR.9000657.
- Rasmi A. 1998. Antiviral activity against Herpes Simplex Virus type 2 of extracts from Cerbera odollam Gaertn., Clausena excavata Burm. F., Coleus amboinicus Lour., Phyla nodiflora (L.) Greene. and Thevetia peruviana Schum [M.Sc. thesis]. Bangkok: Chulalongkorn University.
- Retief E. 2000. Lamiaceae (Labiatae). In: Leistner O.A., editor. Seed plants of Southern Africa. Cape Town: National Botanical Institute; p. 323–334p.
- Revathi A, Thangabalan B, Rao PV, Vadivel K. 2011. Microbiological activity of essential oil extracted from Coleus aromaticus Linn leaves. Res J Pharm Biol Chem Sci. 2(1):12–14.
- Roja G, Pol BB, Subbaraman AS, Chintalwar GJ, Eapen S. 2006. Accumulation of essential oils in tissue cultures of Coleus amboinicus. J Herbs Spice Med Plants. 11(4):37–41. DOI:10.1300/J044v11n04.
- Rout OP, Acharya R, Mishra SK, Sahoo R. 2012. Pathorchur (Coleus aromaticus): A review of the medicinal evidence for its phytochemistry and pharmacology properties. Int J Appl Biol Pharm Technol. 3(4):348–355.
- Ruiz AR, de la Torre RA, Alonso N, Villaescusa A, Betancourt J, Vizoso A. 1996. Screening of medicinal plants for induction of somatic segregation activity in Aspergillus nidulans. J Ethnopharmacol. 52(3):123–127. DOI:10.1016/0378-8741(96)01394-3.
- Sabrina MNE, Razali M, Mirfat AHS, Mohd Shukri MA. 2014. Antimicrobial activity and bioactive evaluation of Plectranthus amboinicus essential oil. Am J Res Commun. 2(12):121–127.
- Sandhya S, Kumar SP, Vinod KR, David B, Kumar K. 2011. Plants as potent anti-diabetic and wound healing agents: A review. Hygeia J Drugs Med. 3:11–19.
- Santos FAV, Serra CG, Roberto JAC, Figueredo FG, Matias EFF, Menezes RA, Jose GM, Henrique DM. 2015. Antibacterial activity of Plectranthus amboinicus Lour (Lamiaceae) essential oil against Streptococcus mutans. Eur J Integr Med. 8(3):293–297. DOI:10.1016/j.eujim.2015.11.021.
- Santosa CM. 2002. The effect of ‘Bangun-Bangun’ leaves (Coleus amboinicus L.) consumption of the potency of milk secretion and its composition of lactating mothers. Indones J Pharm. 13(3):133–139.
- Saraswati S, Katnoria JK, Nagpal AK. 2016. Analytical techniques for phytochemicals screening and bioactivities of some Coleus species: a review. J Pharm Sci Res. 8(4):227–237.
- Senthilkumar A, Venkatesalu V. 2010. Chemical composition and larvicidal activity of the essential oil of Plectranthus amboinicus (Lour.) Spreng against Anopheles stephensi: A malarial vector mosquito. Parasitol Res. 107(5):1275–1278. DOI:10.1007/s00436-010-1996-6.
- Shubha JR, Bhatt P. 2015. Plectranthus amboinicus leaves stimulate growth of probiotic L. plantarum: evidence for ethnobotanical use in diarrhea. J Ethnopharmacol. 166:220–227. DOI:10.1016/j.jep.2015.02.055.
- Singh G, Singh OP, Prasad YR, Lamposona MP, Catalan C. 2002. Studies on essential oils. Part 33. chemical and insecticidal investigations on leaf oil of Coleus amboinicus (Lour). Flavour Frag J. 17:440–442. DOI:10.1002/ffj.1123.
- Soni H, Singhai AK, Sharma S, Malik JK. 2012. Recent updates on the genus Coleus: a review. Asian J Pharm. Clin Res. 5(1):12–17.
- Sur TK, Pandit S, Biswas TK, Ghosh RB, Bhattacharyya D. 2003. Diuretic activity of Coleus aromaticus Benth on rats. Anc Sci Life. 22(4):146–151.
- Suryowati T, Damanik R, Bintang M, Handharyani E. 2013. Antihyperlipidemic activity of torbangun extract (Coleus amboinicus Lour) on diabetic rats induced by streptozotocin. IOSR J Pharm. 5(5):50–54.
- Swamy MK, Sinniah UR. 2015. A comprehensive review on the phytochemical constituents and pharmacological activities of Pogostemon cablin Benth.: an aromatic medicinal plant of industrial importance. Molecules. 20(5):8521–8547. DOI:10.3390/molecules20058521.
- Tempone AG, Sartorelli P, Teixeira DO, Prado F, Calixto LARL, Lorenzi H, Melhem MSC. 2008. Brazilian flora extracts as source of novel antileishmanial and antifungal compounds. Mem Inst Oswaldo Cruz. 103(5):443–449. DOI:10.1590/s0074-02762008000500006.
- Tewari G, Pande C, Kharkwal G, Singh S, Singh C. 2012. Phytochemical study of essential oil from the aerial parts of Coleus aromaticus Benth. Nat Prod Res. 26(2):182–185. DOI:10.1080/14786419.2011.574135.
- Themozhi S, Bhuvana M, Ahmed-John S. 2011. Screening of antimicrobial and phytochemical investigation of Coleus aromaticus (Benth) leaf against five respiratory pathogens. J Pharma Res. 4(7):2261–2262.
- Thilagavathi S, Hariram N. 2016. Coleus aromaticus Benth synthesis of potentially nanomedicine as high nutritive value of human health and immunomodulator. IJSRM Human. 4(4):18–38.
- Uma M, Jothinayaki S, Kumaravel S, Kalaiselvi P. 2011. Determination of bioactive components of Plectranthus amboinicus (Lour) by GC-MS analysis. NY Sci J. 4(8):66–69.
- Uma M, Saraswati X, Kalaiselvi P. 2012. Antimicrobial activity of Plectranthus amboinicus L. leaf extract against respiratory pathogens. J Herbal Med Technol. 6(1):187–193.
- Velasco J, Rojas LB, Díaz T, Usubillaga A. 2009. Chemical composition and antibacterial activity of the essential oil of Coleus amboinicus (Lour.) against enteric pathogens. J Essent Oil Bear Pl. 12(4):453–461. DOI:10.1080/0972060X.2012.10644033.
- Venkatesh G, Baburao K, Rajesh Babu M, Dhanalakshmi S, Indira Priyadarshini G. 2010. Anti urolithiatic and antihyperlipidemic activity of Coleus aromaticus- an explanation of the underlying mechanisms. Int J Phytomed. 2(3):284–291.
- Vera R, Mondon JM, Buttesti JC. 1992. Some medicinal plants of reunion. Proceedings of the 7th Asian Symposium on Medicinal Plants, Spices and other Natural Products, (ASOMPS VII); Manila.
- Verma RS, Padalia RC, Chauhan A. 2012. Essential oil composition of Coleus aromaticus Benth. from Uttarakhand. J Essent Oil Bear. Pl. 15(2):174–179. DOI:10.1080/0972060X.2012.10644033.
- Vijayavel K, Anbuselvam C, Ashokkumar B. 2013. Protective effect of Coleus aromaticus Benth (Lamiaceae) against naphthalene-induced hepatotoxicity. Biomed Environ Sci. 26(4):295–302. DOI:10.3967/0895-3988.2013.04.008.
- Wadikar DD, Patki PE. 2016. Coleus aromaticus: a therapeutic herb with multiple potentials. J Food Sci Technol. 53(7):2895–2901. DOI:10.1007/s13197-016-2292-y.
- Wadikar DD, Premavalli KS. 2011. Appetizer administration stimulates food consumption, weight gain, and leptin levels in male Wistar rats. Appetite. 57(1):131–133. DOI:10.1016/j.appet.2011.04.001.
- Wadikar DD, Premavalli KS. 2013. Development of a hot water reconstitutable appetizer soup mix from Coleus aromaticus using response surface methodology. Int Food Res J. 20(6):3041–3046.
- Wadikar DD, Premavalli KS. 2014. Beverage from Coleus aromaticus reduces leptin levels and improves appetite rating in human volunteers. Nutrition. 30(6):702–705. DOI:10.1016/j.nut.2013.11.02.
- Warrier PK, Nambiar VPK, Ramankutty C, Vasudevan Nair R. 1996. Indian medicinal plants. Vol. 4. Madras: Orient Longman.
- Wu D, Wang W, He X, Jiang M, Lai C, Hu X, Xi J, Wang M. 2019. Biofabrication of nano copper oxide and its aptamer bioconjugate for delivery of mRNA 29b to lung cancer cells. Mater Sci Eng C Mater Biol Appl. 97:827–832. DOI:10.1016/j.msec.2018.12.009.
- Yoganarasimhan SN. 2000. Medicinal plants of India, Tamil Nadu. Srinivasan V., Kosal Ram N., editor. Bangalore: Cyber Media.
- Yoon SJ, Pereira MS, Pavao MSG, Hwang JK, Pyun YR, Mourao PAS. 2002. The medicinal plant Porana volubilis contains polysaccharides with anticoagulant activity mediated by heparin cofactor II. Thromb Res. 106(1):51–58. DOI:10.1016/S0049-3848(02)00071-3.