Abstract
To determine association of severe anemia and red blood cell (RBC) transfusions with bronchopulmonary dysplasia (BPD) in preterm infants with gestational age <32 weeks, a retrospective analysis was conducted on 364 newborns from August 1,2018, until August 31,2020. 135 patients were diagnosed with BPD and the other 229 neonates were included in the non-BPD group. One-to-one propensity score matching (83:83) was used to match the baseline characteristics and confounding factors of patients so as to better evaluate effects of anemia and RBC transfusion. The results showed that the rate of severe anemia was significantly different between the BPD group and non-BPD group [14/83(16.87) vs 4/83(4.82), p = 0.013]. The number and volume of transfusion were significantly lower in the non-BPD group than in the BPD group [number:1(0,2) vs 2(0,4), p = 0.001;volume:30(0,40) vs 40(20,80), p = 0.004]. We find that Among anemic neonates,only severe anemia may increase the risk of developing BPD. Larger volume and higher number of RBC transfusions are related to BPD and its severity. Risk of BPD was neither related to the onset of anemia nor the timing of first transfusion. Medical methods should be adopted to prevent severe anemia and reduce RBC transfusions.
Registration Name of registry: Chinese Clinical Trial Registry, Registry number: ChiCTR 2100042768. Registration date: 28 January, 2021. URL of the trial in the registry database:http://www.chictr.org.cn/listbycreater.aspx.
Introduction
Bronchopulmonary dysplasia (BPD) is one of the most common, complex, and severe diseases in preterm infants. BPD was first described as chronic pulmonary disease in survivors of severe respiratory distress syndrome (RDS) in 1967 by Northway et al., which was also called as the ‘old’ BPD (Northway et al. Citation1967). In recent years, the definition for BPD has developed a lot (Thebaud et al. Citation2019). The National Institute of Child Health and Human Development (NICHD) workshop in 2018 assessed BPD at 36 post-menstrual age (PMA) along with radiographic confirmation and used a severity grading of I–III (Higgins et al. Citation2018). Although with effective surfactant supplement and oxygen support, BPD brings a great challenge to neonatologists.
Anemia remains a frequent occurrence in neonatal intensive care units. Compared with full-term infants, anemia is more common in preterm infants (Ozsoylu Citation2014; Aher et al. Citation2008). The pathogenesis is multi-factorial; it can result from poor iron stores, relatively insufficient Red blood cell (RBC) production, increased destruction of erythrocytes, shortened RBCs lifespan and blood loss for clinical examinations (Aher et al. Citation2008; Colombatti et al. Citation2016; Hellstrom et al. Citation2020; Strauss Citation2010).
Anemia is reported to have a relationship with some respiratory disease, such as asthma, acute lower respiratory tract infections, chronic obstructive pulmonary disease, acute viral bronchiolitis, and coronavirus disease 2019 (Pizzini et al. Citation2020; Xu et al. Citation2020; Ramakrishnan and Borade Citation2010; Hussain et al. Citation2014; Tourniaire et al. Citation2018; Hariyanto and Kurniawan Citation2020). Duan et al. (Citation2016) reported that low hematocrit (Hct) and early anemia were closely related to BPD in preterm infants (Duan et al. Citation2016). RBC transfusions are both common and important therapeutic plans for anemia of prematurity (AOP) (Venkatesh et al. Citation2012). Zhang et al. (Citation2014) stated that RBC transfusion was associated with BPD (Zhang et al. Citation2014). Nevertheless, to our knowledge, the studies on the relation between anemia, RBC transfusions with BPD were very preliminary, and the clinical evidences were weak.
We performed a retrospective study with one-to-one propensity score matching to investigate the association of anemia, especially severe anemia, with the development of BPD in preterm Infants. We also investigated whether the incidence in need of RBC transfusion, number and volume of RBC transfusion are related to the risk of BPD and its severity.
Materials and methods
Population
Patients selection
This retrospective study was conducted in the Department of Neonatology, Children's Hospital of Chongqing Medical University, China. The data were collected from the medical records of the Department of Neonatology from 1 August 2018 until 31 August 2020.
Inclusion criteria
Neonates meeting the following criteria were enrolled: (1) neonates with gestational age less than 32 weeks; (2) neonates who were admitted to the hospital within 48 h after birth.
Exclusion criteria
Neonates meeting at least one of the following criteria were not eligible for the study: (1) neonates with severe congenital heart disease, severe ischemic hypoxic encephalopathy or chromosomal abnormalities; (2) neonates with upper respiratory tract abnormalities, lung malformation or pulmonary hypoplasias; (3) neonates with genetic tests suggesting mutations in the Panel gene of the respiratory system; (4) neonates who died within 14 days or giving up treatment within 36 weeks of corrected gestational age; (5) neonates who missed important clinical information. The study was approved by the Institutional Review Board of Children's Hospital of Chongqing Medical University. The methods were carried out in accordance with the approved guidelines.
Definition of the important diagnoses and concepts
The diagnosis and severity of AOP were made according to the Hct concentration comparing the mean for postnatal age. Anemia was defined as a central venous Hct <39%. The severity of anemia classified in this study were as follows: (1) mild anemia: if Hct was ≥35% but <39%, (2) moderate anemia: if Hct was ≥25% but <35%, (3) severe anemia: if Hct was <25% (Singh et al. Citation2011). BPD was diagnosed at 36 weeks PMA, and diagnostic criteria for severity grading of I–III that incorporated newer modes of non-invasive ventilation was established by NICHD workshop in 2018 (Higgins et al. Citation2018). Diagnostic criteria for the following disease were according to the literature: Intraventricular hemorrhage (IVH) (Radic et al. Citation2015), Necrotizing enterocolitis (NEC) (Sharma and Hudak Citation2013), neonatal respiratory distress syndrome (NRDS) (Sweet et al. Citation2013), Acute respiratory distress syndrome (ARDS) (De Luca et al. Citation2017), premature rupture of membrane (PROM) (Preterm labour and birth Citation2018), maternal pregnancy-Induced hypertension (Brown et al. Citation2018), chorioamnionitis (Tita and Andrews Citation2010), sepsis (Shane et al. Citation2017), and Retinopathy of prematurity (ROP) (Vander et al. Citation2005).
Data collection
Maternal and neonatal data were collected from medical records by trained research staff. We reviewed the patients’ medical records and collected data, including prenatal care, birth history, and postnatal clinical course. The indication of transfusion in our hospital for neonates (≤32 weeks gestational age at birth) followed the Guidelines in 2016 (New et al. Citation2016). Data were again reviewed respectively by two researchers.
Statistical analyses
SPSS 25.0 software (IBM, USA) was used to perform the statistical analysis. All data were tested for normality. Continuous data with a normal distribution was represented standard deviation, and the t-test of independent samples was used for pairwise comparison, while continuous data that did not meet a normal distribution were subjected to the nonparametric rank-sum test. Skewed data was represented as median (quartiles) [M (P25–P75)]. After univariate analysis of the BPD risk factors, factors with P < 0.05 were selected for binary logistic regression analysis to determine the independent factors of BPD (P < 0.05 was considered to be statistically significant). The variables that were considered to have a univariable association (p < 0.05) with BPD were included in multivariate logistic regression analysis to determine the independent factors of BPD (p < 0.05). We evaluated the collinearity before analysis, and when variables were highly correlated, the variable with the highest univariable association with the outcome in the multivariate analysis was retained. Propensity score matching (PSM) was estimated using a logistic regression model in which the following covariates were included: male, gestational age, birth weight, small for gestational age, PROM, chorioamnionitis, maternal pregnancy-induced hypertension, prenatal glucocorticoid, pulmonary hemorrhage, NRDS, sepsis, perinatal asphyxia, pulmonary hypertension, pneumonia. Those covariates were selected based on the previous literature in which an association between those indicators and the incidence of BPD was reported (Higgins et al. Citation2018). A 1:1 ‘nearest neighbor’ case–control match without replacement was used (Austin Citation2009). Each neonate diagnosed with BPD was matched with a non-BPD neonate with the closest estimated propensity scores. All analyses were verified by two statisticians (Xiaohua Liang and Xian Tang).
This study was approved by the Institutional Review Board, Children's Hospital of Chongqing Medical University of China.
Results
Clinical characteristics of participants
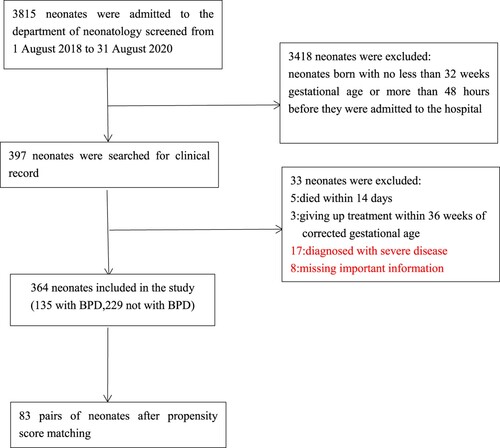
Between August 1 2018 to August 31 2020, 3815 neonates were admitted to the Department of Neonatology, Children's Hospital of Chongqing Medical University. In total, 3418 neonates were born with no less than 32 weeks gestational age or more than 48 h before they were admitted to the hospital. Of the remaining 397, 8 neonates missed important information, 8 neonates died within 14 days or giving up treatment within 36 weeks of corrected gestational age, and 17 neonates were diagnosed with severe disease which were included in exclusion criteria. Ultimately, 364 neonates were included in the study. In total, 135 cases were diagnosed with BPD and 229 cases, not with BPD (Figure ). The demographic and clinical characteristics of them are shown in Table . Before matching, 18 out of the 34 covariates, including gestational age, birth weight, PROM, pulmonary hemorrhage, perinatal asphyxia, pulmonary hypertension, PDA, sepsis, NRDS, IVH ≥ 2nd, ROP ≥ 2nd, AOP, severe anemia, early anemia, RBC transfusion, number of transfusion, volume of transfusion, first transfusion (≤7d), mechanical ventilation (days), were significantly different between the two groups. After matching, 166 cases were included in the PSM model. Fifteen covariates were well balanced, and no significant differences were observed (Table ).
Table 1. Comparison of demographic and clinical characteristics between BPD patients and non-BPD patients.
Table 2. Baseline characteristics and confounding factors of neonates in BPD patients and non-BPD patients groups after matching.
Univariate and multivariate analyses of the risk of BPD
In univariate analysis, variables that were considered to have a univariable association with BPD included gestational age, birth weight, PROM, pulmonary hemorrhage, NRDS, sepsis, perinatal asphyxia, pulmonary hypertension, PDA, AOP, severe anemia, early anemia, RBC transfusion, number of transfusion, volume of transfusion, first transfusion (≤7d), mechanical ventilation, with a p value of less than 0.05 (Table ). Furthermore, in multivariate analysis, variables that retained an independent association with the incidence of BPD included birth weight, NRDS, pulmonary hemorrhage, mechanical ventilation, severe anemia and volume of transfusion (Table ).
Table 3. Risk factors for BPD by multiple logistic regression analysis.
Anemia and BPD
In the study, 294 of 364 infants were diagnosed with anemia. Among the anemic neonates, 164 infants (45.05%) were diagnosed with mild anemia, while 83 infants (22.80%) with moderate anemia and 47 infants (12.91%) with severe anemia. The incidence of anemia in neonates with BPD was 89.63% (121 of 135) and that in non-BPD patients was 75.55% (173 of 229). The rate of severe anemia was significantly lower in non-BPD patients than in BPD patients (severe anemia: 24/135 (17.78%) vs 23/229 (10.04%), p = 0.034, Table ). After matching, the incidence of anemia in neonates with BPD was 86.75% (72 of 83), and the incidence of anemia in non-BPD patients was 80.72% (67 of 83). The rate of anemia was similar in different groups (Table ). However, the rate of severe anemia is significantly higher in BPD-group than in non-BPD group, while the rate of mild and moderate anemia is not significantly different between the two groups (severe anemia:14/83 (16.87%) vs 4/83 (4.82%), p = 0.013; mild anemia: 37/83 (44.58%) vs 40/83 (48.19%), p = 0.641; moderate anemia: 21/83 (25.30%) vs 23/83 (27.71%), p = 0.725). For further analysis, anemia are divided into early (within 7 days after birth), mid-term (7–14 days after birth) and late anemia (14–28 days after birth) according to the occurrence of anemia. There are no differences in the rate of early, mid-term and late anemia between BPD-group and non-BPD group (Table ).
Table 4. Rate of anemia between BPD patients and non-BPD patients after matching.
Transfusion and BPD
We observed that the incidence of RBC transfusion in the non-BPD is lower than BPD groups (Before matching: 117/229 (51.09%) vs 110/135 (81.48%), p < 0.001, Table ; After matching: 49/83 (59.04%) vs 63/135 (75.9%), p = 0.02, Table ). After matching, there is no significant difference in timing of first transfusion (≤7 days after birth) between the two groups (Table ) where there are more infants who received RBC transfusion within 7 days after birth for the first time in BPD group than in non-BPD group before matching (Table ). And there are higher number and large volume of RBC transfusion in BPD group than in non-BPD group (number: 2(0,4) vs 1(0,2), p = 0.001; volume: 40 (20,80) vs 30(0,40), p = 0.004, Table ). To further evaluate the role of transfusion on different grades of BPD, we compared the number, volume of transfusion between the non-BPD, BPD (I) patients and BPD (II/III) patients. As shown in Figure , the number of transfusion is not significantly different between the non-BPD group and BPD (I) group, but significantly different in the non-BPD group and BPD (II/III) group, and BPD (I) group and BPD (II/III) group (non-BPD vs BPD (I) vs BPD (II/III): 1(0,2) vs 2(0,3) vs 4(2,6), Figure ). The outcome of the volume of transfusion in different groups is similar (non-BPD vs BPD (I) vs BPD (II/III): 30 (0,40) vs 40 (0,60) vs 40 (20,101.5), Figure ).
Figure 2. Comparison of the number of RBC transfusions in the non-BPD group and the BPD group (I and II/III). Severity of BPD: 0: non-BPD group (n = 83); 1:BPD (I) group (n = 53); 2:BPD (II/III) group (n = 30).
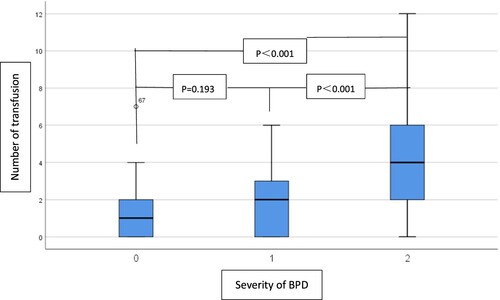
Figure 3. Comparison of the volume of RBC transfusions in the non-BPD group and the BPD group (I and II/III). Severity of BPD:0: non-BPD group (n = 83); 1:BPD(I) group (n = 53); 2:BPD (II/III) group (n = 30).
Table 5. RBC transfusion between BPD patients and non-BPD patients after matching.
Discussion
Anemia is almost universal in the premature population. In our study, 80.77% preterm infants with gestational age <32 weeks were diagnosed with anemia. And the majority of them were mild and moderate anemia; more than half were mild anemia.
Multivariate analysis in the study found that birth weight, NRDS, pulmonary hemorrhage, mechanical ventilation, severe anemia and volume of transfusion were the most significant factors associated with the incidence of BPD. Birth weight is one of the strongest risk factors for BPD (Thebaud et al. Citation2019). Walsh et al. (Citation2004) found that those who were diagnosed with BPD were mostly very low birth weight infants (Walsh et al. Citation2004). The incidence of BPD was higher in infants with NRDS, especially for those with severe NRDS because of lung tissue structure reconstruction after therapy, including mechanical ventilation for those infants (Zhong et al. Citation2019). And pulmonary hemorrhage may increase the risk of BPD by causing lung tissue injury and increasing the need for mechanical ventilation.
To minimize the effects of confounding factors, a propensity score matching was made. After matching, the incidence of anemia in different groups was similar. We observed that neonates in non-BPD group were mostly mild and moderate anemia, while neonates with BPD were with a high incidence of severe anemia. In a previous study, mean Hct in BPD patients was lower than non-BPD patients (Duan et al. Citation2016). The present study evaluates the severity of anemia and compares the association between different severity of anemia and BPD. According to our study, severe anemia was more evident in the BPD group and this was in agreement with Duan et al. Citation2016 (Duan et al. Citation2016). The underlying pathogenesis is multi-factorial:(1) due to inadequate tissue oxygen delivery, severe anemia may produce multi-organ dysfunction, especially respiratory organ dysfunction; (2) inflammation induced by severe anemia also play a role on the development of BPD; (3) severe anemia may cause neonatal feeding difficulties and insufficient weight gain (Hoque et al. Citation2017) and then lead to decreased tolerance to hypoxia and inflammatory reactions. However, we found no relationship between BPD and the onset of anemia, and this was against Duan et al. (Citation2016), where early anemia was associated with the development of BPD (Duan et al. Citation2016). More research are needed to decide the relationship between early anemia and BPD. We suggest that it's the severity of anemia rather than the diagnosis time of anemia to indicate the development of BPD.
It is common and lifesaving for premature neonates to receive RBC transfusion. RBC transfusion is clinically regarded as the first choice for those moderate or severe anemic neonates (Hoque et al. Citation2017; Shanmugha et al. Citation2018). In our study, more than half (62.36%, 227/364) of patients were treated with RBC transfusion. After matching, the incidence of RBC transfusion is still significantly higher in the BPD group. Also, we found that the number and volume of RBC transfusion are significantly larger in BPD group. Greater number and larger volume of transfusions may be related to the development of BPD. In addition, compared to infants in non-BPD group, the median number and volume of transfusions were higher in infants with both BPD (I) and BPD (II/III). But there are no significant differences between non-BPD group and BPD (I) group. It indicates that the volume and number of RBC transfusion is closely related to patients with BPD (II/III). Why RBC transfusion is related to BPD is multi-factorial. Though with RBC supplement and hemoglobin improvement, transfusion may inevitably bring some adverse effects. Transfusion could significantly increase production of pro-inflammatory cytokines (Biedler et al. Citation2002; Schneider et al. Citation2009; Keir et al. Citation2013). In addition, metabolic derangements, especially in the serum Ca2+, K+ and glucose concentrations could accompany RBC transfusions (Abdelghaffar et al. Citation2012; Dani et al. Citation2008). Moreover, hemolytic transfusion reactions, alloimmunization, volume overload, allergic reactions, or iron excess are observed after transfusion (Oakley et al. Citation2015). For those infants with a larger volume of RBC, iron excess could produce overloaded oxygen free radicals and infection (Zhang et al. Citation2014). The above adverse reaction, including inflammation, infection, metabolic derangements and overloaded oxygen free radicals, brought by transfusion would lead to the development of BPD.
According to the current study, we demonstrated that the timing of first transfusion does not contribute to the development of BPD. This was not in accordance to Zhang et al. (Citation2014) where the incidence of BPD was related to early transfusion (Zhang et al. Citation2014). There were confounding factors in the early and late transfusion group in the study, Zhang et al., making the conclusion not convincing. More studies, especially prospective study are needed to investigate the effect of the first RBC transfusion on BPD development.
Recommendation
Anemia is the most common indication for RBC transfusion. For those severe anemic neonates, RBC transfusion is necessary. We should avoid the occurrence and exacerbation of anemia, and thus reduce the number of RBC transfusion in the neonatal period. We can prevent severe anemia by promoting hematopoiesis and reducing blood loss. There are a few methods to promote hematopoiesis, including delayed ligature of the umbilical cord (Committee Opinion No. Citation684 Citation2017; Qian et al. Citation2019), cord milking (Hosono et al. Citation2008), adequate-protein intake (Carroll and Widness Citation2012), early administration of erythropoiesis-stimulating agents and late administration of erythropoietin (starting > eight days) combined with adequate iron supplementation (Ohlsson and Aher Citation2020; Aher and Ohlsson Citation2020; Siddappa et al. Citation2020). To reduce blood loss, a restrictive blood sampling regime in the neonatal period, implementation of a bedside multi-parameter Point of Care Test analyzer (Mahieu et al. Citation2012), and removal of unnecessary indwelling catheters are effective (Hellstrom et al. Citation2020; Carroll and Widness Citation2012). And in a study, earlier full enteral feeding earlier and shorter duration of total parenteral nutrition were effective in reducing AOP and RBC transfusions (Jeon and Sin Citation2013).
Limitations
This study has some limitations. This is a single-center retrospective study. Although we performed a propensity score analysis, some co-effective factors should be further clarified. A multi-centered prospective study with more preterm infants is needed.
Conclusion
This study demonstrated it that severe anemia increased the risk of BPD in preterm infants. The volume and number of RBC transfusions are related to the development of BPD and its severity. And risk of BPD was neither related to the onset of anemia nor the timing of the first transfusion. We should adopt medical methods to prevent severe anemia and reduce RBC transfusions.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board, Children's Hospital of Chongqing Medical University of China.
Acknowledgements
The authors thank statisticians Xiaohua Liang and Xian Tang for assistance in reviewing statistics. Conceptualization, S. S. M., D. L. Z., and Y. S.; methodology, S. S. M., L. C., and Y. S.; data curation, S. S. M. and D. L. Z.; writing original draft preparation, S. S. M. and D. L. Z.; writing review and editing, Y. S. and L. C.; supervision, Y. S. and L. C. All authors have read and agreed to the published version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Yuan Shi is the guarantor of this work and takes responsibility for the integrity of the data and the accuracy of the data analysis. The data that support the findings of this study are available in Mendeley Data repository [https://data.mendeley.com/] at https://doi.org/10.17632/7bvnhn5k22.1.
Correction Statement
This article was originally published with errors, which have now been corrected in the online version. Please see Correction (http://dx.doi.org/10.1080/26895293.2021.1979803)
Additional information
Funding
References
- Abdelghaffar S, Mansi Y, Ibrahim R, Mohamed D. 2012. Red blood transfusion in preterm infants: changes in glucose, electrolytes and acid base balance. Asian J Transfus Sci. 6(1):36–41.
- Aher S, Malwatkar K, Kadam S. 2008. Neonatal anemia. Semin Fetal Neonatal Med. 13(4):239–247.
- Aher SM, Ohlsson A. 2020. Early versus late erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2:CD004865.
- Austin PC. 2009. The relative ability of different propensity score methods to balance measured covariates between treated and untreated subjects in observational studies. Med Decis Making. 29(6):661–677.
- Biedler AE, Schneider SO, Seyfert U, Rensing H, Grenner S, Girndt M, Bauer I, Bauer M. 2002. Impact of alloantigens and storage-associated factors on stimulated cytokine response in an in vitro model of blood transfusion. Anesthesiology. 97(5):1102–1109.
- Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, Hall DR, Warren CE, Adoyi G, Ishaku S. 2018. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. 72(1):24–43.
- Carroll PD, Widness JA. 2012. Nonpharmacological, blood conservation techniques for preventing neonatal anemia--effective and promising strategies for reducing transfusion. Semin Perinatol. 36(4):232–243.
- Colombatti R, Sainati L, Trevisanuto D. 2016. Anemia and transfusion in the neonate. Semin Fetal Neonatal Med. 21(1):2–9.
- Committee Opinion No. 684. 2017. Delayed umbilical cord clamping after birth. Obstet Gynecol. 129(1):1.
- Dani C, Perugi S, Benuzzi A, Corsini I, Bertini G, Pratesi S, Rubaltelli FF. 2008. Effects of red blood cell transfusions during the first week of life on acid-base, glucose, and electrolytes in preterm neonates. Transfusion. 48(11):2302–2307.
- De Luca D, van Kaam AH, Tingay DG, Courtney SE, Danhaive O, Carnielli VP, Zimmermann LJ, Kneyber M, Tissieres P, Brierley J, et al. 2017. The Montreux definition of neonatal ARDS: biological and clinical background behind the description of a new entity. Lancet Respir Med. 5(8):657–666.
- Duan J, Kong X, Li Q, Hua S, Zhang S, Zhang X, Feng Z. 2016. Association between anemia and bronchopulmonary dysplasia in preterm infants. Sci Rep. 6:22717.
- Hariyanto TI, Kurniawan A. 2020. Anemia is associated with severe coronavirus disease 2019 (COVID-19) infection. Transfus Apher Sci. 59(6):102926.
- Hellstrom W, Forssell L, Morsing E, Savman K, Ley D. 2020. Neonatal clinical blood sampling led to major blood loss and was associated with bronchopulmonary dysplasia. Acta Paediatr. 109(4):679–687.
- Higgins RD, Jobe AH, Koso-Thomas M, Bancalari E, Viscardi RM, Hartert TV, Ryan RM, Kallapur SG, Steinhorn RH, Konduri GG, et al. 2018. Bronchopulmonary dysplasia: executive summary of a workshop. J Pediatr. 197:300–308.
- Hoque N, Watts T, Fox G. 2017. Oxford handbook of neonatology, Second edition. Oxford: Oxford University Press.
- Hosono S, Mugishima H, Fujita H, Hosono A, Minato M, Okada T, Takahashi S, Harada K. 2008. Umbilical cord milking reduces the need for red cell transfusions and improves neonatal adaptation in infants born at less than 29 weeks’ gestation: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 93(1):F14–F19.
- Hussain SQ, Ashraf M, Wani JG, Ahmed J. 2014. Low hemoglobin level a risk factor for acute lower respiratory tract infections (ALRTI) in children. J Clin Diagn Res. 8(4):PC01–PC03.
- Jeon GW, Sin JB. 2013. Risk factors of transfusion in anemia of very low birth weight infants. Yonsei Med J. 54(2):366–373.
- Keir AK, McPhee AJ, Andersen CC, Stark MJ. 2013. Plasma cytokines and markers of endothelial activation increase after packed red blood cell transfusion in the preterm infant. Pediatr Res. 73(1):75–79.
- Mahieu L, Marien A, De Dooy J, Mahieu M, Mahieu H, Van Hoof V. 2012. Implementation of a multi-parameter point-of-care-blood test analyzer reduces central laboratory testing and need for blood transfusions in very low birth weight infants. Clin Chim Acta. 413(1-2):325–330.
- New HV, Berryman J, Bolton-Maggs PHB, Cantwell C, Chalmers EA, Davies T, Gottstein R, Kelleher A, Kumar S, Morley SL, et al. 2016. Guidelines on transfusion for fetuses, neonates and older children. Br J Haematol. 175(5):784–828.
- Northway WJ, Rosan RC, Porter DY. 1967. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med. 276(7):357–368.
- Oakley FD, Woods M, Arnold S, Young PP. 2015. Transfusion reactions in pediatric compared with adult patients: a look at rate, reaction type, and associated products. Transfusion. 55(3):563–570.
- Ohlsson A, Aher SM. 2020. Early erythropoiesis-stimulating agents in preterm or low birth weight infants. Cochrane Database Syst Rev. 2:CD004863.
- Ozsoylu S. 2014. Iron deficiency anemia in late preterm infants. Turk J Pediatr. 56(1):119.
- Pizzini A, Aichner M, Sonnweber T, Tancevski I, Weiss G, Loffler-Ragg J. 2020. The significance of iron deficiency and anemia in a real-life COPD cohort. Int J Med Sci. 17(14):2232–2239.
- Preterm labour and birth. London: National Institute for Health and Care Excellence (UK); 2019.
- Qian Y, Ying X, Wang P, Lu Z, Hua Y. 2019. Early versus delayed umbilical cord clamping on maternal and neonatal outcomes. Arch Gynecol Obstet. 300(3):531–543.
- Radic JAE, Vincer M, McNeely PD. 2015. Outcomes of intraventricular hemorrhage and posthemorrhagic hydrocephalus in a population-based cohort of very preterm infants born to residents of Nova Scotia from 1993 to 2010. J Neurosurg Pediatr. 15(6):580–588.
- Ramakrishnan K, Borade A. 2010. Anemia as a risk factor for childhood asthma. Lung India. 27(2):51–53.
- Schneider SO, Rensing H, Graber S, Kreuer S, Kleinschmidt S, Kreimeier S, Muller P, Mathes AM, Biedler AE. 2009. Impact of platelets and fresh frozen plasma in contrast to red cell concentrate on unstimulated and stimulated cytokine release in an in vitro model of transfusion. Scand J Immunol. 70(2):101–105.
- Shane AL, Sanchez PJ, Stoll BJ. 2017. Neonatal sepsis. Lancet. 390(10104):1770–1780.
- Shanmugha PR, Krishnamoorthy R, Panicker VK, Ninan B. 2018. Transfusion support in preterm neonates <1500g and/or <32 weeks in a tertiary care center: a descriptive study. Asian J Transfus Sci. 12(1):34–41.
- Sharma R, Hudak ML. 2013. A clinical perspective of necrotizing enterocolitis: past, present, and future. Clin Perinatol. 40(1):27–51.
- Siddappa AM, Olson RM, Spector M, Northrop E, Zamora T, Brearley AM, Georgieff MK, Rao R. 2020. High prevalence of iron deficiency despite standardized high-dose iron supplementation during recombinant erythropoietin therapy in extremely low gestational age newborns. J Pediatr. 222:98–105.e3.
- Singh R, Visintainer PF, Frantz IR, Shah BL, Meyer KM, Favila SA, Thomas MS, Kent DM. 2011. Association of necrotizing enterocolitis with anemia and packed red blood cell transfusions in preterm infants. J Perinatol. 31(3):176–182.
- Strauss RG. 2010. Anaemia of prematurity: pathophysiology and treatment. Blood Rev. 24(6):221–225.
- Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, Saugstad OD, Simeoni U, Speer CP, Vento M, et al. 2013. European consensus guidelines on the management of neonatal respiratory distress syndrome in preterm infants – 2013 update. Neonatology. 103(4):353–368.
- Thebaud B, Goss KN, Laughon M, Whitsett JA, Abman SH, Steinhorn RH, Aschner JL, Davis PG, McGrath-Morrow SA, Soll RF, et al. 2019. Bronchopulmonary dysplasia. Nat Rev Dis Primers. 5(1):78.
- Tita ATN, Andrews WW. 2010. Diagnosis and management of clinical chorioamnionitis. Clin Perinatol. 37(2):339–354.
- Tourniaire G, Milesi C, Baleine J, Crozier J, Lapeyre C, Combes C, Nagot N, Cambonie G. 2018. [Anemia, a new severity factor in young infants with acute viral bronchiolitis?]. Arch Pediatr. 25(3):189–193.
- Vander JF, McNamara JA, Tasman W, Brown GC. 2005. Revised indications for early treatment of retinopathy of prematurity. Arch Ophthalmol. 123(3):406–407. discussion 409-10.
- Venkatesh V, Khan R, Curley A, Hopewell S, Doree C, Stanworth S. 2012. The safety and efficacy of red cell transfusions in neonates: a systematic review of randomized controlled trials. Br J Haematol. 158(3):370–385.
- Walsh MC, Yao Q, Gettner P, Hale E, Collins M, Hensman A, Everette R, Peters N, Miller N, Muran G, et al. 2004. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics. 114(5):1305–1311.
- Xu Y, Hu T, Ding H, Chen R. 2020. Effects of anemia on the survival of patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Expert Rev Respir Med. 14(12):1267–1277.
- Zhang Z, Huang X, Lu H. 2014. Association between red blood cell transfusion and bronchopulmonary dysplasia in preterm infants. Sci Rep. 4:4340.
- Zhong YY, Li JC, Liu YL, Zhao XB, Male M, Song DK, Bai Y. 2019. Early intratracheal administration of corticosteroid and pulmonary surfactant for preventing bronchopulmonary dysplasia in preterm infants with neonatal respiratory distress syndrome: a meta-analysis. Curr Med Sci. 39(3):493–499.