 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
To investigate the effect of astragalus heart-protecting decoction on rats with dilated cardiomyopathy (DCM). 36 healthy rats were divided into normal control group (n = 12, unmodeled rats without receiving decoction), DCM + control group (n = 12, modeled rats without decoction), and DCM + treatment group (n = 12, modeled rats receiving decoction). Outcomes of the three groups were compared. Serum brain natriuretic peptide and matrix metallopeptidase 10, ventricular remodeling indicators, whole heart weight index and left ventricular weight index were significantly lower in normal control group than in the DMC + treatment and DMC + control groups, so was that in DMC + treatment group compared with those in DMC + control group (P < 0.05). Additionally, the normal control group had higher superoxide dismutase level but lower malondialdehyde and lactate dehydrogenase levels than the DMC + treatment and DMC + control groups (P < 0.05). Regarding myocardial histology or myocardial fibrosis, the DMC + treatment group highlighted better performance than the DMC + control group, with differences from those in the normal control group though. Astragalus heart-protecting decoction has a positive effect in the treatment of rats with DCM.
Introduction
Dilated cardiomyopathy (DCM) involves the myocardium and is characterized by hypofunction of the ventricular systole as well as by left and right ventricular hypertrophy. It is one of the important causes of congestive heart failure and heart transplantation (Schultheiss et al. Citation2019). Epidemiological surveys on the annual incidence of cardiomyopathy have reported that among the 36–66 cardiomyopathy cases reported per hundred thousand, almost half are DCM and that these patients suffer from progressive heart failure, ventricular or supraventricular arrhythmia, and other clinical manifestations (Yu et al. Citation2019). The 5-year mortality rate since its diagnosis is approximately 50%. These manifestations result in heavy burdens on the family and society as a whole (Jammal Addin et al. Citation2019).
Although the pathogenesis of DCM remains unknown, it has been reported that DCM has hereditary characteristics and that myocardial remodeling serves as a pathological feature therein. Hereditary factors play a crucial role in the occurrence and development of DCM. Currently, the identified pathogenic genes include autosomal dominant pathogenic genes, X-linked genetic pathogenic factors, autosomal recessive pathogenic genes, and mitochondrial DNA genes (Schelbert Citation2019). Several complex molecular mechanisms may lead to a significant increase in interstitial collagen volume fraction, excessive deposition or reduced degradation of the extracellular matrix (ECM), and ultimately to the replacement of normal myocardial tissues with fibrous tissues, resulting in changes in the structure, function, and phenotype of the myocardium. This process is clinically called myocardial fibrosis (Chaves et al. Citation2019). This condition gives rise to myocardial remodeling and heart failure. In the treatment of DCM, it is essential to not only inhibit myocardial fibrosis but also to reverse it. Evidence-based studies have indicated the use of β-blockers, angiotensin converting enzyme inhibitors, aldosterone receptor antagonists, and adrenergic II receptor blockers as drugs for the treatment of myocardial fibrosis. Furthermore, a small proportion of related studies have proposed the use of signaling pathway blockers, gene antibodies, and matrix metalloproteinase (MMP) inhibitors; however, these methods have not been widely popularized (Sanz et al. Citation2019; Treibel et al. Citation2019).
Traditional Chinese medicine (TCM) has been used since a long time for the treatment of various diseases. Compounded Chinese medicinal prescriptions with Astragalus membranaceus as the main drug have recently played a promising role in the treatment of several heart diseases (Shan et al. Citation2019). A study on contemporary age has demonstrated that most TCMs for blood circulation promotion to treat blood stasis exert their effects by improving microcirculation, thereby preventing or improving myocardial fibrosis (Hu and Wang Citation2019). In TCM, holistic healing is emphasized by the use of prescriptions with the belief that the principle of ‘benefiting vital energy to regulate the immunity, detoxify, and dissipate any stasis’ should be followed by specifically focusing on the relief of ‘toxicity,’ ‘deficiency,’ and ‘blood stasis.’ Astragalus heart-protecting decoction can relieve qi deficiency and blood stasis as well as stasis associated with toxins (Ma et al. Citation2019). Previous clinical studies and animal experiments have confirmed the therapeutic values of this decoction in DCM. In addition, studies have shown that astragalus heart-protecting decoction can improve the levels of various neuroendocrine factors in patients with DCM (Gao et al. Citation2020). Some studies have also shown that astragalus heart-protecting decoction can improve heart failure in patients with DCM (Lin et al. Citation2018). However, the underlying mechanisms of actions are unknown. Taking these findings into consideration, this study investigated whether astragalus heart-protecting decoction can improve cardiac function in a DCM rat model by investigating the effect of this decoction on ventricular remodeling and myocardial fibrosis in rats with DCM.
Materials and methods
Materials
Animals
Thirty-six healthy clean male rats (180–220 g) were provided by Experimental Animal Center of Xuzhou Medical University [License number: SCXK (Su) 2019-0003] and were housed in separate cages (6 rats per cage) under the conditions of 50% humidity and temperature at approximately 23°C. They were given the special feed supplied by Beijing HFK Bioscience Co., Ltd. with ad libitum access to food and water. The disposal of rats in this study was in accordance with the regulations of animal ethics. This study was approved by Xuzhou Central Hospital.
Drugs
Astragalus heart-protecting decoction is a polyherbal medicine composing of astragalus 30 g, Ophiopogon japonicus 12 g, turtle shell 12 g, Salvia miltiorrhiza 12 g, Poria cocos 12 g, Forsythia suspensa 12 g, Codonopsis pilosula 12 g, cassia twig 6 g, and Schisandra chinensis 6 g; Doxorubicin: [Specifications: 5 ml: 0.5 g (C38H72N2012), Approval No.: SFDA H20020342, Manufacturer: (Suzhou Pharmaceutical Factory of Jiangsu Wuzhong Pharmaceutical Group Corporation)].
Methods
Animal model
The 24 rats were randomly selected from 36 rats for model preparation. The specific methods are as follows: The DCM rat model was established by intraperitoneally injecting Doxorubicin 2.5 (mg/kg·times) three times a week, six times in total for two weeks, with a total weekly dose of 15 mg/kg each week. The needle was inserted 0.5 cm to the right of the umbilicus along the midline of the abdomen.
Grouping and treatment
The 12 unmodeled rats were used as the normal control group without receiving any drugs but received an equal volume of normal saline. The 24 rats successfully modeled were randomly divided into two groups, among which 12 rats were intragastrically administered 8 g/kg/day astragalus heart-protecting decoction (DCM + treatment group), and another 12 rats did not receive astragalus heart-protecting decoction but received an equal volume of normal saline (DCM + control group). Rats were euthanized after 4 weeks of continuous intervention.
Outcome measures
Levels of serum brain natriuretic peptide (BNP) and matrix metallopeptidase 10 (MMP10)
Blood samples were collected from the main abdominal vein of rats, followed by centrifugation. The supernatant was collected. The levels of serum BNP and MMP10 were determined via enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (You et al. Citation2017).
Ventricular remodeling
Color Doppler ultrasound was used to scan two-dimensional image of the standard zone of the left ventricular from the parasternal long-axis of the left ventricular. The indicators of ventricular remodeling, namely left ventricular end-diastolic diameter (LVEDD), left ventricular end-systolic diameter (LVESD), left ventricular ejection fraction (LVEF), fractional shortening (FS), and stroke volume (SV), were determined, and the average value of three cardiac cycles was used as the final result.
Determination of whole heart weight index (HWI) and left ventricular weight index (LVWI)
With curved forceps clamping the lower part of the atrial appendage, the heart was excised and cleared of residual blood. An analytical balance was used to measure heart weight (HW). The right ventricle, atrium, and vessels were then removed for measuring left ventricular weight (LVW). HWI was calculated as HW/BW and LVWI was calculated as LVW/BW (Li et al. Citation2016).
Myocardial tissues: Left ventricular myocardial tissue was collected and processed to obtain the cardiomyocyte suspension. Total protein content was determined using Coomassie Brilliant Blue. The levels of superoxide dismutase (SOD), malondialdehyde (MDA), and lactate dehydrogenase (LDH) were determined using kits according to the manufacturers’ instructions (Zhang et al. Citation2017).
Myocardial histology and myocardial fibrosis
The myocardial tissue was stained using via Masson trichrome staining to evaluate the histological status of the myocardium and the degree of myocardial fibrosis. Myocardial collagen volume fraction (CVF) was calculated as a quantitative indicator of the degree of myocardial fibrosis using the following formula: CVF (%) = Collagen area/Total visual field area ×100% (Omori et al. Citation2020).
Statistical analysis
Statistical analyses were performed using the Statistic Package for the Social Science (SPSS) 22.0 software. Measurement data are expressed as mean ± standard deviation. The groups were using ANOVA. P < 0.05 indicated statistically significant difference.
Results
BNP and MMP10 levels
The levels of BNP and MMP10 were significantly lower in the normal control group than in the DCM + treatment group and DCM + control group (all P < 0.05). The levels of BNP and MMP10 were lower in the DCM + treatment group than in the DCM + control group (P < 0.05) (Table ; Figure ).
Figure 1. Levels of BNP and MMP10 in the three groups of rats The normal control group reported lower levels of BNP (A) and MMP10 (B) compared with the other groups (all P < 0.05). Both BNP and MMP10 were lower in the DCM + treatment group than the DCM + control group (& P < 0.05).
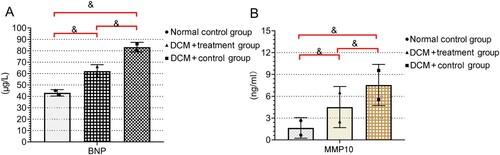
Table 1. Levels of BNP and MMP10 in the three groups of rats (±s).
Ventricular remodeling
LVEDD, LVESD, LVEF, FS, and SV were significantly lower in the normal control group than in the DCM + treatment group and in the DCM + control group (all P < 0.05). On the other hand, LVEDD, LVESD, LVEF, FS, and SV were higher in the DCM + control group than in the DCM + treatment group (P < 0.05) (Table ).
Table 2. Qualitative indexes of ventricular remodeling in the three groups of rats (±s).
HWI & LVWI
The DCM + treatment group had higher BW than the DCM + control group (P < 0.05). However, when compared with the BW of rats in the DCM + treatment or DCM + control groups, the BW of rats in the normal control group was notably higher (P < 0.05). HWI and LVWI were higher in the DCM + control group than in the DCM + treatment group. In addition, HWI and LVWI were higher in the DCM + treatment group than in the normal control group (P < 0.05 for all) (Table ; Figure ).
Figure 2. Comparison of heart weight index (HWI) and left ventricular weight index (LVWI) in the three experimental groups Both HWI and LVWI were lower in the normal control group than in the DCM + treatment and DCM + control groups (*P < 0.05 for all).
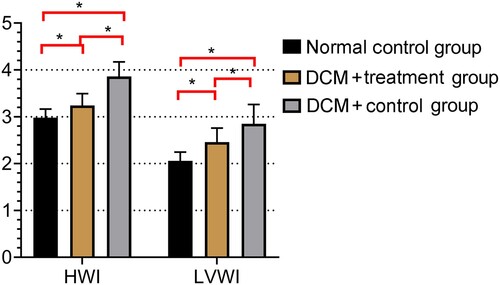
Table 3. BW, HWI, and LVWI of the three groups of rats (±s).
Myocardial tissues
The level of SOD was higher in the normal control group than in the DCM + treatment or DCM + control groups. On the other hand, the levels of MDA and LDH were notably lower in the normal control group than in the DCM + treatment or DCM + control groups (P < 0.05 for all). The DCM + treatment group had higher level of SOD but lower levels of MDA and LDH than the DCM + control group (P < 0.05) (Table ).
Table 4. Effects of astragalus heart-protecting decoction on the levels of oxidative stress markers in the three groups of rats (±s).
Myocardial histology
In the normal control group, myocardial tissues were normally shaped, myocardial fibers were orderly arranged, transverse striations were clearly observed, and intimal cells were intact with centrally located nuclei surround by clear cytoplasm. Small histological changes were apparent (Figure a).
Figure 3. Changes in myocardial histology in the three groups of rats HE staining (×200) showing the pathomorphology of the myocardial tissues in the three groups of rats. (A) Normal histology in the myocardial tissues of the rats in the normal control group. (B) Cardiomyocyte necrosis was observed under the intima of the left ventricle and throughout the myocardium in the DCM + control group. (C) Tissues of rats in the DCM + treatment group exhibited less severe necrotic fibrosis, a small amount of fibrosis adjacent to some cardiomyocytes, and better cardiomyocyte arrangement.
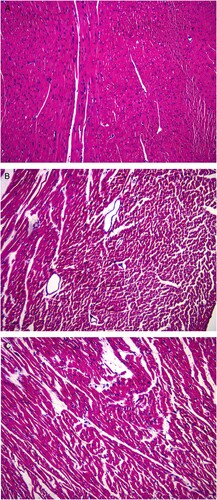
However, in the DCM + control group, cardiomyocyte necrosis was observed under the intima of the left ventricle and in the myocardium, with some fibrotic areas with a small number of myocardiocytes and with vascular proliferation, interstitial edema, and inflammatory cell infiltration. Granulation tissue could be locally found, and adjacent cardiomyocytes were disorganized and hypertrophic (Figure b).
Compared with the lesions in the DCM + control group, those observed in the DCM + treatment group were improved based on the fact that the lesions in the DCM + treatment group had less degree of necrotic fibrosis, a small amount of fibrosis adjacent to some cardiomyocytes, and better cardiomyocyte arrangement. However, they had slight interstitial edema and presence of inflammatory cell infiltration (Figure c).
Myocardial fibrosis
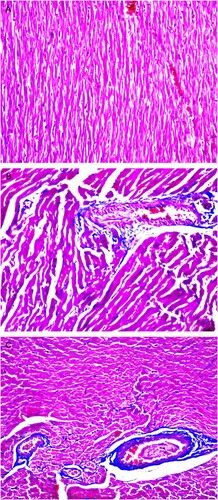
There was little difference between the CVF of the normal control and DCM + treatment groups (P > 0.05). However, the CVF of the DCM + control group was higher than that of the normal control and DCM + treatment groups (P < 0.05). Masson staining mainly showed the presence of myocardial tissues in normal state mingled with a small amount of interstitial collagen fibers in the rats of the control group (Figure a). On the other hand, the rats in the DCM + control group had myocardial interstitial collagen fibers that were obviously hyperplastic and adjacent to the vessels as well as many extracellular matrices (Figure b). The DCM + treatment group had the presence of blue collagen fibers in a wide range, separating the myocardium into islands (Figure c).
Figure 4. Degree of myocardial fibrosis in the three groups of rats Masson staining (×200) showing the degree of myocardial fibrosis in the three groups of rats, Erythrocytes, cardiac muscle fibers, and cytoplasm appear red, whereas myocardial interstitial collagen appears blue. (A) Masson staining mainly showing normal myocardial tissues in the normal control group. (B) Myocardial interstitial collagen fiber accumulation and obvious hyperplasia were observed in the myocardium of the DCM + control group. (C) Blue-stained collagen fibers were observed in the myocardium of the DCM + treatment group.
Discussion
Although the complex pathogenesis of DCM confirmed by a large number of studies has not been elucidated, TCM has unique advantages in its treatment (Reichart et al. Citation2019). Clinical experiences coupled with the integrated thoughts of medical experts have led to the proposition that DCM is characterized by asthenia in origin and excessive superficiality. Asthenia in origin refers to the presence of phlegm and retained fluid, water dampness, and blood stasis. It is characterized by qi and yang deficiencies and commonly involves the heart. The lungs, spleen, and kidneys are also functionally related to it (Liu et al. Citation2014). Qi deficiency is the characteristic of asthenia in origin, whereas toxin accumulation, blood stasis, and water dampness are the characteristics of excessive superficiality. TCM believes thattreatment should be implemented by stages in different disease periods (Bai et al. Citation2013). In the early stage, the syndrome includes qi deficiency in the heart and lungs, blood stasis, blocked phlegm, and fluid retention, with chest tightness, shortness of breath, blood-stained sputum, palpitation after exertion, and panting that does not by resting. In the middle stage, there may be qi deficiency in the heart and spleen, pathogenic factors, blockade of yang and blood circulation, intermingled phlegm and blood stasis, fluid retention in the gastrointestinal tract that changes to phlegm and retained fluid, and edema or abdominal distension. In the late period, pathogenic factors lead to kidney and heart dysfunction, blood stasis that is difficult to reverse, water dampness causing anasarca, intolerance to cold and cold limbs, edema of the lower limbs, and in severe cases, hydrothorax and abdominal dropsy (Shen et al. Citation2016; Sun et al. Citation2017; Wang et al. Citation2018). For the treatment of DCM, TCM emphasizes that both symptoms and root causes should be addressed. In particular, attention should be given to the treatment of symptoms so as to promote blood circulation to remove blood stasis, alleviate water retention for dehumidification, and resolve phlegm accumulation to reduce edema. On the other hand, the treatment of root causes focuses on complementing yin and yang by benefiting vital energy (Liang et al. Citation2004). Astragalus heart-protecting decoction contains Astragalus membranaceus, Forsythia suspensa, Codonopsis pilosula, Cassia twig, Salvia miltiorrhiza, Angelica sinensis, Radix Ophiopogonis, turtle shell, Schisandra chinensis, and Poria cocos. Astragalus membranaceus, Forsythia suspensa, and Codonopsis pilosula help in tonifying vital energy for detoxification. On the other hand, Cassia twig removed coronary collaterals; Salvia miltiorrhiza and Angelica sinensis cool and invigorate blood circulation; Radix Ophiopogonis, turtle shell, and Schisandra chinensis are conductive for tonifying vital energy to nourish yin; and Poria cocos improves splenic function and calms the nerves (Phacharapiyangkul et al. Citation2019). The prescription acts mainly by tonifying vital energy to activate blood circulation, nourishing yin, detoxifying the body, and by promoting dieresis, thereby effectively relieving the symptoms of DCM such as vital energy deficiency, blood stasis, and stasis due to toxicity. Molecular mechanism studies have shown that the components of Astragalus and Codonopsis can scavenge free radicals, enhance SOD activity and play an antioxidant role; the volatile oil, polysaccharide and other components of angelica can play a role in anti-tissue fibrosis, anti-inflammation, and anti-oxidation effects; Schisandra chinensis can regulate the energy metabolism of cardiomyocytes and reduce the degree of oxygen free radical damage to cardiomyocytes (Xu et al. Citation2020).
DCM is followed by left ventricular dilation, hypertrophic or necrotic cardiomyocytes, inflammatory cell infiltration, and fibrous tissue proliferation, which in turn trigger ventricular remodeling (Martens et al. Citation2019). BNP is a hormone released by ventricular cells and serves in mirroring the extent to which heart failure has occurred as well as determined the prognosis of patients (Chin et al. Citation2019). MMP10 is a class of proteins whose generation is mainly due to mechanical stimulation of the myocardium. It is spontaneously generated when there is a strain force. MMP10 can be expressed by normal cardiomyocytes and fibroblasts and is clinically considered an indicator of myocardial fibrosis and myocardial hypertrophy. However, MMP10 level is not closely related to age, BW, or renal function but is related to the prognosis of heart failure (Mirastschijski et al. Citation2019). In the present study, higher levels of BNP and MMP10 in rats with DCM decreased after the use of. In addition, increased ventricular remodeling in rats with DCM was also controlled by the use of this decoction. These results suggest that astragalus heart-protecting decoction is effective in controlling ventricular remodeling in rats with DCM. Similarly, lower weight gain and elevated HWI and LVWI in rats with DCM were improved after treatment with astragalus heart-protecting decoction, indicating that this decoction may increase the HW and ventricular weight of rats with DCM. The levels of SOD, MDA, and LDH mirror oxidative stress. Following treatment with astragalus heart-protecting decoction, the levels of SOD, MDA, and LDH decreased in rats with DCM via antioxidant stress and cardiomyocyte protection. Other studies have also found that the treatment of DCM in rats by astragalus heart-protecting decoction can increase SOD level and reduce LDH level (Wei et al. Citation2019). In addition, after treatment with astragalus heart-protecting decoction in this study, the degree of myocardial fibrosis in rats with DCM was significantly reduced, with the CVF in the normal control group of (6.82 ± 1.23) %, the CVF in the DCM + treatment group of (6.78 ± 1.19) %, and the CVF in the DCM + control group of (20.62 ± 6.83) %, indicating that astragalus heart-protecting decoction can reduce the abnormal changes in myocardial tissues and suppresses the manifestations of myocardial fibrosis. The above results indicated that the treatment of DCM in rats by astragalus heart-protecting decoction could promote the improvement of curative effect.
In summary, astragalus heart-protecting decoction has a positive effect in the treatment of rats with DCM. However, this study still has some limitations. First, astragalus heart-protecting decoction contains nine components; however, this study did not clarify which components play a role in promoting ventricular remodeling, and did not compare differences between different doses, which need to be further studied in the future. Besides, there are few observational indicators in this study, which lacks comprehensiveness. In addition, only rat experiments were conducted, and the translational value of this rat study to humans remains uncertain. However, as this decoction has been widely used in humans with good safety, future clinical studies are warranted to gradually transform the value of astragalus heart-protecting decoction from basic to clinical so that it can play a practical role in the treatment of clinical diseases.
Data availability statement
The data that support the findings of this study are available in figshare at http://doi.org/10.6084/m9.figshare.15112242
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bai H, Li Y, Han K, Gong M, Ma A. 2013. Effectiveness of Chinese herbal medicine as an adjunctive treatment for dilated cardiomyopathy in patients with heart failure. J Altern Complement Med. 19(10):811–819.
- Chaves AT, Menezes CA, Costa HS, Nunes MC, Rocha MO. 2019. Myocardial fibrosis in chagas disease and molecules related to fibrosis. Parasite Immunol. 41(10):e12663.
- Chin KM, Rubin LJ, Channick R, Di Scala L, Gaine S, Galiè N, Ghofrani H-A, Hoeper MM, Lang IM, McLaughlin VV. 2019. Association of N-terminal Pro brain natriuretic peptide and long-term Outcome in patients With pulmonary arterial hypertension: insights from the phase III GRIPHON study. Circulation. 139(21):2440–2450.
- Gao K, Song Y-P, Song A, Chen H, Zhao L-T, Zhang H-W. 2020. Therapeutic efficacy of shenmai injection as an adjuvant treatment in dilated cardiomyopathy: A protocol for systematic review. Medicine (Baltimore). 99(8):e19158.
- Hu Y, Wang J. 2019. Interactions between clopidogrel and traditional Chinese medicine. J Thromb Thrombolysis. 48(3):491–499.
- Jammal Addin MB, Young D, McCarrison S, Hunter L. 2019. Dilated cardiomyopathy in a national paediatric population. Eur J Pediatr. 178(8):1229–1235.
- Li J, Wu S, Tang HT, Zhu GW. 2016. [Effects of electroacupuncture at neiguan (PC6) on protein expressions of Raf-1, ERK 1/2, and p-ERK 1/2 of rats with myocardial hypertrophy]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 36(11):1335–1339.
- Liang H, Müller J, Weng Y-G, Wallukat G, Fu P, Lin H-S, Bartel S, Knosalla C, Pregla R, Hetzer R. 2004. Changes in myocardial collagen content before and after left ventricular assist device application in dilated cardiomyopathy. Chin Med J. 117(3):401–407.
- Lin XF, Luo JW, Liu G, Zhu YB, Jin Z, Lin X. 2018. Genetic mutation of familial dilated cardiomyopathy based on next-generation semiconductor sequencing. Mol Med Rep. 18(5):4271–4280.
- Liu Q, Su X-J, Yu Y, Liu Y-L. 2014. Correlations among persistent viral infection, heart function and Chinese medicine syndromes in dilated cardiomyopathy patients. Chin J Integr Med. 20(12):928–933.
- Ma Y, Chen M, Guo Y, Liu J, Chen W, Guan M, Wang Y, Zhao X, Wang X, Li H. 2019. Prevention and treatment of infectious diseases by traditional Chinese medicine: a commentary. APMIS. 127(5):372–384.
- Martens P, Nuyens D, Rivero-Ayerza M, Van Herendael H, Vercammen J, Ceyssens W, Luwel E, Dupont M, Mullens W. 2019. Sacubitril/valsartan reduces ventricular arrhythmias in parallel with left ventricular reverse remodeling in heart failure with reduced ejection fraction. Clin Res Cardiol. 108(10):1074–1082.
- Mirastschijski U, Dinesh N, Baskaran S, Wedekind D, Gavrilovic J, Murray MY, Bevan D, Kelm S. 2019. Novel specific human and mouse stromelysin-1 (MMP-3) and stromelysin-2 (MMP-10) antibodies for biochemical and immunohistochemical analyses. Wound Repair Regen. 27(4):309–323.
- Omori T, Nakamori S, Fujimoto N, Ishida M, Kitagawa K, Ichikawa Y, Kumagai N, Kurita T, Imanaka-Yoshida K, Hiroe M, Sakuma H. 2020. Myocardial native T(1) predicts load-independent left ventricular chamber stiffness In patients With HFpEF. JACC Cardiovasc Imaging. 13(10):2117–2128.
- Phacharapiyangkul N, Wu L, Lee W, Kuo Y, Wu Y, Liou H, Tsai Y, Lee C. 2019. The extracts of astragalus membranaceus enhance chemosensitivity and reduce tumor indoleamine 2, 3-dioxygenase expression. Int J Med Sci. 16(8):1107–1115.
- Reichart D, Magnussen C, Zeller T, Blankenberg S. 2019. Dilated cardiomyopathy: from epidemiologic to genetic phenotypes: A translational review of current literature. J Intern Med. 286(4):362–372.
- Sanz R, Mazzei L, Manucha W. 2019. Implications of the transcription factor WT1 linked to the pathologic cardiac remodeling post-myocardial infarction. Clin Investig Arterioscler. 31(3):121–127.
- Schelbert EB. 2019. Myocardial scar and fibrosis: The ultimate mediator of outcomes? Heart Fail Clin. 15(2):179–189.
- Schultheiss HP, Fairweather D, Caforio AL, Escher F, Hershberger RE, Lipshultz SE, Liu PP, Matsumori A, Mazzanti A, McMurray J, Priori SG. 2019. Dilated cardiomyopathy. Nat Rev Dis Primers. 5(1):32–32.
- Shan H, Zheng X, Li M. 2019. The Effects of astragalus membranaceus active extracts on autophagy-related diseases. Int J Mol Sci. 20(8):1904.
- Shen L-J, Lu S, Zhou Y-H, Li L, Xing Q-M, Xu Y-L. 2016. Developing a rat model of dilated cardiomyopathy with improved survival. J Zhejiang Univ Sci B. 17(12):975–983.
- Sun R, Wang J, Zheng Y, Li X, Xie T, Li R, Liu M, Cao Y, Lu L, Zhang Q. 2017. Traditional Chinese medicine baoxin decoction improves cardiac fibrosis of rats with dilated cardiomyopathy. Exp Ther Med. 13(5):1900–1906.
- Treibel TA, Badiani S, Lloyd G, Moon JC. 2019. Multimodality Imaging markers of adverse myocardial remodeling in aortic stenosis. JACC Cardiovasc Imaging. 12(8):1532–1548.
- Wang C, Du H, Hou J, Yan S, Yang J, Wang Y, Zhang X, Zhu L, Zhao H. 2018. Chaihulonggumulitang shows psycho-cardiology Therapeutic Effects on acute myocardial infarction by enhancing bone marrow mesenchymal stem cells mobilization. Sci Rep. 8(1):3724–3724.
- Wei W, Peng J, Li J. 2019. Curcumin attenuates hypoxia/reoxygenation-induced myocardial injury. Mol Med Rep. 20(6):4821–4830.
- Xu L, Xu X-Y, Hou X-Q, Wang F-G, Gao S, Zhang H-T. 2020. Adjuvant therapy with astragalus membranaceus for post-stroke fatigue: a systematic review. Metab Brain Dis. 35(1):83–93.
- You M, Lin M, Gong Y, Wang S, Li A, Ji L, Zhao H, Ling K, Wen T, Huang Y, Gao D. 2017. Household fluorescent lateral flow strip platform for sensitive and quantitative prognosis of heart failure using dual-Color upconversion nanoparticles. ACS Nano. 11(6):6261–6270.
- Yu J, Zeng C, Wang Y. 2019. Epigenetics in dilated cardiomyopathy. Curr Opin Cardiol. 34(3):260–269.
- Zhang YY, Yi M, Huang YP. 2017. Oxymatrine ameliorates doxorubicin-induced cardiotoxicity in rats. Cell Physiol Biochem. 43(2):626–635.
