Abstract
Inflammation is a vital process in response to injury and infection. However, hyper-inflammation have been linked to the pathophysiology of diseases such as atherosclerosis, cancers, neuroinflammatory, respiratory, renal diseases and Coronavirus disease 2019. Apocynin is known as a nontoxic herbal compound with anti-inflammatory potential. This review aimed to assess the effects of apocynin in the attenuation of inflammation and identify its mechanism of action based on a narrative review of available evidence. The reviewed literature has shown that apocynin exerts the immunomodulatory effect through reactive oxygen species-dependent and –independent approaches in phagocytic and non-phagocytic cells, respectively. In the reactive oxygen species-dependent pathway, apocynin monomers are converted to dimers, consequently inhibiting NADPH oxidase activation to reduce reactive oxygen species levels. While, in the reactive oxygen species-independent approach, apocynin directly reduces inflammatory cytokines and chemokines and increases nitric oxide without affecting reactive oxygen species levels. In conclusion, apocynin shows various minor macromolecular functions such as reducing chemokine synthesis, inhibiting cell migration and division, suppressing platelet/endothelial interaction and platelet aggregation, blood–brain barrier permeability, and decreasing reduction infarct volume. It is also hypothesized that apocynin might be considered a complementary remedy for Coronavirus disease 2019 due to its role in modulating inflammatory responses.
Introduction
Inflammation is a vital process in response to injury and infection (Hunter Citation2012). However, hyper-inflammation have been linked to the pathophysiology of diseases such as atherosclerosis, cancers, neuroinflammatory, respiratory, renal diseases and Coronavirus disease 2019 (COVID-19). Many drugs are available against inflammation and inflammatory disorders. However, a wide range of adverse effects following the administration of anti-inflammatory medications encourages scientists to consider medicinal plants as alternative choices for treating inflammatory diseases (Gearry et al. Citation2004; Laine et al. Citation2006; Harirforoosh and Jamali Citation2009).
Apocynin, a natural organic compound, was first introduced by Schmiedeberg in 1883 (Stefanska and Pawliczak Citation2008). Several clinical and preclinical studies confirmed the therapeutic effect of apocynin in various inflammatory diseases without any side effect even in long-term administration (Stolk et al. Citation1994b; Wang et al.; Citation2006; Tang et al. Citation2007; Jackman et al. Citation2009; Kelly et al. Citation2009; Stefanska et al. Citation2012; Altintas et al. Citation2013; Khanicheh et al. Citation2013; Kinoshita et al. Citation2013; Hart et al. Citation2014; Oostwoud et al. Citation2016; Jantaree et al. Citation2017), (Jackman et al. Citation2009; Simonyi et al. Citation2012; Altintas et al. Citation2013).
This review aimed to assess the effects of apocynin in the attenuation of inflammatory disorders and identify its mechanism of action based on an integrative review of available evidence. Considering that inflammation plays a significant role in Coronavirus disease 2019 (Cascella et al. Citation2021), this review has proposed the potential of apocynin as a complementary remedy for COVID-19 by discussing its mechanisms of action.
Apocynin pharmacology
Apocynin (4-hydroxy-3-methoxyacetophenone or acetovanillone) is a nontoxic natural organic compound extracted from the roots of the Alpine Himalayan medicinal herb. It is structurally related to vanillin growing in India, Pakistan, Tibet, and Nepal mountainous (Hart et al. Citation2014). The extracted molecule, with a molecular weight of 166.17 g/mol, has a weak vanilla smell, a melting point of 115°C, a boiling point of 233-235°C, and is soluble in hot water, alcohol, benzene and chloroform (Luchtefeld et al. Citation2008). For many years, apocynin has been traditionally used in Ayurvedic medicine to treat different diseases such as liver, heart and lungs disorders (Hart et al. Citation2014). After oral administrations, apocynin is absorbed and excreted rapidly, with small amounts of metabolites in faecal and unmetabolized ring-hydroxylated, demethylated and glucuronide forms in urinary excretion (Gjertsen et al. Citation1988).
Molecular mechanism of apocynin action
Based on in vitro and in vivo studies, apocynin exerts anti-inflammatory effects via various molecular mechanisms, lead to reducing or even preventing inflammatory disorders (Heumuller et al. Citation2008).
It has been shown that apocynin acts contradictory in phagocytic and non-phagocytic cells. Apocynin exerts NADPH oxidase (NOX) inhibitory effect in phagocytic cells while provokes reactive oxygen species (ROS) formation in non-phagocytic cells (Vejrazka et al. Citation2005). However, the anti-inflammatory properties of apocynin in non-phagocytic cells are attributed to alternative approaches (Impellizzeri et al. Citation2011; Kinoshita et al. Citation2013; Nam et al. Citation2016; Oostwoud et al. Citation2016).
NOX enzyme generates superoxide radicals through redox-sensitive signaling pathways in the pathogenesis mechanism of inflammatory-based diseases (Simonyi et al. Citation2012). Numerous isoforms of NOX exist which NOX2 is the most common isoform in phagocytic cells with three cytosolic subunits (p47phox, p67phox and p40phox) and two membrane subunits (gp91 and p22) and a small GTPase (Jackman et al.) protein (Groemping and Rittinger Citation2005) (Figure ).
Figure 1. Schematic illustration of molecular mechanisms involved in COVID-19 pathogenesis in an infected cell. Upon binding of SARS-CoV-2 to angiotensin-converting enzyme 2 (ACE2), the virus enters the cell. The complex of SARS-CoV-2 and ACE2 triggers the renin-angiotensin system (RAS), leading to up-regulation of angiotensin II (Ag II). Binding of Ag II to angiotensin II type I receptor (AT1R) triggers the NADPH oxidase (NOX) activation and oxidative burst via the downstream signaling pathway. Production of ROS leads to DNA strands break which recruits base excision repair (BER) system to repair DNA damage via Poly(ADP-ribose) polymerase (PARP) activity. PARP subsequently modify through transferring ADP-ribose units to itself. Poly(ADP-ribose) glycohydrolase (PARG) which is encoded by the Coronaviridae family, splits ADP-ribose units from PARP, which leads to ADP ribose accumulation in the cytosol. Direct binding of ADP-ribose unites to transient receptor potential channel melastatin 2 (TRPM2) channels, activates these channels in order to release a large amount of Ca2+ ions to the cell. The Ca2+ overload probably results in a distinct form of cell death named parthanatos. In myeloperoxidase (MPO)-containing cells including polymorphonuclears (PMNs), MPO drives dimerization of apocynin. Active apocynin exerts NOX inhibitory function through a disruption of p47phox subunit translocation to NOX complex.
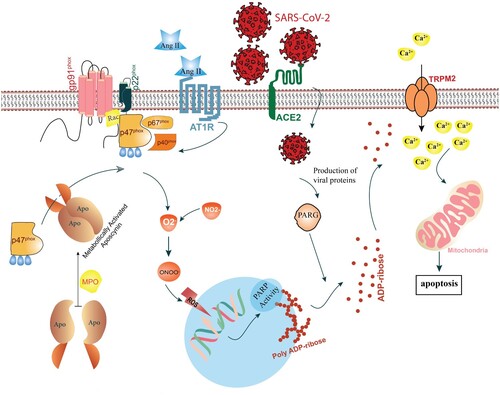
The protective effect of apocynin against NOX is restricted to cells containing myeloperoxidase (MPO) enzymes such as polymorphonuclears (PMNs) phagocytes; thus, there is no inhibitory effect of apocynin in MPO-deficient cells (Ximenes et al. Citation2007; Heumuller et al. Citation2008). MPO functions to convert monomers of apocynin to active di- or trimer forms. A significant NOX-inhibitory effect of dimer apocynin has been observed compared to monomer apocynin (Heumuller et al. Citation2008; Stefanska and Pawliczak Citation2008; Ismail et al. Citation2014). Therefore, apocynin acts as a radical scavenger in the MPO-containing cells and directly prevents the signaling pathways stimulated by ROS without affecting the NOX activity (Heumüller et al. Citation2008).
Active apocynin inhibits the NOX2 activity via disruption of p47phox subunit translocation and attachment to the mitochondrial membrane subunits, resulting in superoxide radical production in leukocytes, monocytes, and endothelial cells (Johnson et al. Citation2002; Tang et al. Citation2007). NOX generates superoxide radicals directly and also various ROS and reactive nitrogen species (RNS) indirectly, which could be diminished by apocynin (Steinkamp-Fenske et al. Citation2007).
The inflammation process initiates with the recruitment of immune cells by endothelial cells, which is concomitant with the secretion of inflammatory cytokines and growth factors, including tumor necrosis factor-alpha (TNFα), interleukin (IL)-1, vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF). The secreted cytokines and growth factors activate intracellular signal transduction via corresponding receptors leading to induction of nuclear factor-κB (NF-κB), mitogen-activated protein kinase (MAPK) activity and synthesis of RNS and ROS in the target cells (Impellizzeri et al. Citation2011). Also, apocynin could act by attenuating some cytokines, chemokines and other pro-inflammatory factors (Kinoshita et al. Citation2013; Nam et al. Citation2016; Oostwoud et al. Citation2016).
One of the key pro-inflammatory cytokines is IL-1, with significant metabolic and haematological effects. Preclinical studies showed that IL-1, through attenuating systemic blood pressure, vascular resistance, high heart rate and leukocyte aggregations exerts hypotensive role (Okusawa et al. Citation1988). IL-1 with inducing endothelial dysfunction causes pathogen infection (Zalinger et al. Citation2017).
On the other hand, IL-1 can stimulate itself and TNF, both promoting pulmonary oedema, thrombosis and bleeding. In addition to hypotension and resistance of systemic blood pressure, IL-1 causes leukopenia and thrombocytopenia. IL-1 induces platelet vascular thrombogenicity in non-endothelial cells by TxA2 production in the inflammation site and leukocyte aggregation and systemic inflammation in activated neutrophils and macrophages by TxB2. As a result, IL-1 increases Tx, leading to the substantial rise in neutrophils adhering to the lungs, which in turn mediates thrombi formation by reducing lymphocytes, vascular inflammation, and platelet aggregation (Conti et al. Citation2020a). Researches have reported primary and secondary prevention of many inflammatory diseases through inhibiting IL-1 family members and IL-6 (Conti et al. Citation2020b). The inhibitory effects of different dose of apocynin on the secretion of TNF-a, IL-1 and IL-6 inflammatory cytokines in murine macrophage cells has been shown. Apocynin activated NF-kB and attenuated pro-inflammatory cytokines generation through suppression of pro-inflammatory cell signaling pathway (Kim et al. Citation2012).
Therapeutic applications of apocynin
Apocynin and respiratory disorders
Oedema, high permeability of pulmonary vessels and blood flow resistance due to airway inflammation and epithelial cell damage are considered lung injury features (Stefanska and Pawliczak Citation2008). In addition to ROS generation as the main pathogenesis mechanism of lung injury (Kellner et al. Citation2017), RNS and especially peroxynitrite reported being responsible for lung injury (Hamid et al. Citation1993; Sadeghi-Hashjin et al. Citation1998). The unstable peroxynitrite is produced following a combination of nitric oxide (NO) and superoxide anion (•O2−). Peroxynitrite seems to function through epithelial damage, mediator generation, and finally, airway hyper-responsiveness (Sadeghi-Hashjin et al. Citation1996). As mentioned earlier, apocynin-induced peroxidase activity is essential for NOX inhibition. Although, the active form of apocynin is improbable in the respiratory system regarding the large surface of airway epithelium (Muijsers et al. Citation2000; Peters et al. Citation2001).
The level of H2O2, NO−2, and NO−3 was diminished following apocynin nebulization (0.5 mg/mL) without any significant effect on other physiological characteristics in nonsmokers patients with mild asthma (Stefanska et al. Citation2012). In this clinical trial, apocynin significantly reduced nitrite (NO-2), nitrate (NO-3) and hydrogen peroxide (H2O2) comparing with placebo. In addition, apocynin could inhibit pulmonary infiltration of ROS- producing PMN and eosinophils by trapping ozone (Peters et al. Citation2001). Another interventional study on healthy subjects demonstrated apocynin functions via reaction with a thiol group of NADPH oxidase subunit, resulting in its direct repression (Stefanska et al. Citation2010).
It was shown that apocynin and ebselen, an active organo-selenium compound, could be considered anti-inflammatory agents, leading to viral clearance in acute exacerbation of chronic obstructive pulmonary disease (AECOPD) mice model alone or in combination with other therapeutic factors (Oostwoud et al. Citation2016). Furthermore, apocynin could significantly decrease ROS generation by neutrophils, neutrophil-mediated human umbilical vein endothelial cell (HUVEC) injury and sepsis-induced lung injury when administered at the concentration of 10–100 µg /mL in guinea pigs (Wang et al. Citation1994).
In vivo animal studies revealed that apocynin can improve acute lung inflammation through attenuating neutrophils penetration into the lung, as well as generation of various pro-inflammatory related factors including MAPK, NF-κB, inducible nitric oxide synthase nitrotyrosine and poly-ADP-ribosyl polymerase (PARP), TNF-α, IL-1, intercellular adhesion molecule (ICAM-1) and platelet-adhesion molecule (P-selectin) in the lung tissue (Kinoshita et al.).
Apocynin inhibits oxidative-mediated inactivation of secretory leukocyte proteinase inhibitor (SLPI) in neutrophils, thus has introduced as a potential therapeutic value in neutrophil-induced lung injury (Stolk et al. Citation1994a). Moreover, orally administered apocynin showed inhibition of lipopolysaccharide (LPS)-induced emphysema (Stolk et al. Citation1994b).
In pathogen-dependent lung injury models, in addition to protective effects against lung vascular permeability following ischemia and reperfusion, apocynin modulated inflammatory responses in a dose-dependent manner (Dodd-o et al. Citation2004). It caused LPS–induced lung injuries such as increasing levels of CD31, H2O2, TNF-α, IL-1, myeloperoxidase, P38, and NF-B (Chian et al. Citation2012).
Apocynin reduced ovalbumin-induced airway hyperresponsiveness and inflammation in an asthma mouse model, as evidenced by a reduction in total inflammatory cells such as macrophages and eosinophils. Also, apocynin reduced soluble components of IL-4, IL-5, IL-12, IL-13, and TNF concentration in the bronchoalveolar lavage fluid and lungs tissue (Kim et al. Citation2012).
In summary, apocynin exerts anti-inflammatory effects in the respiratory system through different mechanisms. The most important mechanisms include inhibiting ROS and RNS production and regulating inflammatory factors like pro-inflammatory cytokines and NF-B production.
Apocynin and atherosclerosis
Atherosclerosis is a chronic inflammatory disease caused by dysfunction between lipid metabolism and immune system response. Inflammation is the major process in forming atherosclerotic plaques, which is dependent on oxidized lipoprotein, shear stress, and oxidative stress (Gregersen and Halvorsen Citation2017). Based on the immunomodulatory role of apocynin, various studies were conducted to illustrate apocynin function in endothelial cells. These studies revealed that the mechanism of action for apocynin in endothelial cells is similar to that in phagocytic cells (Johnson et al. Citation2002; Touyz Citation2008).
The decreased vascular inflammation following 4 mg/kg of apocynin administration in the mice model showed not to be associated with reduced NOX activity; thus, apocynin action mode proposed to be ROS-independent (Khanicheh et al. Citation2013).
Moreover, apocynin attenuated platelet-endothelial interactions through downregulation of endothelial adhesion molecules, platelet adhesion, and ultimately plaque growth (Hawrylowicz et al. Citation1991; Henn et al. Citation1998; Hundelshausen et al. Citation2001). In this context, the apocynin immunoregulatory effect exerts through inhibition of platelet aggregation via downregulation of thromboxane (Tx) A2 synthesis (Engels et al. Citation1992). Although the mechanism by which apocynin functions to diminish endothelial-platelet interaction is not fully understood (Khanicheh et al. Citation2013).
On the other hand, apocynin acts in a ROS-dependent manner in the late stage of atherosclerosis. The action mode of apocynin reported reducing ROS generation in the first step, which is followed by vascular remodeling through a decrease of intima thickness and cell proliferation in a sequential manner. Moreover, with the growing NO production rate (Riganti et al. Citation2008), not only endothelial vasodilation accelerates, but also LDL oxidation and atherosclerosis process decline significantly (Hogg et al. Citation1993).
NOX- derived superoxide radicals induce intimal thickness and cell proliferation which are major players in vascular remodeling scenarios. Apocynin administration in animal models presented to have a regulatory effect on arterial remodeling related to the early stages of atherosclerosis (Dodd-o et al. Citation2004; Chan et al. Citation2007).
Apocynin reduced the production of H2O2, thus showing an anti-atherogenic potential in the presence of serum low-density lipoprotein (LDL) in endothelial cells (Holland et al. Citation1997; Holland et al. Citation1998; Holland Citation1999). It has been proposed that apocynin exerts anti-oxidant activity via NOX suppression in endothelial cells, thus could be considered as a protective strategy against the onset of atherosclerosis (Stefanska and Pawliczak Citation2008).
An experimental study on the hypercholesteremic mice model of atherosclerosis presented a decelerate atherosclerotic plaque formation process by inhibiting NOX following administration of 500 mg apocynin/ L of drinking water for 17–18 weeks (Kinkade et al. Citation2013).
In peritoneal macrophages of atherosclerosis-prone apoE–/–mice, apocynin prevented atherosclerosis development by inhibiting Ox-LDL-mediated ROS production, monoattractant chemokine protein (MCP)-1, IL-6 and granulocyte/macrophage colony-stimulating factor expression, and other pro-inflammatory factors, as well as cell proliferation (Kinkade et al. Citation2013).
Moreover, apocynin reduced NADH-provoked O2−anion production in rat and human arteries and accelerated NO bioavailability; leading to improved endothelium-dependent vasodilation; and enhancement of NO generation from saphenous vein (Heumüller et al.) endothelial cells (Hamilton et al. Citation2002).
An in vivo study on myocardial ischemia/reperfusion (I/R) injury reported that apocynin reduced MPO (as neutrophil infiltration index), iNOS and toll-like receptor 4 (TLR4) (as inflammatory intermediaries), and asymmetric dimethylarginine (ADMA) in myocardial tissue (Riganti et al.). All together, apocynin acts as a suppressive factor for MIR injury by modulating oxidative stress and inflammatory mediators (Uysal et al. Citation2015). Apocynin also reduced the activity of iNOS in addition to downregulation of iNOS level in MIR injury (Uysal et al. Citation2015).
Apocynin mode of action in vascular smooth muscle cells has been reported to inhibit the activation of redox-sensitive kinases p38-mitogen-activate protein kinase (MAPK), Protein kinase B (Akt), and extracellular signal-regulated kinase 1/2 through reduction of hydrogen peroxide and menadione, intracellular radical generator, generation (Heumuller et al. Citation2008).
An in vivo experimental study illustrated the dual function of apocynin in rat ventricular myocytes. The major role of apocynin is to modulate the L-type calcium channel (Ica,L), responsible for contractility regulation in corresponding muscles. Based on redox-related action, apocynin reduced H2O2 and glutathione (GSH), which further inhibited Ica,L. The minor role of apocynin was reported to be prooxidant, leading to H2O2 production, which subsequently facilitates Ica,L in ventricular myocytes (Ochi et al. Citation2010; Ochi and Gupte Citation2011).
Apocynin and hypertension and renal diseases
Renal oxidative stress plays a critical role in hypertension, and elevated ROS levels have been reported in several hypertensive models. Two main sources of ROS origination in the hypertensive kidney are NOX and defective anti-oxidant (Araujo and Wilcox Citation2014).
In vivo renal ischemia models, apocynin showed to inhibit inflammatory damage through attenuating iNOS activity while enhancing eNOS-induced NO levels and regulating the pro-inflammatory cytokine and chemokine production through inhibition of TLR4 in TLR4/ NF-B / MAPK signaling cascade (Li and Wang Citation2015).
The suppression of NF-B activity was also observed in the left ventricle of the hypertensive rat model following apocynin therapy (Pechanova et al. Citation2009). Administration of .5 mmol/L and 100 µm apocynin in deoxycorticosterone acetate salt- (DOCA-) induced hypertensive rats revealed the reduction of blood pressure as well as superoxide production in isolated arteries (Beswick et al. Citation2001; Ghosh et al. Citation2004). The superoxide anion showed kidney regulatory function through adjustment of urinary volume and sodium excretion, which has been mediated by the thick ascending loop of Henle as the major contributor of sodium retention (Hogg et al. Citation1993; Ghosh et al. Citation2004).
In another experiment on Dahl salt-sensitive rat models, control of hypertension occurred through inhibition of superoxide generation following apocynin therapy in the renal medulla (Taylor et al. Citation2006). Moreover, apocynin exerted antihypertensive properties in male rats with dexamethasone-induced hypertension by inhibiting NADPH oxidase activation and superoxide production without affecting endothelial NO synthase (Hu et al. Citation2006). It has also been proposed apocynin might have a protective effect in humoral-mediated hypertension like those induced by angiotensin II and aldosterone (Stefanska and Pawliczak Citation2008).
The findings of an experimental study indicated apocynin (20 mg/kg, i.p.) to improve I/R-induced kidney damage. The protective effect of apocynin in renal injury, especially during ischemia, is the reduction of renal tissue malondialdehyde (MDA) and MPO, and the increase of glutathione peroxidase (GPX), which are considered as indicators of histological damages (Altintas et al. Citation2013).
In addition, an animal study showed pretreatment with 50 mg/kg of apocynin could partially prevent renal I/R injury in rats via stimulation of Src homology-2 domain-containing phosphatase-1 (SHP-1) expression, which is involved in the negative regulation of signaling pathways of oxidative stress injury (Li and Wang Citation2015).
Apocynin and neuroinflammatory disorders
NOX, known as the primary source of oxidative stress in the brain, is upregulated in neuroinflammatory disorders, including ischemic stroke, Alzheimer's, Parkinson's diseases, HIV dementia, multiple sclerosis, and psychiatric diseases (Bedard and Krause Citation2007; Simonyi et al. Citation2012). The maximum concentrations of apocynin found in liver and brain tissues nearly one hour after injection. The presence of apocynin in the brain indicated the potency of apocynin to interrupt the blood–brain barrier (BBB) and made it as one of the best potential therapeutic choices in neurodegenerative diseases (Wang et al. Citation2008).
Oral administration of apocynin (2.5 mg/kg) was observed to diminish infarct volume and cerebral haemorrhage and increase BBB permeability, leading to improved neurological outcomes in the male R/I mice model. Another finding of this study was the high amounts of superoxide anions which were interestingly produced by neuron and microglia cells/monocytes. The prescribed apocynin at a dose of 2.5 mg/kg was capable of decreasing O.- levels in the brain, while higher doses of apocynin not only did not exert inhibitory effect but also exacerbated the brain haemorrhage (Tang et al. Citation2007).
The effect of pre- and post-ischemia- administration of 2.5 mg/kg apocynin in a transient cerebral ischemia mice model was evaluated by Jackman and colleagues (Jackman et al. Citation2009). Unlike post-treatment with apocynin, pretreatment attenuated infarct volume, neurological damage and mortality rate only in wild type mice. Moreover, a high amount of superoxide anions generated in the brain after 24 h of cerebral I/R injury decreased in the wild type mice following apocynin use, while they remained intact in the Nox2 (-/-) mice (Jackman et al. Citation2009), which provides evidence for apocynin role as an anion scavenger.
In a gerbil cerebral ischemic model, primary lipid peroxidation in the hippocampus decreased after 5 mg/kg apocynin injection into the peritoneal cavity. Consequently, the reduction of I/R and neuronal decay was observed simultaneously with delay in neuron death and glial cell activation (Wang et al. Citation2006).
Another preclinical study has reported that apocynin treatment dramatically improved the average lifespan of the familial ALS model (Harraz et al. Citation2008). Surprisingly, findings of a research provided strong evidence of the relationship between age and the protective effect of apocynin in stroke outcomes. The aged rats interestingly showed increased mortality rate, total infarct volume, oedema formation and BBB permeability and ultimately worsening of the functional outcome once treated with 5 mg/kg of apocynin (Kelly et al. Citation2009).
Compared to neurons, glial cells are considered as the sources of NOX2 (Kahles et al. Citation2010), with a high capacity of ROS production leading to detrimental effects on neurons (Brown and Neher Citation2010). The released ROS might serve as a second messenger to facilitate the signaling of MAPKs, PI3 K/Akt and NF-kappaB pathways during an inflammatory response (Simonyi et al. Citation2012). Moreover, NO-mediated peroxynitrite production and ROS activities in glial cells potentially act as neuron killer factors (Brown Citation2007).
NOX controls the production of NADPH oxidase subunits in a negative feedback loop and also have a regulatory effect on secretory phospholipase A2 (sPLA2-IIA), cyclooxygenase-2 (COX-2), and iNOS. Therefore, as a NOX inhibitor, apocynin protects neurons against inflammation-induced toxicity (Gao et al. Citation2002; Pawate et al. Citation2004; Shibata et al. Citation2006; Li et al. Citation2008; Jensen et al. Citation2009; Simonyi et al. Citation2012).
Apocynin and cancer and gastrointestinal inflammatory diseases
Oxidative stress plays a potent role in the pathogenesis of various cancers and could be considered a therapeutic approach upon inhibition (Reuter et al. Citation2010; Mileo and Miccadei Citation2016). In vivo and in vitro experiments evaluated the anti-cancerous effects of apocynin on androgen-independent rat prostate cancer cell line (PLS10). Apocynin significantly reduced PLS10 cell proliferation through cell cycle arrest in the G1 phase without affecting ROS levels. Moreover, this compound inhibited phosphorylation of Rac1, a component of NOX complex, and NF-B and cyclin D1. Additionally, apocynin was reported to reduce vascular endothelial growth factor (VEGF) levels in a dose-dependent manner, thus revealing the protective effect on tumor development and metastasis in a prostate cancer xenograft model of PLS10 (Suzuki et al. Citation2013a).
In order to investigate the possible effect of apocynin on prostate cancer, Suzuki et al. employed the transgenic rat model for adenocarcinoma of the prostate (TRAP). Apocynin was found to significantly reduces the carcinoma cell number in ventral and lateral aspects of the prostate in a dose-dependent manner. Moreover, ROS production and tumor cell proliferation were attenuated, and the MEK-ERK1/2 signaling pathway was blocked in the TRAP model. In the human prostate cancer cell line, LNCaP, apocynin administration showed similar outcomes, including reduction of ROS generation and G0/G1 phase of cell cycle arrest via downregulation of clusterin and cyclin D1(Suzuki et al. Citation2013b).
Obesity is known as one of the major risk factors for colorectal cancer. High levels of insulin in abdominal obesity stimulates NOX and consequently ROS production in large quantities. Administration of 250 mg/L or 500 mg/L of apocynin in mice drinking water for seven weeks presented to decrease the number of colorectal aberrant crypt foci and intestinal polyps in KK-Ay and ApcMin/+mice, respectively. Furthermore, apocynin was reported to reduce oxidized serum LDL and 8-oxo-2′-deoxyguanosine in adipose tissue in both groups while downregulates iNOS expression only in ApcMin+ mice model (Komiya et al. Citation2015). Additionally, in vitro studies also indicated the inhibitory function of apocynin for NF-B transcriptional activity (Barbieri et al. Citation2004; Suzuki et al. Citation2013a), which is responsible for COX-2 induction in monocytes (Barbieri et al. Citation2004).
The nontoxic concentration of apocynin derivatives showed to block the migration of the breast cancer cell line, MDA-MB-435. The apocynin derivatives probably stimulate actin cytoskeleton rearrangement and also reduce the active form of G-protein-associated molecules, Rac1 and Cdc42, and ultimately regulate cell division and migration (Klees et al. Citation2006). Based on the negative correlation of apocynin and Rac1 activation, the apocynin derivation seems to be capable of preventing Rac1-mediated tumor cell migration (Hamilton et al. Citation2002; Klees et al. Citation2006).
The role of apocynin in some gastrointestinal inflammatory diseases has also been reported. It is known that ulcerative colitis occurs through various signaling pathways (Guan and Zhang Citation2017; Lee et al. Citation2018). When the permeability of the intestinal epithelium is enhanced, following disturbance of the epithelial obstacles, increased uptake of luminal antigens occurs; the macrophages and dendritic cells are activated; NF-kB signal transduction pathway is initiated, and finally, pro-inflammatory cytokines of TNF-α, IL-1, and IL-6 are produced (Marin et al. Citation2013; Lee et al. Citation2018). The pro-inflammatory cytokines catalyze ROS production by NADPH-oxidase, which leads to endothelial dysfunction and DNA, protein, and lipid damage. NOS and COXs play a catalyzer role in the production of ROS (Lam et al. Citation2015; Tian et al. Citation2017).
In an animal study, the protective effect of apocynin against chronic inflammatory bowel disease was observed. The weight and length of the colon were alleviated, and intestinal damage, especially inflammatory cell infiltration into the colonic mucosa, was ameliorated. In addition, this molecule caused a significant decrease in colonic iNOS, COX-2, TNF-αand MCP-1 expression. Moreover, apocynin activated anti-inflammatory mediators of Nrf2 and HO-1 (Hwang et al. Citation2019). Also, treatment with apocynin in rats reduced colon damage as well as the enzymatic activity of myeloperoxidase, which is linked to inflammation. The number of macrophages and polymorphonuclear leukocytes in the colon were likewise reduced by apocynin (Van den Worm et al. Citation2001).
Moreover, several studies have illustrated the anti-oxidant therapeutic role of apocynin in chronic inflammatory bowel disease (Moura et al. Citation2015; Tian et al. Citation2017). Oxidative stress causes Nrf2 to prevent protein kinase C activation and reduce NADPH-oxidase function and finally ROS generation (Khor et al. Citation2008; Kruse et al. Citation2016). Also, apocynin launches a defensive mechanism via heme oxygenase-1 activation, diminishing inflammation and tissue damage in the intestinal mucosa (Naito et al. Citation2004; Stefanska et al. Citation2012). Accordingly, apocynin is expected to present a significant therapeutic effect in bowel disease by reducing pro-inflammatory cytokines and inducing anti-inflammatory molecules of Nrf2 and hem oxygenase-1 (Hwang et al. Citation2019). In an experimental murine colitis model, the protective effect of apocynin was exerted via generating NO, PGE2, iNOS and COX-2 expression (Marin et al. Citation2013). Apocynin revealed its inhibitory effects through NF-B and STAT3 signaling pathways. Also, in vitro and in vivo investigations showed the protective effect of apocynin on acute colitis by TNF-α reduction (Mouzaoui et al. Citation2014; Ramonaite et al. Citation2014).
Apocynin and other inflammatory disorders
Apocynin improved insulin resistance in high-fat diet obese mice by controlling inflammation. In apocynin treated mice, a significant decrease in serum TNF-α, IL-6, and leptin concentration, as well as diverse plasma inflammatory cytokines, were observed (Meng et al. Citation2010). Apocynin exerted these changes by downregulating TNF-α, IL-6, MCP-1 and leptin genes in the liver and adipose tissue. Moreover, apocynin ameliorated insulin sensitivity, inhibited the activity of transcription factor NF-B in liver tissue, subsequently improved insulin resistance (Meng et al. Citation2010).
Orally administered apocynin in a mouse model of zymosan-induced acute arthritis, partially suppressed proteoglycan, synthesis in articular cartilage of the arthritic joint, via inhibiting peroxynitrite production. It seems that apocynin exerts this protective role by decreasing inflammatory mediators and COX through NF-B pathway. Therefore, apocynin was proposed as a therapeutic agent for chronic inflammatory joint diseases like osteoarthritis or rheumatoid arthritis (Hougee et al. Citation2006).
The combination of apocynin and paeonol (APPA) inhibited human neutrophil degranulation and reduced ROS production and the neutrophil extracellular traps formation in vitro (Cross et al. Citation2020). APPA, through suppressing TNFα- and GM-CSF-driven signaling pathways, down-regulated cytokine-stimulated gene expression. Therefore, APPA with such anti-inflammatory properties can use as a therapeutic agent in controlling inflammatory diseases that involve neutrophils and TNFα signaling as underlying pathogenesis, such as rheumatoid arthritis (Cross et al. Citation2020).
In arthritic animal models, apocynin improved the arthritic lesions by inhibiting TNFα and IL-1 expression, preventing cell migration and PGE2 synthesizing by leucocytes (Pandey et al. Citation2009). The anti-inflammatory activity of apocynin in drinking water was also investigated, starting at the onset of joint swelling in arthritis models in rats and ending 14 days later when joint swelling in the control group was at its peak. The apocynin-treated rats had normal plasma levels of collagen-specific antibodies, but their joint swelling was significantly reduced (Hart et al. Citation1990).
Apocynin as a therapeutic agent for COVID-19
Since December 2019, a novel infectious viral disease, COVID-19, has been emerged by a new species of coronavirus (nCoV-19) in Wuhan (Xu et al. Citation2020), which has been announced as a pandemic by World Health Organization (WHO) (Organization Citation2020).
COVID-19 is an acute disease that begins with different manifestations, including fever and nonproductive cough, which in some cases further develops into difficult breathing due to lung tissue fibrosis and death (Cascella et al. Citation2021). Unfortunately, there is no definite medication for COVID-19 yet, although the molecular mechanism of nCoV-19 pathogenesis has been demonstrated to be a complex inflammatory disease (Kouhpayeh et al. Citation2020).
The COVID-19 pathogenesis generally involves four main pathways: renin-angiotensin (RAS) signaling pathway, oxidative stress and cell death, cytokines storm, and endothelial dysfunction (Kouhpayeh et al. Citation2020). Extensive oxidative/nitrosative stress, due to RAS activation and subsequent damage to DNA following nCoV-19 binding to ACE2, is considered the major event at the onset of acute respiratory distress syndrome (ARDS). RAS activation induces oxidative stress, tissue and DNA damage and endothelial cell dysfunction. Altogether, these processes lead to activation of PARP-1, viral macrodomain (NSP3) poly (ADP-ribose) glycohydrolase (PARG) and transient receptor potential channel, melastatin 2 (TRPM2) and eventually apoptosis and necrosis in a sequential manner. In this detrimental circuit, oxidative stress plays an initiating role which is further developed by NOX. This circuit consumes NAD, reduces anti-oxidant capacity and consequently increases cytokine release and inflammation (Rajman et al. Citation2018). Subsequently, NAD depletion affects the function of important NAD consuming genes like SIRT1 and CD38 (Mendelsohn and Larrick Citation2017). SIRT1 requires NAD to suppress some particular genes expression, including NF-kB and pro-inflammatory cytokine genes, through deacetylase activity. Interestingly, despite RAS overactivation and subsequent hypervolemia and hypertension, the patients with COVID-19 experience hypovolemia and hypoaldosteronism due to serotonin depletion, contributing to aldosterone secretion displays positive cardiovascular effects (Kouhpayeh et al. Citation2020).
In addition to direct effects, nCoV via activating macrophages stimulates pro-inflammatory cytokines such as IL-1, metalloproteinases and other proteolytic enzymes release, which indirectly creates thrombosis and severe respiratory dysfunction. In addition, IL-1, by stimulating the generation of itself and TNF, causes shock syndrome in COVID-19. Hence, blocking this cytokine seems the better way to prevent thrombi formation and improving COVID-19 complications (Conti et al. Citation2020a).
The molecular mechanism of COVID-19 pathogenesis provides strong evidence for the inflammatory nature, leading to categorizing COVID-19 as systemic inflammatory response syndrome (SIRS). In this view, apocynin with ROS and RNS attenuation function may lead to decreased H2O2, nitrite, and nitrate ion generation in lung tissue. Another mechanism of apocynin function in ROS reduction is the inhibition of polymorphonuclear and eosinophils penetration into the lung. Furthermore, apocynin, through inhibiting pro-inflammatory cytokines especially IL-1 could prevent thrombosis.
As mentioned previously, apocynin was found to exert anti-inflammatory function on various inflammatory diseases through different mechanisms (Ghosh et al. Citation2004; Impellizzeri et al. Citation2011; Hart et al. Citation2014). Evidence shows that post-treatment with moderate doses of apocynin in non-aged patients in some inflammatory diseases exert optimum anti-inflammatory effects (Tang et al. Citation2007; Jackman et al. Citation2009; Kelly et al. Citation2009). Apocynin with NOX inhibitory effect could prevent DNA damage, cell death and apoptosis (Pan and Qian Citation2018). Moreover, apocynin can inhibit endothelial dysfunction via enhancing NO synthesis (Riganti et al. Citation2008), which ameliorates symptoms of COVID-19.
Cytokines storm is a landmark of COVID-19 development which is mediated by leukocytes and endothelial cells in an inflammatory microenvironment.
Investigations on some inflammatory disorders illustrated apocynin as capable of suppressing cytokines and even chemokines production and improves the sign and symptoms of the disease (Impellizzeri et al. Citation2011; Li and Wang Citation2015).
Data analyses
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Conclusion
Apocynin exerts anti-inflammatory effects through a ROS-dependent approach in atherosclerosis, respiratory, renal and neuroinflammatory diseases as well as cancers. During the chronic inflammatory, several pro-inflammatory related factors such as MAPK, iNOS, NF-kB, nitrotyrosine, PARP, TNF-α, ICAM-1, p-selectin, CD31, P38, MPO, MCP-1, IL-6 are contributed which could be targeted by apocynin.
In the case of COVID-19, patients usually experience severe acute respiratory infection and cardiovascular damage. Thus, the employed therapeutic approach for this viral disease should target these two major pathogenesis steps. Apocynin might be an appropriate therapeutic candidate by reducing endothelial damage and suppressing mediator generation and airway hyper-responsiveness. Treating COVID-19 patients with apocynin might be beneficial due to anti-inflammatory potencies of apocynin, including reducing oxidative/nitrosative stress and attenuation of some pro-inflammatory cytokines and chemokines, inhibition of cell proliferation and platelet aggregation, suppression of platelet/endothelial interaction, inactivation of macrophages and lowering vascular permeability. However, there are still some doubts to be addressed, including the proper dosage of apocynin administration, the age range of patients, and the course of the disease in which apocynin is administered. In addition, available evidence on the optimal anti-inflammatory effects of moderate doses of apocynin in non-aged patients supports the idea that apocynin can be used as a therapeutic agent and not a preventive compound in COVID-19 patients.
Acknowledgements
We would like to thank our colleagues at Isfahan University of Medical Sciences and its research centers for their efforts in identifying any potential drug combinations that could be used in the fight against COVID 19.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Altintas R, Polat A, Vardi N, Oguz F, Beytur A, Sagir M, Yildiz A, Parlakpinar H. 2013. The protective effects of apocynin on kidney damage caused by renal ischemia/reperfusion. J Endourol. 27:617–624.
- Araujo M, Wilcox CS. 2014. Oxidative stress in hypertension: role of the kidney. Antioxid Redox Signal. 20:74–101.
- Barbieri SS, Cavalca V, Eligini S, Brambilla M, Caiani A, Tremoli E, Colli S. 2004. Apocynin prevents cyclooxygenase 2 expression in human monocytes through NADPH oxidase and glutathione redox-dependent mechanisms. Free Radic Biol Med. 37:156–165.
- Bedard K, Krause KH. 2007. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 87:245–313.
- Beswick RA, Dorrance AM, Leite R, Webb RC. 2001. NADH/NADPH oxidase and enhanced superoxide production in the mineralocorticoid hypertensive rat. Hypertension. 38:1107–1111.
- Brown GC. 2007. Mechanisms of inflammatory neurodegeneration: iNOS and NADPH oxidase. Biochem Soc Trans. 35:1119–1121.
- Brown GC, Neher JJ. 2010. Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol Neurobiol. 41:242–247.
- Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. 2021. Features, evaluation and treatment Coronavirus (COVID-19). StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK554776/.
- Chan EC, Datla SR, Dilley R, Hickey H, Drummond GR, Dusting GJ. 2007. Adventitial application of the NADPH oxidase inhibitor apocynin in vivo reduces neointima formation and endothelial dysfunction in rabbits. Cardiovasc Res. 75:710–718.
- Chian CF, Chiang CH, Yuan-Jung C, Chuang CH, Liu SL, Yi-Han J, Zhang H, Ryu JH. 2012. Apocynin attenuates lipopolysaccharide-induced lung injury in an isolated and perfused rat lung model. Shock. 38:196–202.
- Conti P, Caraffa A, Gallenga CE, Ross R, Kritas SK, Frydas I, Younes A, Di Emidio P, Ronconi G, Toniato E. 2020a. IL-1 induces throboxane-A2 (TxA2) in COVID-19 causing inflammation and micro-thrombi: inhibitory effect of the IL-1 receptor antagonist (IL-1Ra). J Biol Regul Homeost Agents. 34:1623–1627.
- Conti P, Ronconi G, Caraffa A, Gallenga CE, Ross R, Frydas I, Kritas SK. 2020b. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. 34:327–331.
- Cross AL, Hawkes J, Wright HL, Moots RJ, Edwards SW. 2020. APPA (apocynin and paeonol) modulates pathological aspects of human neutrophil function, without supressing antimicrobial ability, and inhibits TNFalpha expression and signalling. Inflammopharmacology. 28:1223–1235.
- Dodd-O JM, Welsh LE, Salazar JD, Walinsky PL, Peck EA, Shake JG, Caparrelli DJ, Ziegelstein RC, Zweier JL, Baumgartner WA, Pearse DB. 2004. Effect of NADPH oxidase inhibition on cardiopulmonary bypass-induced lung injury. Am J Physiol Heart Circ Physiol. 287:H927–H936.
- Engels F, Renirie BF, Hart BA, Labadie RP, Nijkamp FP. 1992. Effects of apocynin, a drug isolated from the roots of picrorhiza kurroa, on arachidonic acid metabolism. FEBS Lett. 305:254–256.
- Gao HM, Jiang J, Wilson B, Zhang W, Hong JS, Liu B. 2002. Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson's disease. J Neurochem. 81:1285–1297.
- Gearry RB, Barclay ML, Burt MJ, Collett JA, Chapman BA. 2004. Thiopurine drug adverse effects in a population of New Zealand patients with inflammatory bowel disease. Pharmacoepidemiol Drug Saf. 13:563–567.
- Ghosh M, Wang HD, Mcneill JR. 2004. Role of oxidative stress and nitric oxide in regulation of spontaneous tone in aorta of DOCA-salt hypertensive rats. Br J Pharmacol. 141:562–573.
- Gjertsen FB, Solheim E, Scheline RR. 1988. Metabolism of aromatic plant ketones in rats: acetovanillone and paeonol. Xenobiotica. 18:225–234.
- Gregersen BI, Halvorsen B. 2017. Inflammatory mechanisms in atherosclerosis. Atherosclerosis - Yesterday, Today and Tomorrow. 7:328–331.
- Groemping Y, Rittinger K. 2005. Activation and assembly of the NADPH oxidase: a structural perspective. Biochem J. 386:401–416.
- Guan Q, Zhang J. 2017. Recent advances: The imbalance of cytokines in the pathogenesis of inflammatory bowel disease. Mediators Inflamm. 2017:4810258.
- Hamid Q, Springall DR, Riveros-Moreno V, Chanez P, Howarth P, Redington A, Bousquet J, Godard P, Holgate S, Polak JM. 1993. Induction of nitric oxide synthase in asthma. Lancet. 342:1510–1513.
- Hamilton CA, Brosnan MJ, Al-Benna S, Berg G, Dominiczak AF. 2002. NAD(p)H oxidase inhibition improves endothelial function in rat and human blood vessels. Hypertension. 40:755–762.
- Harirforoosh S, Jamali F. 2009. Renal adverse effects of nonsteroidal anti-inflammatory drugs. Expert Opin Drug Saf. 8:669–681.
- Harraz MM, Marden JJ, Zhou W, Zhang Y, Williams A, Sharov VS, Nelson K, Luo M, Paulson H, Schöneich C, Engelhardt JF. 2008. SOD1 mutations disrupt redox-sensitive Rac regulation of NADPH oxidase in a familial ALS model. J Clin Invest. 118:659–670.
- Hart BA, Copray S, Philippens I. 2014. Apocynin, a low molecular oral treatment for neurodegenerative disease. Biomed Res Int. 2014:298020.
- Hart T, B A, Simons JM, Shoshan K-S, Bakker NPM, Labadie RP. 1990. Antiarthritic activity of the newly developed neutrophil oxidative burst antagonist apocynin. Free Radical Biol Med. 9:127–131.
- Hawrylowicz CM, Howells GL, Feldmann M. 1991. Platelet-derived interleukin 1 induces human endothelial adhesion molecule expression and cytokine production. J Exp Med. 174:785–790.
- Henn V, Slupsky JR, Grafe M, Anagnostopoulos I, Forster R, Muller-Berghaus G, Kroczek RA. 1998. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 391:591–594.
- Heumüller S, Wind S, Barbosa-Sicard E, Schmidt HHHW, Busse R, Schröder K, Brandes RP. 2008. Apocynin Is Not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 51:211–217.
- Hogg N, Kalyanaraman B, Joseph J, Struck A, Parthasarathy S. 1993. Inhibition of low-density lipoprotein oxidation by nitric oxide. potential role in atherogenesis. FEBS Lett. 334:170–174.
- Holland JA. 1999. Prevention of atherosclerosis using NADPH oxidase inhibitors. US patent no. 5902831.
- Holland JA, Meyer JW, Chang MM, O'donnell RW, Johnson DK, Ziegler LM. 1998. Thrombin stimulated reactive oxygen species production in cultured human endothelial cells. Endothelium. 6:113–121.
- Holland JA, Meyer JW, Schmitt ME, Sauro MD, Johnson DK, Abdul-Karim RW, Patel V, Ziegler LM, Schillinger KJ, Small RF, Lemanski LF. 1997. Low-density lipoprotein stimulated peroxide production and endocytosis in cultured human endothelial cells: mechanisms of action. Endothelium. 5:191–207.
- Hougee S, Hartog A, Sanders A, Graus YM, Hoijer MA, Garssen J, Van Den Berg WB, Van Beuningen HM, Smit HF. 2006. Oral administration of the NADPH-oxidase inhibitor apocynin partially restores diminished cartilage proteoglycan synthesis and reduces inflammation in mice. Eur J Pharmacol. 531:264–269.
- Hu L, Zhang Y, Lim PS, Miao Y, Tan C, Mckenzie KU, Schyvens CG, Whitworth JA. 2006. Apocynin but not L-arginine prevents and reverses dexamethasone-induced hypertension in the rat. Am J Hypertens. 19:413–418.
- Hundelshausen PV, Weber KSC, Huo Y, Proudfoot AEI, Nelson PJ, Ley K, Weber C. 2001. RANTES Deposition by Platelets Triggers Monocyte Arrest on Inflamed and Atherosclerotic Endothelium. Circulation. 103:1772–1777.
- Hunter P. 2012. The inflammation theory of disease. The growing realization that chronic inflammation is crucial in many diseases opens new avenues for treatment. EMBO Rep. 13:968–970.
- Hwang YJ, Nam SJ, Chun W, Kim SI, Park SC, Kang CD, Lee SJ. 2019. Anti-inflammatory effects of apocynin on dextran sulfate sodium-induced mouse colitis model. PLoS One. 14:e0217642.
- Impellizzeri D, Esposito E, Mazzon E, Paterniti I, Di Paola R, Bramanti P, Cuzzocrea S. 2011. Effect of apocynin, a NADPH oxidase inhibitor, on acute lung inflammation. Biochem Pharmacol. 81:636–648.
- Ismail HM, Scapozza L, Ruegg UT, Dorchies OM. 2014. Diapocynin, a dimer of the NADPH oxidase inhibitor apocynin, reduces ROS production and prevents force loss in eccentrically contracting dystrophic muscle. PLOS ONE. 9:e110708.
- Jackman KA, Miller AA, De Silva TM, Crack PJ, Drummond GR, Sobey CG. 2009. Reduction of cerebral infarct volume by apocynin requires pretreatment and is absent in Nox2-deficient mice. Br J Pharmacol. 156:680–688.
- Jantaree P, Lirdprapamongkol K, Kaewsri W, Thongsornkleeb C, Choowongkomon K, Atjanasuppat K, Ruchirawat S, Svasti J. 2017. Homodimers of vanillin and apocynin decrease the metastatic potential of human cancer cells by inhibiting the FAK/PI3 K/Akt signaling pathway. J Agric Food Chem. 65:2299–2306.
- Jensen MD, Sheng W, Simonyi A, Johnson GS, Sun AY, Sun GY. 2009. Involvement of oxidative pathways in cytokine-induced secretory phospholipase A2-IIA in astrocytes. Neurochem Int. 55:362–368.
- Johnson DK, Schillinger KJ, Kwait DM, Hughes CV, Mcnamara EJ, Ishmael F, O'donnell RW, Chang MM, Hogg MG, Dordick JS, et al. 2002. Inhibition of NADPH oxidase activation in endothelial cells by ortho-methoxy-substituted catechols. Endothelium. 9:191–203.
- Kahles T, Kohnen A, Heumueller S, Rappert A, Bechmann I, Liebner S, Wittko IM, Neumann-Haefelin T, Steinmetz H, Schroeder K, Brandes RP. 2010. NADPH oxidase Nox1 contributes to ischemic injury in experimental stroke in mice. Neurobiol Dis. 40:185–192.
- Kellner M, Noonepalle S, Lu Q, Srivastava A, Zemskov E, Black SM. 2017. ROS signaling in the pathogenesis of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). Adv Exp Med Biol. 967:105–137.
- Kelly KA, Li X, Tan Z, Vangilder RL, Rosen CL, Huber JD. 2009. NOX2 inhibition with apocynin worsens stroke outcome in aged rats. Brain Res. 1292:165–172.
- Khanicheh E, Qi Y, Xie A, Mitterhuber M, Xu L, Mochizuki M, Daali Y, Jaquet V, Krause KH, Ruggeri ZM, et al. 2013. Molecular imaging reveals rapid reduction of endothelial activation in early atherosclerosis with apocynin independent of antioxidative properties. Arterioscler Thromb Vasc Biol. 33:2187–2192.
- Khor TO, Huang MT, Prawan A, Liu Y, Hao X, Yu S, Cheung WK, Chan JY, Reddy BS, Yang CS, Kong AN. 2008. Increased susceptibility of Nrf2 knockout mice to colitis-associated colorectal cancer. Cancer Prev Res (Phila). 1:187–191.
- Kim SY, Moon KA, Jo HY, Jeong S, Seon SH, Jung E, Cho YS, Chun E, Lee KY. 2012. Anti-inflammatory effects of apocynin, an inhibitor of NADPH oxidase, in airway inflammation. Immunol Cell Biol. 90:441–448.
- Kinkade K, Streeter J, Miller FJ. 2013. Inhibition of NADPH oxidase by apocynin attenuates progression of atherosclerosis. Int J Mol Sci. 14:17017–17028.
- Kinoshita H, Matsumura T, Ishii N, Fukuda K, Senokuchi T, Motoshima H, Kondo T, Taketa K, Kawasaki S, Hanatani S, et al. 2013. Apocynin suppresses the progression of atherosclerosis in apoE-deficient mice by inactivation of macrophages. Biochem Biophys Res Commun. 431:124–130.
- Klees RF, De Marco PC, Salasznyk RM, Ahuja D, Hogg M, Antoniotti S, Kamath L, Dordick JS, Plopper GE. 2006. Apocynin derivatives interrupt intracellular signaling resulting in decreased migration in breast cancer cells. J Biomed Biotechnol. 2006:87246.
- Komiya M, Fujii G, Miyamoto S, Takahashi M, Ishigamori R, Onuma W, Ishino K, Totsuka Y, Fujimoto K, Mutoh M. 2015. Suppressive effects of the NADPH oxidase inhibitor apocynin on intestinal tumorigenesis in obese KK-A(y) and Apc mutant Min mice. Cancer Sci. 106:1499–1505.
- Kouhpayeh S, Shariati L, Boshtam M, Al E. 2020. The molecular story of COVID-19; NAD+ depletion addresses all questions in this infection Med & Pharmacol, preprint.
- Kruse ML, Friedrich M, Arlt A, Rocken C, Egberts JH, Sebens S, Schafer H. 2016. Colonic lamina propria inflammatory cells from patients with IBD induce the nuclear factor-E2 related factor-2 thereby leading to greater proteasome activity and apoptosis protection in human colonocytes. Inflamm Bowel Dis. 22:2593–2606.
- Laine L, Smith R, Min K, Chen C, Dubois RW. 2006. Systematic review: the lower gastrointestinal adverse effects of non-steroidal anti-inflammatory drugs. Aliment Pharmacol Ther. 24:751–767.
- Lam G, Apostolopoulos V, Zulli A, Nurgali K. 2015. NADPH oxidases and inflammatory bowel disease. Curr Med Chem. 22:2100–2109.
- Lee SH, Kwon JE, Cho ML. 2018. Immunological pathogenesis of inflammatory bowel disease. Intest Res. 16:26–42.
- Li B, Guo YS, Sun MM, Dong H, Wu SY, Wu DX, Li CY. 2008. The NADPH oxidase is involved in lipopolysaccharide-mediated motor neuron injury. Brain Res. 1226:199–208.
- Li Z, Wang Y. 2015. Effect of NADPH oxidase inhibitor-apocynin on the expression of Src homology-2 domain-containing phosphatase-1 (SHP-1) exposed renal ischemia/reperfusion injury in rats. Toxicol Rep. 2:1111–1116.
- Luchtefeld R, Luo R, Stine K, Alt ML, Chernovitz PA, Smith RE. 2008. Dose formulation and analysis of diapocynin. J Agric Food Chem. 56:301–306.
- Marin M, Giner RM, Rios JL, Recio Mdel C. 2013. Protective effect of apocynin in a mouse model of chemically-induced colitis. Planta Med. 79:1392–1400.
- Mendelsohn AR, Larrick JW. 2017. The NAD+/PARP1/SIRT1 axis in aging. Rejuvenation Res. 20:244–247.
- Meng R, Zhu DL, Bi Y, Yang DH, Wang YP. 2010. Apocynin improves insulin resistance through suppressing inflammation in high-fat diet-induced obese mice. Mediators Inflamm. 2010:858735.
- Mileo AM, Miccadei S. 2016. Polyphenols as modulator of oxidative stress in cancer disease: New therapeutic strategies. Oxid Med Cell Longev. 2016:6475624.
- Moura FA, De Andrade KQ, Dos Santos JCF, Araujo ORP, Goulart MOF. 2015. Antioxidant therapy for treatment of inflammatory bowel disease: does it work? Redox Biol. 6:617–639.
- Mouzaoui S, Djerdjouri B, Makhezer N, Kroviarski Y, El-Benna J, Dang PM. 2014. Tumor necrosis factor-alpha-induced colitis increases NADPH oxidase 1 expression, oxidative stress, and neutrophil recruitment in the colon: preventive effect of apocynin. Mediators Inflamm. 2014:312484.
- Muijsers RB, Van Den Worm E, Folkerts G, Beukelman CJ, Koster AS, Postma DS, Nijkamp FP. 2000. Apocynin inhibits peroxynitrite formation by murine macrophages. Br J Pharmacol. 130:932–936.
- Naito Y, Takagi T, Yoshikawa T. 2004. Heme oxygenase-1: a new therapeutic target for inflammatory bowel disease. Aliment Pharmacol Ther. 20(Suppl 1):177–184.
- Nam YJ, Kim A, Sohn DS, Lee CS. 2016. Apocynin inhibits toll-like receptor-4-mediated activation of NF-kappaB by suppressing the Akt and mTOR pathways. Naunyn Schmiedebergs Arch Pharmacol. 389:1267–1277.
- Ochi R, Gupte R, Murayama T, Kurebayashi N, Gupte S. 2010. Apocynin reversibly inhibits L-type Ca2+ channel current involvement of reactive oxygen species. Biophysical J. 98:134a–135a.
- Ochi R, Gupte S. 2011. Apocynin induces rapid inhibition and slow facilitation of ICa, L and decrease and increase of reactive oxygen species in rat ventricular myocytes. Biophysical J. 100:197a.
- Okusawa S, Gelfand JA, Ikejima T, Connolly RJ, Dinarello CA. 1988. Interleukin 1 induces a shock-like state in rabbits. synergism with tumor necrosis factor and the effect of cyclooxygenase inhibition. J Clin Invest. 81:1162–1172.
- Oostwoud LC, Gunasinghe P, Seow HJ, Ye JM, Selemidis S, Bozinovski S, Vlahos R. 2016. Apocynin and ebselen reduce influenza A virus-induced lung inflammation in cigarette smoke-exposed mice. Sci Rep. 6:20983.
- Pan L, Qian S. 2018. Apocynin promotes neural function recovery and suppresses neuronal apoptosis by inhibiting Tlr4/NF-B signaling pathway in a rat model of cerebral infarction. Int J Immunopathol Pharmacol. 32:1–11.
- Pandey A, Kour K, Bani S, Suri KA, Satti NK, Sharma P, Qazi GN. 2009. Amelioration of adjuvant induced arthritis by apocynin. Phytother Res. 23:1462–1468.
- Pawate S, Shen Q, Fan F, Bhat NR. 2004. Redox regulation of glial inflammatory response to lipopolysaccharide and interferongamma. J Neurosci Res. 77:540–551.
- Pechanova O, Jendekova L, Vrankova S. 2009. Effect of chronic apocynin treatment on nitric oxide and reactive oxygen species production in borderline and spontaneous hypertension. Pharmacol Rep. 61:116–122.
- Peters E, Hiltermann J, Stolk J. 2001. Effectofapocynin onozone-inducedairwayhyperresponsivenesstomethacholine in asthmatics. Free Radical Biol Med. 31:1442–1447.
- Rajman L, Chwalek K, Sinclair DA. 2018. Therapeutic potential of NAD-boosting molecules: The In vivo evidence. Cell Metab. 27:529–547.
- Ramonaite R, Skieceviciene J, Juzenas S, Salteniene V, Kupcinskas J, Matusevicius P, Borutaite V, Kupcinskas L. 2014. Protective action of NADPH oxidase inhibitors and role of NADPH oxidase in pathogenesis of colon inflammation in mice. World J Gastroenterol. 20:12533–12541.
- Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. 2010. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 49:1603–1616.
- Riganti C, Costamagna C, Doublier S, Miraglia E, Polimeni M, Bosia A, Ghigo D. 2008. The NADPH oxidase inhibitor apocynin induces nitric oxide synthesis via oxidative stress. Toxicol Appl Pharmacol. 228:277–285.
- Sadeghi-Hashjin G, Folkerts G, Henricks PA, Muijsers RB, Nijkamp FP. 1998. Peroxynitrite in airway diseases. Clin Exp Allergy. 28:1464–1473.
- Sadeghi-Hashjin G, Folkerts G, Henricks PA, Verheyen AK, Van Der Linde HJ, Van Ark I, Coene A, Nijkamp FP. 1996. Peroxynitrite induces airway hyperresponsiveness in Guinea pigs in vitro and in vivo. Am J Respir Crit Care Med. 153:1697–1701.
- Shibata H, Katsuki H, Okawara M, Kume T, Akaike A. 2006. . c-Jun N-terminal kinase inhibition and alpha-tocopherol protect midbrain dopaminergic neurons from interferon-gamma/lipopolysaccharide-induced injury without affecting nitric oxide production. J Neurosci Res. 83:102–109.
- Simonyi A, Serfozo P, Lehmidi TM, Cui J, Gu Z, Lubahn DB, Sun AY, Sun GY. 2012. The neuroprotective effects of apocynin. Front Biosci (Elite Ed). 4:2183–2193.
- Stefanska J, Pawliczak R. 2008. Apocynin: molecular aptitudes. Mediators Inflamm. 2008:106507.
- Stefanska J, Sarniak A, Wlodarczyk A, Sokolowska M, Pniewska E, Doniec Z, Nowak D, Pawliczak R. 2012. Apocynin reduces reactive oxygen species concentrations in exhaled breath condensate in asthmatics. Exp Lung Res. 38:90–99.
- Stefanska J, Sokolowska M, Sarniak A, Wlodarczyk A, Doniec Z, Nowak D, Pawliczak R. 2010. Apocynin decreases hydrogen peroxide and nitrate concentrations in exhaled breath in healthy subjects. Pulm Pharmacol Ther. 23:48–54.
- Steinkamp-Fenske K, Bollinger L, Xu H, Yao Y, Horke S, Forstermann U, Li H. 2007. Reciprocal regulation of endothelial nitric-oxide synthase and NADPH oxidase by betulinic acid in human endothelial cells. J Pharmacol Exp Ther. 322:836–842.
- Stolk J, Hiltermann TJ, Dijkman JH, Verhoeven AJ. 1994a. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am J Respir Cell Mol Biol. 11:95–102.
- Stolk J, Rossie W, Dijkman JH. 1994b. Apocynin improves the efficacy of secretory leukocyte protease inhibitor in experimental emphysema. Am J Respir Crit Care Med. 150:1628–1631.
- Suzuki S, Pitchakarn P, Sato S, Shirai T, Takahashi S. 2013a. Apocynin, an NADPH oxidase inhibitor, suppresses progression of prostate cancer via Rac1 dephosphorylation. Exp Toxicol Pathol. 65:1035–1041.
- Suzuki S, Shiraga K, Sato S, Punfa W, Naiki-Ito A, Yamashita Y, Shirai T, Takahashi S. 2013b. Apocynin, an NADPH oxidase inhibitor, suppresses rat prostate carcinogenesis. Cancer Sci. 104:1711–1717.
- Tang LL, Ye K, Yang XF, Zheng JS. 2007. Apocynin attenuates cerebral infarction after transient focal ischaemia in rats. J Int Med Res. 35:517–522.
- Taylor NE, Glocka P, Liang M, Cowley AW, Jr. 2006. NADPH oxidase in the renal medulla causes oxidative stress and contributes to salt-sensitive hypertension in Dahl S rats. Hypertension. 47:692–698.
- Tian T, Wang Z, Zhang J. 2017. Pathomechanisms of oxidative stress in inflammatory bowel disease and potential Antioxidant therapies. Oxid Med Cell Longev. 2017:4535194.
- Touyz RM. 2008. Apocynin, NADPH oxidase, and vascular cells: a complex matter. Hypertension. 51:172–174.
- Uysal A, Sahna E, Ozguler IM, Burma O, Ilhan N. 2015. Effects of apocynin, an NADPH oxidase inhibitor, on levels of ADMA, MPO, iNOS and TLR4 induced by myocardial ischemia reperfusion. Perfusion. 30:472–477.
- Van den Worm E, Beukelman CJ, Van den Berg AJ, Kroes BH, Labadie RP, Van Dijk H. 2001. Effects of methoxylation of apocynin and analogs on the inhibition of reactive oxygen species production by stimulated human neutrophils. Eur J Pharmacol. 433:225–230.
- Vejrazka M, Micek R, Stipek S. 2005. Apocynin inhibits NADPH oxidase in phagocytes but stimulates ROS production in non-phagocytic cells. Biochim Biophys Acta. 1722:143–147.
- Wang Q, Smith RE, Luchtefeld R, Sun AY, Simonyi A, Luo R, Sun GY. 2008. Bioavailability of apocynin through its conversion to glycoconjugate but not to diapocynin. Phytomedicine. 15:496–503.
- Wang Q, Tompkins KD, Simonyi A, Korthuis RJ, Sun AY, Sun GY. 2006. Apocynin protects against global cerebral ischemia-reperfusion-induced oxidative stress and injury in the gerbil hippocampus. Brain Res. 1090:182–189.
- Wang W, Suzuki Y, Tanigaki T, Rank DR, Raffin TA. 1994. Effect of the NADPH oxidase inhibitor apocynin on septic lung injury in Guinea pigs. Am J Respir Crit Care Med. 150:1449–1452.
- World Health Organization. 2020. Coronavirus disease 2019 (COVID-19). Situation Report – 67.
- Ximenes VF, Kanegae MP, Rissato SR, Galhiane MS. 2007. The oxidation of apocynin catalyzed by myeloperoxidase: proposal for NADPH oxidase inhibition. Arch Biochem Biophys. 457:134–141.
- Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, et al. 2020. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 8:420–422.
- Zalinger ZB, Elliott R, Weiss SR. 2017. Role of the inflamma some-related cytokines Il-1 and Il-18 during infection with murine coronavirus. J Neurovirol. 23:845–854.
