ABSTRACT
The contamination of foods with aflatoxins, a group of carcinogenic compounds produced by some filamentous fungi belonging to Aspergillus section Flavi, is the major food safety concern worldwide. Various pre- and post-harvest techniques have been employed to minimize the level of aflatoxins in food commodities. The present study aimed to explore the potential of essential oils (EOs) derived from the medicinal herbs viz., Heliotropium bacciferum, Ocimum dhofarense and Zataria multiflora to detoxify aflatoxin B1 (AFB1). The EOs extracted from H. bacciferum, O. dhofarense and Z. multiflora exhibited 82.6, 92.0 and 67.9% degradation of AFB1, respectively as determined by ELISA. In the laboratory tests, EO of Z. multiflora was very effective in inhibiting the growth of Aspegillus flavus, whereas EOs of H. bacciferum and O. dhofarense did not show inhibitory activity towards A. flavus. Gas chromatography-mass spectrometry analysis of the EOs showed the presence of α-pinene (32.9%) and β-myrcene (9.4%) in H. bacciferum, germacrene D (41%), bicyclogermacrene (16.4%) and germacrene B (13.7%) in O. dhofarense and linalool (27.5%) and bornyl acetate (15.4%) in Z. multiflora as the major components. To our knowledge, this is the first study demonstrating detoxification of AFB1 by EOs of medicinal plants.
GRAPHICAL ABSTRACT
Introduction
In recent years, foodborne illnesses have emerged as an important public health concern worldwide. The consumption of mycotoxin-contaminated foods is often recognized as the principal source of foodborne sickness in humans (Adeyeye Citation2016; Sarma et al. Citation2017). Aflatoxins, fumonisins, ochratoxins and trichothecenes are the major foodborne mycotoxins and Aspergillus, Fusarium, Alternaria and Penicillium are the main producers of foodborne mycotoxins (Wu et al. Citation2014). Among the various mycotoxins, aflatoxin B1 (AFB1) produced predominantly by Aspergillus flavus Link and A. parasiticus Speare has been described as the highest carcinogenic mycotoxin (Ahmed Adam et al. Citation2017). These toxigenic fungi invade the agricultural commodities such as peanut, corn, cottonseed, tree nuts, rice and spices at pre-harvest and/or post-harvest stages and release aflatoxins under favourable conditions (Kumar et al. Citation2017).
Aflatoxins are highly stable under different storage conditions and hence complete removal or destruction of the toxins is very difficult after contamination of agricultural commodities. However, by adopting appropriate decontamination/detoxification techniques, the aflatoxins in the agricultural products can be either degraded or the amounts of aflatoxins can be reduced to a safe level (Aiko and Mehta Citation2015; Ismail et al. Citation2018). Physical methods such as heating, extrusion and irradiation, chemical methods such as ammonization, treatment with hydrogen peroxide, sodium bisulphite, ozonization and biological methods such as the use of microbial enzymes, live bacterial and yeast cells have been used for degradation of aflatoxins in foods (Velazhahan Citation2017; Ismail et al. Citation2018); however, each method has its own limitations. Plant products are considered as a source of biologically safe, cost-effective and complementary approach for detoxification of aflatoxins (Hajare et al. Citation2005; Sandosskumar et al. Citation2007; Velazhahan et al. Citation2010; Panda and Mehta Citation2013; Kannan and Velazhahan Citation2014; Vijayanandraj et al. Citation2014; Iram et al. Citation2016). In the course of screening of Omani traditional medicinal plants for in vitro detoxification of AFB1, we observed that the aqueous extracts of Heliotropium bacciferum Forssk. (Boraginaceae), Ocimum dhofarense (Sebald) A.J. Paton (Lamiaceae) and Zataria multiflora Boiss. (Lamiaceae) were able to degrade over 90% of AFB1 (Velazhahan et al. unpublished). The aforementioned medicinal plants are known to contain essential oils (Saleem et al. Citation2004; Ismail Citation2006; Carovic-Stanko et al. Citation2010; Raeisi et al. Citation2016). The antimicrobial activity of essential oils of plants has been reported by many researchers (Cox et al. Citation2000; Dorman and Deans Citation2000; Bankole and Joda Citation2004; Oxenham et al. Citation2005; Sharma and Tripathi Citation2006; Szczerbanik et al. Citation2007; Gandomi et al. Citation2009; Vilela et al. Citation2009; Huang et al. Citation2010; Tolouee et al. Citation2010; Combrinck et al. Citation2011; Tian et al. Citation2012a; Kedia et al. Citation2015; Kiran et al. Citation2016; Ghaffari et al. Citation2019; Chaudhari et al. Citation2020; Das et al. Citation2020; Rabib et al. Citation2020). However, AFB1- detoxifying ability of essential oils has not been reported so far. The objective of this study was to evaluate the AFB1-detoxifying potential of essential oils of H. bacciferum, O. dhofarense and Z. multiflora.
Materials and methods
Plant material
H. bacciferum Forssk. (Boraginaceae) (Accession number 201600290), O. dhofarense (Sebald) A.J.Paton (Lamiaceae) (Accession number 202000071) and Z. multiflora Boiss. (Lamiaceae) (Accession number 201100114) plants were obtained from Oman Botanic Garden, Muscat, Sultanate of Oman.
Extraction of essential oils
One kg of the leaves and/or stem of the medicinal plants were added with 1.5 l of distilled water in a glass reactor and extracted by using ETHOS X microwave extraction system (Milestone Inc., Shelton, CT, USA) as described by Filly et al. (Citation2014). The extracted essential oils were stored at −20°C in small amber glass vials.
Test for detoxification of aflatoxins by essential oils
The essential oils were diluted with methanol (1:10, v/v) and 250 μl of EO was mixed with AFB1 (50 μg/l) in a microcentrifuge. Following a 24-h incubation at 25°C, AFB1 in the mixture was extracted with 250 μl of chloroform. The chloroform fraction was evaporated at 60°C using a water bath and the residue was quantified by enzyme-linked immunosorbent assay using a commercial kit (RIDASCREEN Aflatoxin B1; R-Biopharm AG, Darmstadt, Germany). The absorbance of the samples was measured at 450 nm using a microplate reader. Analysis was performed in triplicate for each sample. The experiment was repeated twice.
Testing antifungal activity
The antifungal activity of EOs against A. flavus was tested using agar-diffusion technique (Al-Maawali et al. Citation2021). Potato dextrose agar (Oxoid Ltd., Basingstoke, UK) plates were inoculated with 100 μl of A. flavus (GenBank accession number MW386304) spore suspension (108 spores/ml), spread uniformly with a spreader and then sterile filter paper discs (6 mm) were placed on the agar surfaces. The paper disks were applied with 10 μl of EOs. The plates were incubated at room temperature (25 ± 2°C) and the zone of inhibition was observed after 7–10 days of incubation. All tests were performed in triplicate.
Analysis of essential oils
The EOs were analyzed on a Shimadzu GC-2010 Plus, fitted with a Rtx-5MS capillary column (30 m ×0.25 mm; 0.25 μm), coupled to a GCMS-QP2010 ULTRA MS as described by Hanif et al. (Citation2011). Ultra-high purity helium (99.9999%) was used as carrier gas at a flow rate of 1.0 ml/min. The injection, transfer line and ion source temperatures were 280°C, 270°C and 230°C, respectively. The ionizing energy was 70 eV. The mass spectra were recorded in the scan range of 40–550 amu. The injected sample volume was 1 μl with a split ratio of 100:1. The oven temperature was programmed to increase from 42°C to 330°C at a rate of 5.5°C/min with a final hold for 10 min. The total run time was 63.5 min. NIST 2011 v.2.3 and Wiley 9th edition mass spectrum libraries were used for the identification of compounds. The EO components were confirmed using Kovat’s indices (KI).
Results and discussion
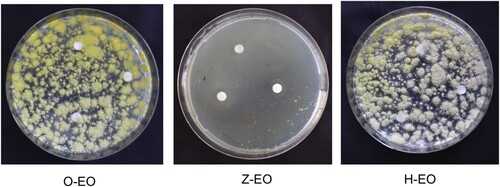
The essential oils were extracted from the leaves of O. dhofarense and leaves and stems of H. bacciferum and Z. multiflora by microwave extraction method and their yields were 0.05%, 0.07% and 0.5%, respectively. The EO of Z. multiflora strongly inhibited the growth of A. flavus and formed clear zones around the paper disc applied with EO in the disc diffusion test (Figure ). Our results are consistent with those of Gandomi et al. (Citation2009) who reported that EO of Z. multiflora inhibited the growth and production of spores and aflatoxin by A. flavus. The antifungal activity of EOs of several plant species including Cinnamomum zeylanicum (Kiran et al. Citation2016), Cinnamomum jensenianum (Tian et al. Citation2012a), Pimenta dioica (Chaudhari et al. Citation2020), Trachyspermum ammi (Kedia et al. Citation2015), Eucalyptus globulus (Vilela et al. Citation2009) and Cuminum cyminum (Kedia et al. Citation2014) against A. flavus has been reported. Cox et al. (Citation2000) while studying the mode of action of Melaleuca alternifolia essential oil on Candida albicans, Staphylococcus aureus and Escherichia coli reported that the EO suppressed the respiration and augmented the permeability of yeast plasma membranes and bacterial cytoplasmic membranes. Tian et al. (Citation2012b) demonstrated that the EO extracted from Anethum graveolens seeds induced morphological changes in A. flavus cells and caused decrease in ergosterol content, ATPase and dehydrogenase activities, increase in mitochondrial membrane potential and production of reactive oxygen species. Chaudhari et al. (Citation2020) reported that the EO of Pimenta dioica completely inhibited the growth of A. flavus and aflatoxin B1 production. The oil caused reduction of methylglyoxal, a signalling molecule that can trigger aflatoxin biosynthesis gene aflR, enhanced leakage of cellular ions and ergosterol content of fungal plasma membrane suggesting plasma membrane of fungi as the action site. In this study, EOs of H. bacciferum and O. dhofarense did not show inhibitory activity towards A. flavus. However, Kumar et al. (Citation2010) reported that EO of O. sanctum and its major constituent, eugenol inhibited the growth of A. flavus and AFB1 production.
Figure 1. Inhibition of Aspergillus flavus by essential oils of H. bacciferum, O. dhofarense and Z. multiflora. H-EO, H. bacciferum essential oil; O-EO, O. dhofarense essential oil; Z-EO, Z. multiflora essential oil.
The results of this study also revealed that the EOs of H. bacciferum, O. dhofarense and Z. multiflora caused 82.6, 92.0 and 67.9% degradation of AFB1, respectively (Table ). The GC/MS analysis of EO of H. bacciferum revealed the presence of α-pinene (32.9%) and β-myrcene (9.4%) as the major components (Table ; Figure (a)). In the essential oil of O. dhofarense, the main constituents were germacrene D (41%), bicyclogermacrene (16.4%) and germacrene B (13.7%) (Table ; Figure (b)). Linalool (27.5%) and bornyl acetate (15.4%) were the main components in the EO of Z. Multiflora (Table ; Figure (c)). α-pinene, a terpene, has been shown to have a variety of pharmacological activities including antimicrobial, antioxidant, anti-inflammatory, antitumour and anticoagulant (Salehi et al. Citation2019). The monoterpene β-myrcene has been reported to prevent peptic ulcer disease (Bonamin et al. Citation2014). Germacrene D is a precursor of many sesquiterpenes (Bulow and Konig Citation2000). Mosquito larvicidal potential of bicyclogermacrene (a sesquiterpene) has been reported (Govindarajan and Benelli Citation2016). Antimicrobial effect of linalool, an acyclic monoterpene (Park et al. Citation2012) and anti-inflammatory activity of linalool-containing essential oils has been reported earlier (Peana et al. Citation2002). Bornyl acetate has been identified as the main constituent of EO of Tetraclinis articulata that showed antibacterial activities (Rabib et al. Citation2020). However, the role of these chemical constituents in detoxification of AFB1 remains to be elucidated. Aflatoxin detoxification potential of aqueous extracts of O. tenuiflorum (Panda and Mehta Citation2013) and O. basilicum (Iram et al. Citation2016) has been reported. However, no information about the detoxification potential of essential oils of O. dhofarense is available in the literature. To our knowledge, this is the first study demonstrating detoxification of AFB1 by EOs of medicinal herbs. The chemical composition of EO of Z. multiflora has been reported earlier (Shafiee and Javidnia Citation1997). However, this is the first report describing the constituents of O. dhofarense and H. bacciferum.
Figure 2. GC-MS chromatogram of essential oils of H. bacciferum (A), O. dhofarense (B) and Z. multiflora (C).
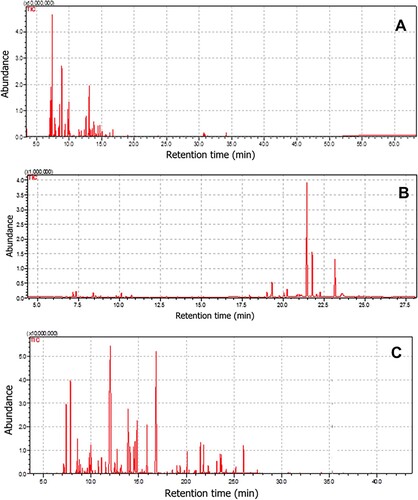
Table 1. Detoxification of AFB1 by essential oils of medicinal herbs.
Table 2. Chemical composition of the essential oil of H. bacciferum.
Table 3. Chemical composition of the essential oil of O. dhofarense.
Table 4. Chemical composition of the essential oil of Z. multiflora.
Z. multiflora is generally used as a flavour ingredient in foods (Sajed et al. Citation2013). A number of medicinal properties of Z. multiflora including antibacterial, antiseptic, anaesthetic, antioxidant and immunomodulatory activities have been reported (Sajed et al. Citation2013). Phenolic compounds such as thymol and carvacrol have been identified in Z. multiflora (Shafiee and Javidnia Citation1997). In this study, the EO of Z. multiflora showed direct antifungal activity against A. flavus and AFB1 detoxification potential. This EO can be used as a natural food preservative to suppress the growth of A. flavus and detoxification of aflatoxins.
Conclusions
Our results showed that the EOs extracted from H. bacciferum, O. dhofarense and Z. multiflora were capable of detoxifying AFB1. These EOs showed a wide variation in their chemical compositions. Further studies are required to assess the possible role of the constituents identified in the EO of each medicinal plant in the detoxification of AFB1, evaluate the efficacy of these EOs in the detoxification of other major aflatoxins viz., AFB2, AFG1 and AFG2 and to determine the biological toxicity of the degraded products of aflatoxins.
The data reported in this study showed that Z. multiflora EO exhibited strong inhibitory effect on A. flavus. These findings suggest that Z. multiflora EO could be considered as a potential plant-based antimicrobial agent for the protection of food products from A. flavus and aflatoxin contamination. Further studies are needed to determine the mechanism of activity of Z. multiflora EO as well as its constituents against A. flavus.
Compliance with ethical standards
Ethical approval
The authors confirm that there are no ethical issues in the publication of the manuscript.
Acknowledgements
We thank Oman Botanic Garden for providing medicinal plants. This work was supported by research grants from The Research Council (RC/RG-AGR/CROP/19/02) and Sultan Qaboos University (IG/AGR/CROP/21/03).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data supporting the findings of this study are available in Mendeley Data, DOI: 10.17632/86g3w9vhbn.2 (https://data.mendeley.com/datasets/86g3w9vhbn/2)
Additional information
Funding
References
- Adeyeye SA. 2016. Fungal mycotoxins in foods: a review. Cogent Food Agric. 2:1213127.
- Ahmed Adam MA, Tabana YM, Musa KB, Sandai DA. 2017. Effects of different mycotoxins on humans, cell genome and their involvement in cancer. Oncol Rep. 37:1321–1336.
- Aiko V, Mehta A. 2015. Occurrence, detection and detoxification of mycotoxins. J Biosci. 40:943–954.
- Al-Maawali SS, Al-Sadi AM, Sathish Babu SP, Velazhahan R. 2021. In vitro tolerance to antifungal glycoalkaloids and biofilm forming ability of the antagonistic yeast Meyerozyma guilliermondii strain SQUCC-33Y. Indian Phytopathol. 74:817–821.
- Bankole SA, Joda AO. 2004. Effect of lemon grass (Cymbopogon citratus Stapf) powder and essential oil on mould deterioration and aflatoxin contamination of melon seeds (Colocynthis citrullus L.). Afr J Biotechnol. 3:52–59.
- Bonamin F, Moraes TM, Dos Santos RC, Kushima H, Faria FM, Silva MA, Junior IV, Nogueira L, Bauab TM, Brito ARS, da Rocha LR. 2014. The effect of a minor constituent of essential oil from Citrus aurantium: the role of β-myrcene in preventing peptic ulcer disease. Chem Biol Interact. 212:11–19.
- Bulow N, Konig WA. 2000. The role of germacrene D as a precursor in sesquiterpene biosynthesis: investigations of acid catalyzed, photochemically and thermally induced rearrangements. Phytochemistry. 55:141–168.
- Carovic-Stanko K, Orlic S, Politeo O, Strikic F, Kolak I, Milos M, Satovic Z. 2010. Composition and antibacterial activities of essential oils of seven Ocimum taxa. Food Chem. 119:196–201.
- Chaudhari AK, Singh VK, Dwivedy AK, Das S, Upadhyay N, Singh A, Dkhar MS, Kayang H, Prakash B, Dubey NK. 2020. Chemically characterised Pimenta dioica (L.) Merr. essential oil as a novel plant based antimicrobial against fungal and aflatoxin B1 contamination of stored maize and its possible mode of action. Nat Prod Res. 34:745–749.
- Combrinck S, Regnier T, Kamatou GP. 2011. In vitro activity of eighteen essential oils and some major components against common postharvest fungal pathogens of fruit. Ind Crops Prod. 33:344–349.
- Cox SD, Mann CM, Markham JL, Bell HC, Gustafson JE, Warmington JR, Wyllie SG. 2000. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil). J Appl Microbiol. 88:170–175.
- Das S, Kumar Singh V, Kumar Dwivedy A, Kumar Chaudhari A, Upadhyay N, Singh A, Krishna Saha A, Ray Chaudhury S, Prakash B, Dubey NK. 2020. Assessment of chemically characterised Myristica fragrans essential oil against fungi contaminating stored scented rice and its mode of action as novel aflatoxin inhibitor. Nat Prod Res. 34:1611–1615.
- Dorman HJD, Deans SG. 2000. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol. 88:308–316.
- Filly A, Fernandez X, Minuti M, Visinoni F, Cravotto G, Chemat F. 2014. Solvent-free microwave extraction of essential oil from aromatic herbs: from laboratory to pilot and industrial scale. Food Chem. 150:193–198.
- Gandomi H, Misaghi A, Basti AA, Bokaei S, Khosravi A, Abbasifar A, Javan AJ. 2009. Effect of Zataria multiflora Boiss. essential oil on growth and aflatoxin formation by Aspergillus flavus in culture media and cheese. Food Chem Toxicol. 47:2397–2400.
- Ghaffari T, Kafil HS, Asnaashari S, Farajnia S, Delazar A, Baek SC, Hamishehkar H, Kim KH. 2019. Chemical composition and antimicrobial activity of essential oils from the aerial parts of Pinus eldarica grown in Northwestern Iran. Molecules. 24:3203.
- Govindarajan M, Benelli G. 2016. Eco-friendly larvicides from Indian plants: effectiveness of lavandulyl acetate and bicyclogermacrene on malaria, dengue and Japanese encephalitis mosquito vectors. Ecotoxicol Environ Saf. 133:395–402.
- Hajare SS, Hajare SH, Sharma A. 2005. Aflatoxin inactivation using aqueous extract of Ajowan (Trachyspermum ammi) seeds. J Food Sci. 70:29–34.
- Hanif MA, Al-Maskari MY, Al-Maskari A, Al-Shukaili A, Al-Maskari AY, Al-Sabahi JN. 2011. Essential oil composition, antimicrobial and antioxidant activities of unexplored Omani basil. J Med Plant Res. 5:751–757.
- Huang Y, Zhao J, Zhou L, Wang J, Gong Y, Chen X, Guo Z, Wang Q, Jiang W. 2010. Antifungal activity of the essential oil of Illicium verum fruit and its main component trans-anethole. Molecules. 15:7558–7569.
- Iram W, Anjum T, Iqbal M, Ghaffar A, Abbas M, Khan AM. 2016. Structural analysis and biological toxicity of aflatoxins B1 and B2 degradation products following detoxification of Ocimum basilicum and Cassia fistula aqueous extracts. Front Microbiol. 7:1105.
- Ismail A, Gonçalves BL, de Neeff DV, Ponzilacqua B, Coppa CFSC, Hintzsche H, Sajid M, Cruz AG, Corassin CH, Oliveira CAF. 2018. Aflatoxin in foodstuffs: Occurrence and recent advances in decontamination. Food Res Int. 113:74–85.
- Ismail M. 2006. Central properties and chemical composition of Ocimum basilicum essential oil. Pharm Biol. 44:619–626.
- Kannan K, Velazhahan R. 2014. The potential of leaf extract of Barleria lupulina for detoxification of aflatoxins. Indian Phytopath. 67:298–302.
- Kedia A, Prakash B, Mishra PK, Dubey NK. 2014. Antifungal and antiaflatoxigenic properties of Cuminum cyminum (L.) seed essential oil and its efficacy as a preservative in stored commodities. Int J Food Microbiol. 168:1–7.
- Kedia A, Prakash B, Mishra PK, Dwivedy AK, Dubey NK. 2015. Trachyspermum ammi L. essential oil as plant based preservative in food system. Ind Crop Prod. 69:104–109.
- Kiran S, Kujur A, Prakash B. 2016. Assessment of preservative potential of Cinnamomum zeylanicum Blume essential oil against food borne molds, aflatoxin B1 synthesis, its functional properties and mode of action. Innov Food Sci Emerg Technol. 37:184–191.
- Kumar A, Shukla R, Singh P, Dubey NK. 2010. Chemical composition, antifungal and antiaflatoxigenic activities of Ocimum sanctum L. essential oil and its safety assessment as plant based antimicrobial. Food Chem Toxicol. 48:539–543.
- Kumar P, Mahato DK, Kamle M, Mohanta TK, Kang SG. 2017. Aflatoxins: a global concern for food safety, human health and their management. Front Microbiol. 7:2170.
- Oxenham SK, Svoboda KP, Walters DR. 2005. Antifungal activity of the essential oil of basil (Ocimum basilicum). J Phytopathol. 153:174–180.
- Panda P, Mehta A. 2013. Aflatoxin detoxification potential of Ocimum tenuiflorum. J Food Saf. 33:265–272.
- Park SN, Lim YK, Freire MO, Cho E, Jin D, Kook JK. 2012. Antimicrobial effect of linalool and α-terpineol against periodontopathic and cariogenic bacteria. Anaerobe. 18:369–372.
- Peana AT, D'Aquila PS, Panin F, Serra G, Pippia P, Moretti MDL. 2002. Anti-inflammatory activity of linalool and linalyl acetate constituents of essential oils. Phytomedicine. 9:721–726.
- Rabib H, Elagdi C, Hsaine M, Fougrach H, Koussa T, Badri W. 2020. Antioxidant and antibacterial activities of the essential oil of Moroccan Tetraclinis articulata (Vahl) Masters. Biochem Res Int. 2020:9638548.
- Raeisi M, Tajik H, Rohani SMR, Tepe B, Kiani H, Khoshbakht R, Aski HS, Tadrisi H. 2016. Inhibitory effect of Zataria multiflora Boiss. essential oil, alone and in combination with monolaurin, on Listeria monocytogenes. Vet Res Forum. 7:7–11.
- Sajed H, Sahebkar A, Iranshahi M. 2013. Zataria multiflora Boiss. (Shirazi thyme)- an ancient condiment with modern pharmaceutical uses. J Ethnopharmacol. 145:686–698.
- Saleem M, Nazli R, Afza N, Sami A, Shaiq Ali M. 2004. Biological significance of essential oil of Zataria multiflora Boiss. Nat Prod Res. 18:493–497.
- Salehi B, Upadhyay S, Orhan IE, Jugran AK, Jayaweera SLD, Dias DA, Sharopov F, Taheri Y, Martins N, Baghalpour N, et al. 2019. Therapeutic potential of α-and β-pinene: a miracle gift of nature. Biomolecules. 9:738.
- Sandosskumar R, Karthikeyan M, Mathiyazhagan S, Mohankumar M, Chandrasekar G, Velazhahan R. 2007. Inhibition of Aspergillus flavus growth and detoxification of aflatoxin B1 by the medicinal plant zimmu (Allium sativum L. x Allium cepa L.). World J Microbiol Biotechnol. 23:1007–1014.
- Sarma UP, Bhetaria PJ, Devi P, Varma A. 2017. Aflatoxins: implications on health. Indian J Clin Biochem. 32:124–133.
- Shafiee A, Javidnia K. 1997. Composition of essential oil of Zataria multiflora. Planta Med. 63:371–372.
- Sharma N, Tripathi A. 2006. Fungitoxicity of the essential oil of Citrus sinensis on post-harvest pathogens. World J Microbiol Biotechnol. 22:587–593.
- Szczerbanik M, Jobling J, Morris S, Holford P. 2007. Essential oil vapours control some common postharvest fungal pathogens. Aust J Exp Agric. 47:103–109.
- Tian J, Ban X, Zeng H, He J, Chen Y, Wang Y. 2012b. The mechanism of antifungal action of essential oil from dill (Anethum graveolens L.) on Aspergillus flavus. PLoS One. 7:e30147.
- Tian J, Huang B, Luo X, Zeng H, Ban X, He J, Wang Y. 2012a. The control of Aspergillus flavus with Cinnamomum jensenianum Hand.-Mazz essential oil and its potential use as a food preservative. Food Chem. 130:520–527.
- Tolouee M, Alinezhad S, Saberi R, Eslamifar A, Zad SJ, Jaimand K, Taeb J, Rezaee MB, Kawachi M, Shams-Ghahfarokhi M, Razzaghi-Abyaneh M. 2010. Effect of Matricaria chamomilla L. flower essential oil on the growth and ultrastructure of Aspergillus niger van Tieghem. Int J Food Microbiol. 139:127–133.
- Velazhahan R. 2017. Bioprospecting of medicinal plants for detoxification of aflatoxins. Int J Nutr Pharmacol Neurol Dis. 7:60–63.
- Velazhahan R, Vijayanandraj S, Vijayasamundeeswari A, Paranidharan V, Samiyappan R, Iwamoto T, Friebe B, Muthukrishnan S. 2010. Detoxification of aflatoxins by seed extracts of the medicinal plant, Trachyspermum ammi (L.) Sprague ex Turrill- structural analysis and biological toxicity of degradation product of aflatoxin G1. Food Control. 21:719–725.
- Vijayanandraj S, Brinda R, Kannan K, Adhithya R, Vinothini S, Senthil K, Ramakoteswara Rao C, Paranidharan V, Velazhahan R. 2014. Detoxification of aflatoxin B1 by an aqueous extract from leaves of Adhatoda vasica Nees. Microbiol Res. 169:294–300.
- Vilela GR, de Almeida GS, D'Arce MABR, Moraes MHD, Brito JO, da Silva MFDG, Silva SC, de Stefano Piedade SM, Calori-Domingues MA, da Gloria EM. 2009. Activity of essential oil and its major compound, 1, 8-cineole, from Eucalyptus globulus Labill., against the storage fungi Aspergillus flavus Link and Aspergillus parasiticus Speare. J Stored Prod Res. 45:108–111.
- Wu F, Groopman JD, Pestka JJ. 2014. Public health impacts of foodborne mycotoxins. Annu Review Food Sci Technol. 5:351–372.
