Abstract
In this study, we investigated the association of six single nucleotide polymorphisms (SNPs) in HSD11B1 with the progression of liver cirrhosis (LC) and occurrence of hepatocellular carcinoma (HCC) in a Han Chinese population. In this retrospective case–control study, a total of 656 ethnic Han Chinese patients with chronic hepatitis B virus infection were recruited. The clinicopathological characteristics of patients were obtained. This study group consisted of 104 patients with chronic hepatitis B, 266 patients with LC, and 286 patients with HCC. Six SNPs (rs846908, rs701950, rs846910, rs3753519, rs4844488, and rs932335) were genotyped using a MassArray system. Compared to the wild genotype GG, the CC genotype of rs932335 was significantly related to an increased risk of LC after adjusting for age, sex, smoking, and drinking (odds ratios = 7.89, 95% confidence interval: 1.04–60.06). Moreover, the CC genotype had increased serum levels of alanine transaminase and aspartate transaminase and elevated MELD scores in patients with LC. No significant differences were found among other SNPs. This study provides epidemiological evidence that the rs932335 variant of HSD11B1 may lead to the progression of LC and worse prognosis for patients with LC. No significant correlation was found between HSD11B1 genetic polymorphism and the progression of HCC.
Introduction
Cirrhosis is the end stage of a large number of chronic liver conditions that share common features of chronic necroinflammation and activation of hepatic stellate cells with ensuing fibrogenic processes, which modify the normal liver structure to reduce its functional mass and alter the vascular architecture (Bertrand et al. Citation1986; Tsochatzis et al. Citation2014). Cirrhosis has become a major public health problem and a significant cause of morbidity and mortality (Hernandez-Gea and Friedman Citation2011; Stasi et al. Citation2015), causing more than one million deaths each year (Safaei et al. Citation2016). Additionally, more than 50,000 deaths occur due to hepatocellular carcinoma (HCC) each year, which usually arises as a result of liver cirrhosis (LC) (Forner et al. Citation2018). The most common primary etiology for cirrhosis and HCC is chronic hepatitis B (CHB) (Mokdad et al. Citation2014).
Glucocorticoids (GCs) have a wide range of functions that modulate many of the pathological processes that occur during tissue injury and repair and contribute to liver fibrosis (Fede et al. Citation2012). Tissue GC levels are regulated by the intracellular enzyme 11-beta-hydroxysteroid dehydrogenase 1 (HSD11B1), which converts inactive cortisone into active cortisol in humans and is highly abundant in the liver (Seckl and Walker Citation2001). HSD11B1 overexpression in the liver can cause hepatic steatosis and dyslipidemia (Morton et al. Citation2001; Paterson et al. Citation2004). However, GCs can increase the survival rate of patients with severe hepatitis B virus (HBV) infection, and the deficient production or action of GCs is associated with an increased mortality rate due to LC (Harry et al. Citation2002; He et al. Citation2013; Tsai et al. Citation2006). Additionally, GC therapy in the early stages of HBV-precipitated acute-on-chronic liver failure resulted in remarkably improved short-term survival and is associated with the restoration of myeloid dendritic cells, suggesting that an appropriate amount of GCs is beneficial to treat HBV-related liver disease (Fujiwara et al. Citation2008; Zhao et al. Citation2012; Zhao et al. Citation2018). Furthermore, HSD11B1 deficiency enhances myofibroblast activation and promotes initial fibrosis (Wen et al. Citation2018).
The relationship among HSD11B1 polymorphism and GC-related diseases, such as obesity, type 2 diabetes, metabolic syndrome, and polycystic ovary syndrome, has been widely investigated, resulting in the suggestion of a wide distribution of the HSD11B1 polymorphism and its influence on gene expression (Devang et al. Citation2017; Gandhi et al. Citation2013; Grolmusz et al. Citation2014; Ju et al. Citation2015; Quteineh et al. Citation2015). However, the SNPs in HSD11B1 with the occurrence of HCC have not been investigated previously. The aim of this study was to evaluate the association of six single nucleotide polymorphisms (SNPs) with the progression of liver fibrosis and HBV-related HCC occurrence in a Chinese Han population.
Methods
Study population
Between January 2012 and December 2018, 656 patients from the First Hospital of Jilin University were enrolled in the present study. This study group consisted of 104 patients with CHB, 266 patients with HBV-related LC, and 286 patients with HBV-related HCC. CHB and HBV-related LC patients were diagnosed according to the AASLD 2018 Hepatitis B Guidance (Terrault et al. Citation2018). HBV-related HCC was defined based on 2018 practice guidance of hepatocellular carcinoma of AASLD (Marrero et al. Citation2018). The non-HCC patients included CHB and LC patients, characterized by active necro-inflammatory liver disease without/with fibrosis on imaging examination without evidence of HCC. Patients infected with human immunodeficiency virus or other viruses, such as hepatitis A, C, D, and E, were excluded from this study. In addition, patients with combined autoimmune diseases or other liver diseases, such as intra- and extra- hepatic bile duct stones, alcoholic liver diseases, and hemorrhagic liver diseases, were excluded. General characteristics, including sex, age, smoking and drinking history, and HBV infection and treatment history, were obtained using a standardized questionnaire.
Whole blood (5 mL) was collected from the veins of each patient within 48 h of hospital admission and their HBV profile was compiled, including HBV s antigen (HBsAg), HBV s antibody (HBsAb), HBV e antigen (HBeAg), HBV e antibody (HBeAb), HBV core antibody (HBc), anti-hepatitis C antibody, and HBV DNA quantification. Additionally, clinical laboratory and imaging examinations were carried out, and patients with LC were assessed using the Child–Pugh and MELD scores (Peng et al. Citation2016). This study was carried out in accordance with the Helsinki Declaration of 1975, as revised in 2000 and approved by the First Hospital Ethical Committee of Jilin University. Written informed consent was obtained from all the participants.
SNP selection and genotyping
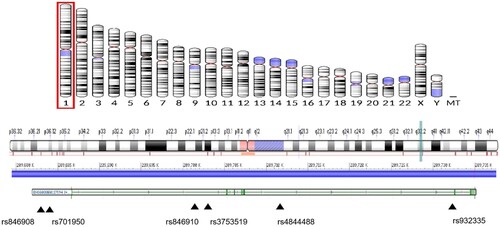
Geneview of the NCBI databases was used to select the SNPs from the promoter proxy (upstream variant 2KB), 5'UTR, exon (missense, synonymous), and 3'UTR functional region of ECM1 (minor allele frequency (MAF) > 5% in CHB data), and the function was forecasted using the following website: http://snpinfo.niehs.nih.gov/. Then, LD analysis was conducted for the selected SNPs using the website: http://asia.ensembl.org/Homo_sapiens/Tools/LD?db=core. We selected one of the complete linkage sites if R2 = 1. The validated and hot SNPs that had a relationship with the disease were then selected, and the MAF > 5% in CHB was verified. rs3834087 and rs3754217 are located in the promoter region, whereas rs3754217 was predicted to be located at the transcription factor binding site (TFBS), and they may play a role in genetic transcription. rs3737240 and rs13294 were located at the exon missense mutation, which may affect the expression and function of the gene. The locations of the ECM1 gene and four selected SNPs are shown in Figure . HSD11B1 SNPs were selected from the functional regions of the promoter proxy (upstream variant 2KB), 5'UTR, exon (missense, synonymous), and 3'UTR functional region (Draper et al. Citation2002) based on the Hapmap (https://www.genome.gov/10001688/international-hapmap-project) and 1,000 Genomes database (http://www.internationalgenome.org/), with a MAF of > 5%. The SNPs rs846908 and rs701950 were selected as they were located in the TFBS of the promoter region and may play a role in genetic transcription. The validated and hot SNPs that have a relationship with the disease were then selected, and the MAF > 5% in CHB was verified. rs3753519, rs846910, rs4844488, and rs932335 were located at the intron and were related to the occurrence of the diseases, meaning they might affect gene function. The locations of HSD11B1 and selected SNPs are shown in Figure .
Genomic DNA was isolated from the whole blood sample using a blood genomic DNA kit (Sigma-Aldrich; Merck KGaA) according to the manufacturer's instructions. SNP genotyping was performed using a MassArray system (Sequenom, San diego, CA, USA) according to the manufacturer's protocol. All SNP primers were designed using Assay Designer (http://assay.archerdx.com/, version 3.2; Table ).
Table 1. Primer sequences and reaction conditions for genotyping HSD11B1 polymorphisms.
Statistical analysis
All data were analyzed using SPSS version 17.0 (IBM, Chicago, IL, USA). Continuous variables were expressed as the median and interquartile range (25% and 75%). Categorical variables were expressed as a percentage (%). Differences among the multiple groups were compared using analysis of variance and least significant difference multiple comparisons test. Haplotype analysis was performed using UNPHASED (version 3.1.4). The two-sided χ2 test or Fisher's exact test was used to compare allele distributions. Multivariate logistic regression analysis was performed to calculate odds ratios (OR) and 95% confidence intervals (CIs) after adjusting the factors for aging, smoking, drinking, and sex differences. The Hardy-Weinberg equilibrium (H-WE) test was used to assess independent segregation of alleles. Hetrozygosity and polymorphic information content (PIC) were calculated by plink. PIC > 0.1 indicated the selected SNPs were informative for genetic statistical analysis. P < 0.05 indicated a statistically significant difference.
Results
General characteristics of the study population
The main general and clinical characteristics of the study population are summarized in Table . No statistical differences were observed between the sex, age, smoking status, or alcohol consumption status in the CHB and LC patient groups (P < 0.05). Furthermore, there was a greater number of smokers in the HCC patient group than in the LC patient group (P = 0.000); however, no significant difference was observed for age, sex, or alcohol consumption. In comparison, there were significant differences in age, sex, and numbers of smokers and alcoholics between the LC and HCC patient groups (P = 0.048; P = 0.022; P = 0.000; P = 0.023, respectively). The serum HBV-DNA positive rate and HBV load; the serum levels of alanine transaminase (ALT), aspartate transaminase (AST), glutamyl transpeptidase (GGT), prealbumin, albumin, and cholinesterase; and the platelet counts of the patients with CHB were significantly higher compared to those of the patients with LC (P < 0.05), suggesting that hepatocellular damage was more severe in the patients with CHB, but the liver reserve function was better. Moreover, the serum levels of AST, GGT, albumin, and cholinesterase and the platelet counts of the patients with LC were significantly lower compared to those of the patients with HCC, which may be due to the action of the tumor, meaning that the patients with HCC had better liver reserve functions. The GGT and AST levels of the patients with HCC were higher than those of the non-HCC patients, but the other indicators did not demonstrate a substantial difference. Furthermore, all five SNPs (rs846908, rs701950, rs846910, rs3753519, and rs4844488) among each group were in equilibrium, as determined by the HWE test, except for the rs932335 in LC group and non-HCC group (P = 0.001, P = 0.025, respectively, Table ).
Table 2. General and clinical characteristics of study subjects.
Table 3. The Hardy-Weinberg equilibrium of each SNP in each group.
Associations between genotype and allele frequency in HSD11B1 SNPs
The genotype and allele frequency of the HSD11B1 polymorphisms in each group are displayed in Table . PICs of all SNPs were larger than 0.1, indicating the selected SNPs were informative for genetic statistical analysis. No significant associations were detected between the genotype and allele frequency of the rs846910, rs701950, rs846910, rs3753519, and rs4844488 with CHB progression or HCC occurrence after adjusting for sex, age, smoking status, and drinking status. Compared to wild genotype GG, we found a significant relationship between the CC genotype of rs932335 and an increased risk of CHB progression with an OR of 7.89 (95% CI: 1.04–60.06). Also, recessive model analysis showed the CC genotype have high risk of CHB progression compare with CG plus GG genotype (OR=8.37, 95% CI:1.11–63.39).
Table 4. Genotype and allele frequencies of SNPs in the HSD11B1 gene in each group.
Haplotype analysis of HSD11B1
We analyzed differences in haplotype distributions between patients with CHB and those with LC, patients with LC and those with HCC, and patients without HCC and those with HCC. However, we did not find positive alleles related to the progression of chronic liver disease and the occurrence of liver cancer.
Associations of rs932335 genotypes and LC
Compared to the wild genotype GG, the CC genotype of rs932335 was related to an increased risk of CHB progression with an OR of 7.89 (95% CI: 1.04–60.06). Therefore, we considered whether the genotypes of rs932335 may play a role in the severity of LC. The clinical characteristics of different genotypes of rs932335 in the LC group are summarized in Table . In comparison, the CC genotype had increased serum levels of ALT and AST, but no difference in the serum HBV-DNA positive rate, HBV load, HBeAg positive rate, GGT, ALP, albumin, prealbumin, total bilirubin, cholinesterase, or platelet count levels. The Child–Pugh and MELD scores are widely used to determine the prognosis in patients with LC (Asrani and Kamath Citation2015; Peng et al. Citation2016). In this study, the CC genotype showed higher MELD scores, but no difference in Child–Pugh scores.
Table 5. The impact of rs932335 genotypes on the clinical indicators of liver cirrhosis.
The heterozygosity of SNPs in each group
We also calculated the heterozygosity of each gene in each group (Table ). The observed heterozygosity of rs932335 was significantly less than expected heterozygosity (P=0.001071) in LC group, suggesting the formation and occurrence of LC might be related to the loss of heterozygosity of the rs932335.
Table 6 Genotype, allele frequency and genetic diversity of six SNP locus of HSD11B1.
Discussion
The aim of this study was to evaluate the association of six SNPs with the progression of liver fibrosis and HBV-related HCC occurrence in a Chinese Han population. A total of 656 patients were included in the study, consisting of 104 patients with CHB, 266 patients with HBV-related LC, and 286 patients with HBV-related HCC. Of the six investigated variants, only rs932335 was significantly associated with an increased risk of LC. Besides, the loss of heterozygosity and the H-W imbalance of rs932335 in LC group suggest that rs932335 is related to the formation of LC.
HSD11B1, located on 1q32-41, encodes the enzyme HSD11B1, which converts inactive steroid cortisone into active GCs principally in the liver and adipose tissue (Draper et al. Citation2002). HSD11B1 deficiency enhances myofibroblast activation and promotes initial fibrosis. Therefore, HSD11B1 is considered a protective factor against liver fibrosis (Wen et al. Citation2018). HSD11B1 is a possible candidate gene for weight change (Fox et al. Citation2005). Most polymorphisms of HSD11B1 are associated with increased weight-related diseases, such as type 2 diabetes, metabolic syndrome, and tumor occurrence (Chedid et al. Citation2019; Do Nascimento et al. Citation2015; Feigelson et al. Citation2008; Gronau et al. Citation2002; Ter-Minassian et al. Citation2012). Contradictory results have been obtained on the efficacy of exogenous glucocorticoid treatment for hepatitis B. Several studies have reported that the early application of glucocorticoids in severe hepatitis may prevent the necrosis of liver cells and provide a possibility of liver regeneration. However, this treatment might enhance HBV replication, aggravate the condition, and lead to liver failure (Yang et al. Citation2007; Zhang et al. Citation2010). A meta-analysis result confirmed that glucocorticoids can increase the survival rate of patients with severe viral hepatitis B (He et al. Citation2013). So we were wondering the effort of small dose of endogenous hormones during the development of fibrosis.
Although there are many studies on genetic polymorphism of HSD11B1 on other glucocorticoid-related disease, its correlation with the progression of LC and the occurrence of HCC has not yet been reported. The CC genotype of rs932335 has decreased mRNA levels of HSD11B1 (Wang et al. Citation2013). Compared to wild genotype GG, the CC genotype of rs932335 was related to an increased risk of CHB progression to liver fibrosis with an OR of 7.89 (95% CI: 1.04-60.06). Moreover, the CC genotype had higher levels of ALT and AST in patients with LC. Additionally, the CC genotype showed higher MELD scores, suggesting that low levels of GC expression caused by mutations may lead to the progression of LC and a worse prognosis for patients with LC. These findings suggest that the functional polymorphism rs932335 at intron 4 of HSD11B1 may influence the progression of LC through reducing endogenous hormone level in a Han Chinese population.
The primary limitation of this study was its relatively small CHB sample size and therefore P value was not adjusted by Bonferroni methods. Although the results suggest that HSD11B1 may be associated with a risk of LC, it cannot be excluded that these results are due to chance. Furthermore, this case–control study was a hospital-based study; thus, selection bias may have occurred. In addition, due to technical problems in functional studies, we did not find direct evidence that this intronic polymorphism regulates or influences HSD11B1 expression. Therefore, further functional studies are warranted.
Conclusion
This study provides epidemiological evidence that the rs932335 variant of HSD11B1 may lead to the progression of LC and worse prognosis for patients with LC in Chinese Han population. No significant correlation between the other SNPs, including rs846908, rs701950, rs846910, rs3753519 and rs4844488, and formation of LC was found. No significant correlation was found between HSD11B1 genetic polymorphism and the progression of HCC.
Ethics approval and consent to participate
This study was approved by the First Hospital Ethical Committee of Jilin University. Written informed consent was obtained from all the participants.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Due to the nature of this research, the participants of this study did not agree to their data being shared publicly, so supporting data is not available.
Additional information
Funding
References
- Asrani SK, Kamath PS. 2015. Model for end-stage liver disease score and MELD exceptions: 15 years later. Hepatol Int. 9:346–354.
- Bertrand P, Guigliarelli B, Gayda JP. 1986. An analysis of the g values in semi-met forms of hemerythrin and in related proteins. Arch Biochem Biophys. 245:305–307.
- Chedid MF, Do Nascimento FV, De Oliveira FS, De Souza BM, Kruel CRP, Gurski RR, Canani LH, Crispim D, Gerchman F. 2019. Interaction of HSD11B1 and H6PD polymorphisms in subjects with type 2 diabetes are protective factors against obesity: a cross-sectional study. Diabetol Metab Syndr. 11:78.
- Devang N, Satyamoorthy K, Rai PS, Nandini M, Rao S, Phani NM, Adhikari P. 2017. Association of HSD11B1 gene polymorphisms with type 2 diabetes and metabolic syndrome in South Indian population. Diabetes Res Clin Pract. 131:142–148.
- Do Nascimento FV, Piccoli V, Beer MA, Von Frankenberg AD, Crispim D, Gerchman F. 2015. Association of HSD11B1 polymorphic variants and adipose tissue gene expression with metabolic syndrome, obesity and type 2 diabetes mellitus: a systematic review. Diabetol Metab Syndr. 7:38.
- Draper N, Echwald SM, Lavery GG, Walker EA, Fraser R, Davies E, Sørensen TI, Astrup A, Adamski J, Hewison M, et al. 2002. Association studies between microsatellite markers within the gene encoding human 11beta-hydroxysteroid dehydrogenase type 1 and body mass index, waist to hip ratio, and glucocorticoid metabolism. J Clin Endocrinol Metab. 87:4984–4990.
- Fede G, Spadaro L, Tomaselli T, Privitera G, Germani G, Tsochatzis E, Thomas M, Bouloux PM, Burroughs AK, Purrello F. 2012. Adrenocortical dysfunction in liver disease: a systematic review. Hepatology (Baltimore, Md.). 55:1282–1291.
- Feigelson HS, Teras LR, Diver WR, Tang W, Patel AV, Stevens VL, Calle EE, Thun MJ, Bouzyk M. 2008. Genetic variation in candidate obesity genes ADRB2, ADRB3, GHRL, HSD11B1, IRS1, IRS2, and SHC1 and risk for breast cancer in the Cancer Prevention study II. Breast Cancer Research: BCR. 10:R57.
- Forner A, Reig M, Bruix J. 2018. Hepatocellular carcinoma. Lancet (London, England). 391:1301–1314.
- Fox CS, Heard-Costa NL, Vasan RS, Murabito JM, D'agostino Sr. RB, Atwood LD. 2005. Genomewide linkage analysis of weight change in the Framingham Heart Study. J Clin Endocrinol Metab. 90:3197–3201.
- Fujiwara K, Yasui S, Yonemitsu Y, Fukai K, Arai M, Imazeki F, Suzuki A, Suzuki H, Sadahiro T, Oda S, Yokosuka O. 2008. Efficacy of combination therapy of antiviral and immunosuppressive drugs for the treatment of severe acute exacerbation of chronic hepatitis B. J Gastroenterol. 43:711–719.
- Gandhi K, Adhikari P, Basu A, Achappa B. 2013. Association between a 11β-hydroxysteroid dehydrogenase type 1 gene polymorphism and metabolic syndrome in a South Indian population. Metab Syndr Relat Disord. 11:397–402.
- Grolmusz VK, Acs OD, Feldman-Kovács K, Szappanos Á, Stenczer B, Fekete T, Szendei G, Reismann P, Rácz K, Patócs A. 2014. Genetic variants of the HSD11B1 gene promoter may be protective against polycystic ovary syndrome. Mol Biol Rep. 41:5961–5969.
- Gronau S, Koenig Greger D, Jerg M, Riechelmann H. 2002..11Beta-hydroxysteroid dehydrogenase 1 expression in squamous cell carcinomas of the head and neck. Clin Otolaryngol Allied Sci. 27:453–457.
- Harry R, Auzinger G, Wendon J. 2002. The clinical importance of adrenal insufficiency in acute hepatic dysfunction. Hepatology (Baltimore, Md.). 36:395–402.
- He B, Zhang Y, Lü MH, Cao YL, Fan YH, Deng JQ, Yang SM. 2013. Glucocorticoids can increase the survival rate of patients with severe viral hepatitis B: a meta-analysis. Eur J Gastroenterol Hepatol. 25:926–934.
- Hernandez-Gea V, Friedman SL. 2011. Pathogenesis of liver fibrosis. Annu Rev Pathol. 6:425–456.
- Ju R, Wu W, Tang Q, Wu D, Xia Y, Wu J, Wang X. 2015. Association analysis between the polymorphisms of HSD11B1 and H6PD and risk of polycystic ovary syndrome in Chinese population. PloS one. 10:e0140326.
- Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. 2018. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the study of liver diseases. Hepatology. 68:723–750.
- Mokdad AA, Lopez AD, Shahraz S, Lozano R, Mokdad AH, Stanaway J, Murray CJ, Naghavi M. 2014. Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Med. 12:145.
- Morton NM, Holmes MC, Fiévet C, Staels B, Tailleux A, Mullins JJ, Seckl JR. 2001. Improved lipid and lipoprotein profile, hepatic insulin sensitivity, and glucose tolerance in 11beta-hydroxysteroid dehydrogenase type 1 null mice. J Biol Chem. 276:41293–41300.
- Paterson JM, Morton NM, Fievet C, Kenyon CJ, Holmes MC, Staels B, Seckl JR, Mullins JJ. 2004. Metabolic syndrome without obesity: hepatic overexpression of 11beta-hydroxysteroid dehydrogenase type 1 in transgenic mice. Proc Natl Acad Sci U S A. 101:7088–7093.
- Peng Y, Qi X, Guo X. 2016. Child-Pugh versus MELD score for the assessment of prognosis in liver cirrhosis: A systematic review and meta-analysis of observational studies. Medicine (Baltimore). 95:e2877.
- Quteineh L, Vandenberghe F, Saigi Morgui N, Delacrétaz A, Choong E, Gholam-Rezaee M, Magistretti P, Bondolfi G, Von Gunten A, Preisig M, et al. 2015. Impact of HSD11B1 polymorphisms on BMI and components of the metabolic syndrome in patients receiving psychotropic treatments. Pharmacogenet Genomics. 25:246–258.
- Safaei A, Arefi Oskouie A, Mohebbi SR, Rezaei-Tavirani M, Mahboubi M, Peyvandi M, Okhovatian F, Zamanian-Azodi M. 2016. Metabolomic analysis of human cirrhosis, hepatocellular carcinoma, non-alcoholic fatty liver disease and non-alcoholic steatohepatitis diseases. Gastroenterol Hepatol Bed Bench. 9:158–173.
- Seckl JR, Walker BR. 2001. Minireview: 11beta-hydroxysteroid dehydrogenase type 1- a tissue-specific amplifier of glucocorticoid action. Endocrinology. 142:1371–1376.
- Stasi C, Silvestri C, Voller F, Cipriani F. 2015. Epidemiology of liver cirrhosis. J Clin Exp Hepatol. 5:272.
- Ter-Minassian M, Asomaning K, Zhao Y, Chen F, Su L, Carmella SG, Lin X, Hecht SS, Christiani DC. 2012. Genetic variability in the metabolism of the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) to 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL). Int J Cancer. 130:1338–1346.
- Terrault NA, Lok ASF, Mcmahon BJ, Chang KM, Hwang JP, Jonas MM, Brown Jr. RS, Bzowej NH, Wong JB. 2018. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 67:1560–1599.
- Tsai MH, Peng YS, Chen YC, Liu NJ, Ho YP, Fang JT, Lien JM, Yang C, Chen PC, Wu CS. 2006. Adrenal insufficiency in patients with cirrhosis, severe sepsis and septic shock. Hepatology (Baltimore, Md.). 43:673–681.
- Tsochatzis EA, Bosch J, Burroughs AK. 2014. Liver cirrhosis. Lancet (London, England). 383:1749–1761.
- Wang J, Gao Y, Wang L, Liu X, Li J, Wang Z, Zhou J, Wang K. 2013. A variant (rs932335) in the HSD11B1 gene is associated with colorectal cancer in a Chinese population. Eur J Cancer Prev. 22:523–528.
- Wen D, Zou W, Wen X, Yang Y, Chen Y, He Y, Wang G, Shan B. 2018. Urban-rural disparity in colorectal cancer incidence and increasing trend in relation to socioeconomic development and urbanization in China. J Int Med Res. 46:4181–4196.
- Yang CH, Wu TS, Chiu CT. 2007. Chronic hepatitis B reactivation: a word of caution regarding the use of systemic glucocorticosteroid therapy. Br J Dermatol. 157:587–590.
- Zhang B, Wang J, Xu W, Wang L, Ni W. 2010. Fatal reactivation of occult hepatitis B virus infection after rituximab and chemotherapy in lymphoma: necessity of antiviral prophylaxis. Onkologie. 33:537–539.
- Zhao J, Zhang JY, Yu HW, He YL, Zhao JJ, Li J, Zhu YK, Yao QW, Wang JH, Liu HX, et al. 2012. Improved survival ratios correlate with myeloid dendritic cell restoration in acute-on-chronic liver failure patients receiving methylprednisolone therapy. Cell Mol Immunol. 9:417–422.
- Zhao RH, Shi Y, Zhao H, Wu W, Sheng JF. 2018. Acute-on-chronic liver failure in chronic hepatitis B: an update. Expert Rev Gastroenterol Hepatol. 12:341–350.