Abstract
To investigate the effects of different doses of levosimendan combined with Shenmai injection on immune function and galectin-3 and heat shock protein 70 (HSP70) levels in rats with acute heart failure. Forty eight specific-pathogen free Sprague Dawley male rats were selected and divided into 6 groups according to different administration schemes after the heart failure model was established. The left ventricular end diastolic diameter (LVEDD) and left ventricular ejection fraction (LVEF) of rats in each group were observed before and after treatment. After treatment, rats in groups A–D and the model group (MG) showed decreased LVEDDs compared with the control group (CG), and group D showed the highest reduction. In contrast, rats in groups A–D and the MG showed increased LVEFs compared with the CG, and group D showed the highest increase. The MG and groups A–D rats showed an increased percent of CD3+ T cells and CD4+/CD8+ T cell ratio compared with the CG. Levosimendan combined with Shenmai injection optimized the maintenance of immune and cardiac function in rats with acute heart failure compared with Shenmai injection alone. The higher dose of levosimendan combined with Shenmai injection improved the inflammatory reaction and levels of galectin-3 and HSP70.
Introduction
Cardiovascular disease is the major cause of death globally and is a health problem in low-, middle-, and high-income countries. The cumulative burden of systemic vascular injury caused by heart failure is the fastest growing form of cardiovascular diseases (Cheng and Rong Citation2019; Tulay et al. Citation2019; Noale et al. Citation2020). Although medical care has improved, heart disease patients with sudden heart failure events remain the main causes of mortality and morbidity worldwide (Mazloumi et al. Citation2019; Raczkiewicz et al. Citation2019; Xu et al. Citation2019). In addition to interventional therapies, such as coronary artery bypass grafting and bone tissue engineering, myocardial cell-based therapy can also lead to improvements in regeneration. Unfortunately, most of these advanced methods require many long and expensive preparation stages without significant clinical benefits (Fu et al. Citation2019; Johnson Citation2019; Chen et al. Citation2020b).
Herbal medicine injections have demonstrated outstanding clinical effects in treating cardiovascular diseases (Shi et al. Citation2019; Wang et al. Citation2019b). Among them, Astragalus membranaceus, Shenmai, and Shenqi Fuzheng injection are common clinical drugs (Chen et al. Citation2019; Hong et al. Citation2020; Pu et al. Citation2020). Shenmai injection has been widely used to treat cardiovascular diseases such as heart failure and myocardial ischemia (Gu et al. Citation2020). Shenmai injection can reduce oxidative damage in myocardial cells and protect mitochondria from oxidative stress (Yang et al. Citation2020). Huang et al. found that Shenmai injection can improve adriamycin-induced cardiomyopathy (You et al. Citation2006). Levosimendan exerts a vasodilation via opening of the ATP-dependent potassium channels in the smooth musculature of the vessels (Hansen et al. Citation2018; Papp et al. Citation2020). Studies have shown that levosimendan can reduce the plasma NT-proBNP level of patients with acute heart failure (Li et al. Citation2004; Comín-Colet et al. Citation2018; Yang et al. Citation2019). Here, we aimed to investigate the effects of different doses of levosimendan combined with Shenmai injection on immune function and galectin-3 and heat shock protein 70 (HSP70) levels in acute heart failure model rats.
Material and methods
Preparation of heart failure rat model
Forty-eight specific-pathogen free Sprague Dawley male rats were selected for cardiac function test. Healthy cardiac function was confirmed for each rat. After adaptive feeding for one week, eight rats were randomly selected as blank control group (CG), and the other 40 rats were used to establish a heart failure model as previously described (Jiang et al. Citation2018) which used 2.5% pentobarbital at 35 mg/kg. Every procedure was approved by the Animal Care and Use Committee of the Fifth People’s Hospital of Ji’nan.
Grouping and administration of animals
Rats in the successfully established model were randomly divided into the control, model, and medication groups, the latter was further divided into groups A, B, C, and D according to different medication schemes. Rats in the control and MGs were administered equal volume distilled water for gastric perfusion.
Rats in groups A, B, C, and D rats were administered Shenmai injection (Zhengda Qingchunbao Pharmaceutical Co., Ltd., SFDA Approval No. Z33020020) by gavage at a dosage of 2.4 mL/300 g/d (calculated at 10 g/100 mL), once a day for 4 weeks. Groups B–D were also treated with levosimendan (Qilu Pharmaceutical Co., Ltd., Chinese medicine standard H20100043). Group B was treated with levosimendan at a dosage of 1 mg/kg/d in drinking water daily. Group C was treated with levosimendan at a dosage of 2 mg/kg/d in drinking water daily. Group D was treated with levosimendan at a dosage of 3 mg/kg/d in drinking water daily.
Outcome measures
The cardiac function indexes of rats in each group before and after treatment were observed. The 48 surviving rats in each group were examined by echocardiography after the last intragastric administration. No rats die in the experiment process. General anesthesia was performed by intraperitoneal injection of 10% chloral hydrate solution (100 mg/kg) (Mohamed et al. Citation2020). Rat exhibited no signs of peritonitis, pain or discomfort following i.p. administration of 10% chloral hydrate. The rats were euthanized. After fixation, left anterior chest skin preparation was performed. Echocardiography and 8 MHz wire-controlled probe were used for ultrasonic detection and left ventricular end diastolic inner diameter (LVEDD) and left ventricular ejection fraction (LVEF) were measured. The measured value was the average of three cardiac cycles. The immune function indexes of rats in each group before and after treatment were observed by assessing the levels of CD3+, CD4+, and CD8+ T cells in the peripheral blood of rats using flow cytometry. Blood is taken from the fundus venous plexus before and after treatment. A 220 g rat can take at least 0.5 ml of blood at a time. The serum levels of recombinant human galectin-3 (ab269555,abcam), HSP70 (ab133060,abcam), and inflammatory factors, such as interleukin (IL)-8 (ab214030,abcam) and IL-10 (Interleukin-10, ab185986,abcam), of rats in each group were determined using enzyme linked immunosorbent assay (ELISA).
Statistical methods
SPSS 20.0 (SPSS, Inc., Chicago, IL, USA) was used for performing statistical analyses. Normal distribution data were expressed as mean ± standard deviation (means ± SD). Comparison between the two groups was conducted using Student’s t test. The difference was considered statistically significant at P < 0.05.
Results
Cardiac function of rats in each group
LVEDD index changes
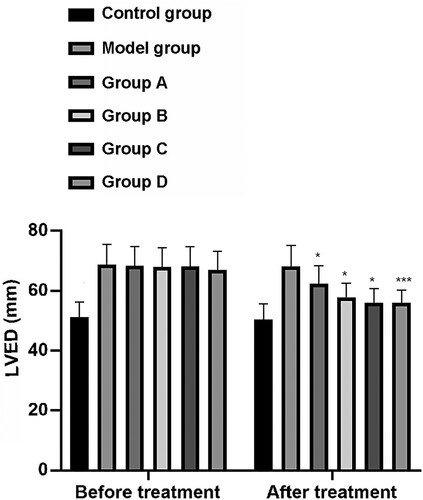
The LVEDD values (mm) of rats in the CG, MG, and groups A–D before treatment were 51.00 ± 5.30, 68.70 ± 6.70, 68.30 ± 6.50, 67.92 ± 6.50, 68.09 ± 6.65, and 67.01 ± 6.23 respectively. After treatment, the LVEDD (mm) values for rats in these groups were 50.20 ± 5.40, 68.20 ± 6.90, 62.27 ± 6.00, 57.80 ± 4.70, 56.09 ± 4.65, and 55.95 ± 4.38, respectively. Before treatment, there was no significant difference in LVEDD values among the MG and groups A–D, but they were all significantly higher than those of the CG (P < 0.001). After treatment, the MG and groups A–D showed decreased LVEDD values compared with the CG, with group D showing the largest decrease (P < 0.001) (Figure ).
LVEF index changes
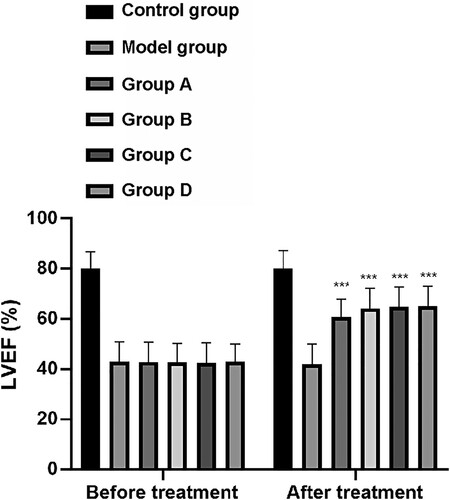
Echocardiography results showed that the LVEF (%) for rats in the CG, MG, and groups A–D before treatment was 80.00 ± 6.90, 43.00 ± 7.86, 42.80 ± 7.90, 42.70 ± 7.50, 42.50 ± 8.00, and 43.00 ± 7.00, respectively. After treatment, the LVEF (%) value for rats in these groups was 80.10 ± 7.20, 42.00 ± 8.00, 60.80 ± 7.00, 64.20 ± 8.00, 64.90 ± 7.90, and 65.00 ±8.00, respectively. Similarly to the LVEDD results prior to treatment, there was no significant difference in LVEF levels among the MG and groups A–D, although these levels were all significantly lower than those in the CG (P < 0.001). After treatment, the MG and groups A–D showed increased LVEF levels compared with the CG, of which group D had the largest increase (P < 0.001, Figure ).
Immune function indexes of rats in each group
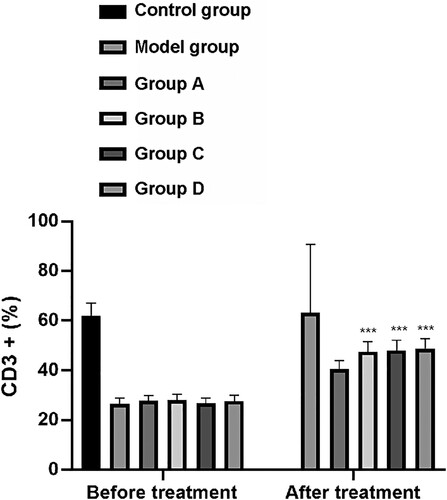
Flow cytometry analysis showed that the CD3+ T cells (%) in peripheral blood prior to treatment of rats in the CG, MG, and groups A–D was 62.00 ± 5.00, 26.58 ± 2.20, 27.90 ± 2.00, 28.00 ± 2.30, 26.70 ± 2.10, and 27.67 ± 2.40, respectively. After treatment, CD3+ T cells (%) in rats in these groups was 63.30 ± 4.80, 27.40 ± 2.00, 40.50 ± 3.50, 47.60 ± 3.97, 48.00 ± 4.10, and 48.80 ± 4.00, respectively, (Figure ).
Figure 3. Changes of CD3+. After treatment, CD3+ in model group, group A, group B, group C and group D was significantly increased. Among them, the up-regulation of group A was significantly larger than that of model group, while the up-regulation of group B, group C and group D was significantly larger than that of group A (P < 0.001). Group A, group B, group C and group D were compare with the model group. ***means P < 0.001.
The CD4+/CD8+ T cell ratio of rats in the CG, MG, and group A–D before treatment was 2.01 ± 0.40, 1.10 ± 0.30, 1.11 ± 0.32, 1.12 ± 0.35, 1.11 ± 0.40, and 1.10 ± 0.47, respectively. After treatment, the CD4+/CD8+ T cell ratio of rats in these groups was 2.01 ± 0.40, 1.10 ± 0.30, 1.16 ± 0.33, 1.22 ± 0.45, 1.23± 0.44, and 1.24 ± 0.47, respectively (Figure ).
Figure 4. Changes of CD4+/CD8+. After treatment, CD4+/CD8+ in model group, group A, group B, group C and group D rats were significantly increased. Among them, the up-regulation of group A was significantly larger than that of model group, while the up-regulation of group B, group C and group D was significantly larger than that of group A (P < 0.05). Group A, group B, group C and group D compare were with the model group. *means P < 0.05.
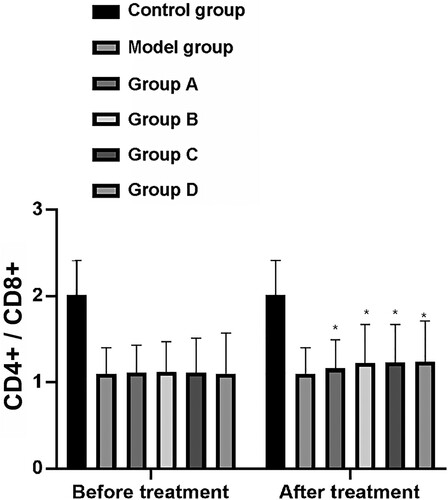
Before treatment, the levels of CD3+ T cells and the CD4+/CD8+ T cell ratio between the MG and groups A–D lacked statistical significance but were significantly lower than those in the CG (P < 0.001). After treatment, the groups A–D and the MG shoed increased levels of CD3+ T cells and the CD4+/CD8+ T cell ratio compared with the CG. The increase in levels of CD3+ T cells and the CD4+/CD8+ T cell ratio in group A was significantly larger than those of the MG, whereas the increase of these in groups B–D was significantly larger than those of group A (P < 0.001).
Changes of serum galectin-3 and HSP70 in rats of each group
Galectin-3 index changes
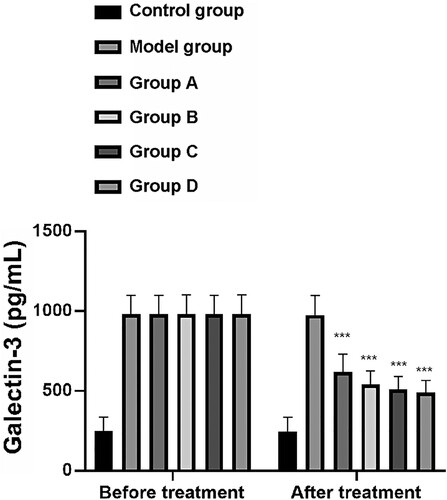
Galectin-3 levels (pg/mL) of rats in the CG, MG, and groups A–D before treatment were 246.30 ± 90.00, 980.24 ± 118.00, 982.00 ± 117.90, 981.00 ± 120.45, 981.40 ± 117.00, and 980.69 ± 120.58, respectively. Following treatment, galectin-3 (pg/mL) serum levels of rats in these groups were 245.20 ± 89.00, 976.40 ± 120.45, 620.19 ± 110.50, 540.20 ± 86.00, 510. 19 ± 80.00, and 490.23 ± 76.00, respectively. Before treatment, there was no significant difference in galectin-3 levels between the MG and groups A–D, although these levels were significantly higher than those in the CG (P < 0.001). After treatment, the groups A–D and the MG showed decreased galectin-3 levels compared with those of the MG, of which group D had the largest decrease (P < 0.001) (Figure ).
HSP70 index change
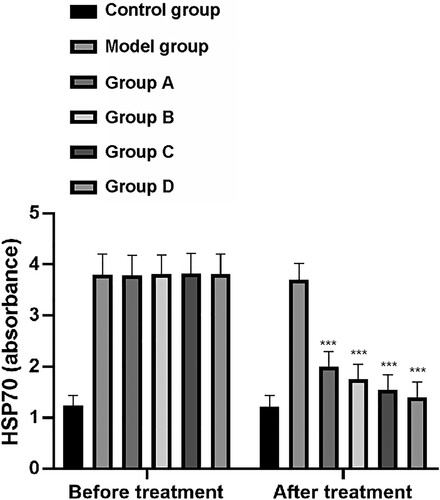
Levels of HSP70 prior to treatment of rats in the CG, MG, and groups A–D were 1.23 ± 0.20, 3.80 ± 0.40, 3.79 ± 0.39, 3.81 ± 0.38, 3.82 ± 0.40, and 3.81 ± 0.39, respectively. Following treatment, HSP70 levels in these groups were 1.22 ± 0.21, 3.70 ± 0.32, 2.0 ± 0.30, 1.75 ± 0.30, 1.55 ± 0.29, and 1.40 ± 0.30, respectively. There was no significant difference in HSP70 levels among the MG and groups A–D, but these were significantly higher than those of the CG (P < 0.001). After treatment, the MG and groups A–D showed decreased HSP70 levels compared with the CG, of which group D showed the greatest decrease (P < 0.001) (Figure ).
Changes of serum inflammatory factors in rats of each group
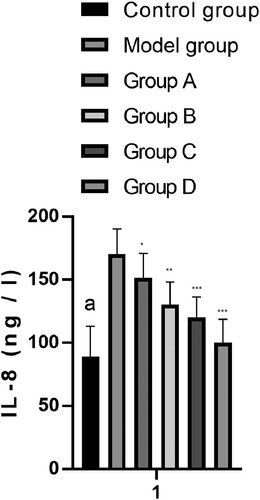
IL-8 index changes
The serum levels of IL-8 (ng/L) from rats in the CG, MG, and groups A–D after treatment were 89.20 ± 24.00, 170.20 ± 20.00, 151.40 ± 19.58, 130.20± 18.00, 120.19 ± 16.20, and 100.30 ± 18.40, respectively. The MG and groups A–D showed increased IL-8 levels compared with the CG. Groups A–D showed decreased IL-8 levels compared with the MG. The levels of IL-8 in group D were the lowest, which were significantly lower than those in the combined group (P < 0.001) (Figure ).
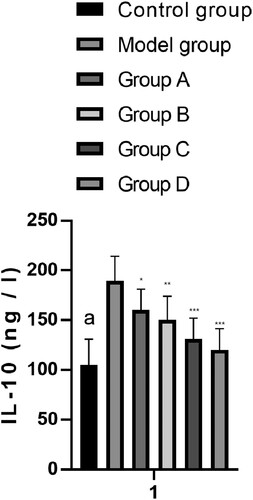
IL-10 index changes
The serum levels of IL-10 (ng/L) of rats in the CG, MG, and groups A–D after treatment were 105.20 ± 25.77, 189.30 ± 24.70, 160.20 ± 21.00, 150.20 ± 23.80, 131.20± 20.88, and 120.19 ± 21.20, respectively. The MG and groups A–D showed increased IL-10 levels compared with the CG. Groups A–D showed decreased IL-10 levels compared with the MG. Among them, the levels of IL-10 in group D were the lowest, which were significantly lower than those in the combined group (P < 0.001) (Figure ).
Discussion
With the rapid aging of human society, heart failure has become one of the important causes of illness and death among the elderly (Gulyaev et al. Citation2020). Levosimendan is a new calcium sensitizer, but its use is often restricted because of common adverse reactions (Wang et al. Citation2019a). To ensure the safety of clinical medication, this study investigated the effects of different doses of levosimendan combined with Shenmai injection on immune function and galectin-3 and HSP70 levels in acute heart failure model rats.
In this study, we first assessed the cardiac function indexes of each group of rats using echocardiography. Prior to treatment, the levels of LVEDD and LVEF among the groups of acute heart failure model rats did not significantly vary. However, the MGs had significantly higher values of LVEDD and significantly lower levels of LVEF in comparison with those of the healthy control rats. After treatment, the values of LVEDD among groups treated with Shenmai alone or in combination with levosimendan significantly decreased compared with the LVEDD values of the MG, and the levels of LVEF significantly increased in these groups in comparison with the LVEF values of the MG. The high-dose levosimendan group combined with Shenmai injection had the largest decrease in LVEDD and largest increase in LVEF. A comparative study of the effects of different doses of levosimendan on patients with chronic heart failure showed that levosimendan can improve cardiac function of patients with heart failure more effectively, and the improvement effect of large dose of levosimendan is greater than that of a smaller dose (Levijoki et al. Citation2001). Shenmai injection stimulates the heart, boosting blood pressure, and therefore, it is a common injection for patients with clinical heart failure (Li et al. Citation2020).
We then analyzed the immune function indexes of rats in each group by flow cytometry. The results showed that there was no significant difference in the levels of CD3+ T cells or CD4+/CD8+ T cell ratio between the groups of acute heart failure model rats before treatment; however, these were both significantly lower than those of normal control rats. After treatment, levels of CD3+ T cells and the CD4+/CD8+ cell ratio in rats in the acute heart failure model were both significantly increased, and these were significantly higher in rats with the single Shenmai injection compared with those of the MG. The increase in the levels of CD3+ T cells and the CD4+/CD8+ cell ratio in groups with the Shenmai injection and levosimendan combined treatment was also significantly larger than these results from rats that only received Shenmai injection. Auxiliary T cells can infiltrate the myocardium following damage, promoting myocardial cellular dysfunction and inducing heart failure. Combined with the results of this study, we believe that levosimendan combined with Shenmai injection was effective in regulating immune function in rats with acute heart failure.
Finally, we observed the changes in the levels of serum inflammatory factors (IL-8 and IL-10) and galectin-3 and HSP70 in each group of rats. There was no significant difference between levels of galectin-3, HSP70, and serum inflammatory factors in acute heart failure MGs before treatment, but these were all significantly higher than those in control rats. After treatment, the levels of galectin-3, HSP70, and serum inflammatory factors in acute heart failure model rats were significantly reduced, with the maximum reduction in the group with high-dose levosimendan combined with Shenmai injection. Heart failure is closely related to the production and release of proinflammatory cytokines and the activation process of inflammatory response. High levels of proinflammatory cytokines directly affect the severity of heart failure (Eskandari et al. Citation2018; Lino et al. Citation2019). The concentration of inflammatory mediators has been confirmed to be related to cardiac function. In patients with heart failure, the concentration of proinflammatory interleukins increases, and IL-6, IL-8, and IL-10 levels can all serve as predictors of heart failure (Segiet et al. Citation2019; Cabiati et al. Citation2020). Studies on myocardial cells have suggested that extracellular HSP70 released by injured myocardial cells act as an inflammatory mediator, leading to cell death (Song et al. Citation2020). HSP70 plays an anti-inflammatory role by inhibiting proinflammatory signaling pathways and simultaneously regulating signal transduction kinase pathways to inhibit apoptosis (Chen et al. Citation2020a). Galectin-3 is a member of β-galactoside binding lectin family, and it has been demonstrated to be a biomarker for cardiac remodeling and is involved in the progression of heart failure (Baggen et al. Citation2018). Galectin-3 participates in the regulation of inflammation, angiogenesis, and fibrosis by regulating high glucose-induced myocardial injury (Shen et al. Citation2019). An in vivo rat study found that the cardiac injury of streptozotocin-induced diabetic rats was weakened by silencing galectin-3, which may be a potential target for treating myocardial disorders of diabetic patients (Zhang et al. Citation2016). Based on the results of this study, we speculated that different doses of levosimendan combined with Shenmai injection had different effects on the levels of galectin-3 and HSP70 in rats with acute heart failure, and the higher dose of levosimendan combined with Shenmai injection had an improved inhibitory effect on inflammatory reaction and the levels of galectin-3 and HSP70 in rats with acute heart failure.
In this study, the acute heart failure rat model was established, and different doses of levosimendan combined with Shenmai injection were administered. However, there were a few limitations, such as incomplete display of other biochemical indexes of the rats. In addition, the setting of observation time points for galectin-3 and HSP70 levels in rats could be more specific.
In conclusion, levosimendan optimized the stabilization of immune and cardiac function induced by Shenmai injection in rats with acute heart failure. In addition, the higher dose of levosimendan combined with Shenmai injection improved inflammatory reaction and galectin-3 and HSP70 levels.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are openly available in figshare at http://doi.org/10.6084/m9.figshare.16800871.
References
- Baggen VJM, van den Bosch AE, Eindhoven JA, Menting ME, Witsenburg M, Cuypers J, Boersma E, Roos-Hesselink JW. 2018. Prognostic value of galectin-3 in adults with congenital heart disease. Heart. 104(5):394–400.
- Cabiati M, Botta L, Caselli C, Del Ry S. 2020. Transcriptional evaluation of relaxin and endothelin-1 axis in heart failure patients: first evidence of its involvement during left ventricular assist device support. Int J Cardiol. 306:109–115.
- Chen LJ, Zhang RG, Yu DD, Wu G, Dong XR. 2019. Shenqi Fuzheng injection ameliorates radiation-induced brain injury. Curr Med Sci. 39(6):965–971.
- Chen Q, Huang M, Wu J, Jiang Q, Zheng X. 2020a. Exosomes isolated from the plasma of remote ischemic conditioning rats improved cardiac function and angiogenesis after myocardial infarction through targeting Hsp70. Aging. 12(4):3682–3693.
- Chen RJ, Rui QL, Wang Q, Tian F, Wu J, Kong XQ. 2020b. Shenfu injection attenuates lipopolysaccharide-induced myocardial inflammation and apoptosis in rats. Chin J Nat Med. 18(3):226–233.
- Cheng Y, Rong J. 2019. Pro-resolving lipid mediators as therapeutic leads for cardiovascular diseases. Expert Opin Ther Targets. 23(5):423–436.
- Comín-Colet J, Manito N, Segovia-Cubero J, Delgado J, García Pinilla JM, Almenar L, Crespo-Leiro MG, Sionis A, Blasco T, Pascual-Figal D, et al. 2018. Efficacy and safety of intermittent intravenous outpatient administration of levosimendan in patients with advanced heart failure: the LION-HEART multicentre randomised trial. Eur J Heart Fail. 20(7):1128–1136.
- Eskandari V, Amirzargar AA, Mahmoudi MJ, Rahnemoon Z, Rahmani F, Sadati S, Rahmati Z, Gorzin F, Hedayat M, Rezaei N. 2018. Gene expression and levels of IL-6 and TNFα in PBMCs correlate with severity and functional class in patients with chronic heart failure. Ir J Med Sci. 187(2):359–368.
- Fu R, Noguchi H, Kaneko S, Kawamura A, Kang C, Takahashi H, Tamiya N. 2019. How do cardiovascular diseases harm labor force participation? Evidence of nationally representative survey data from Japan, a super-aged society. PLoS One. 14(7):e0219149.
- Gu T, Wang RH, Wu T, Ke ZH, Yang H, Wang D. 2020. [Therapeutic effect on mild perimenopausal depression treated with acupuncture at the “thirteen ghost points” and kaixin powder]. Zhongguo Zhen Jiu. 40(3):267–271.
- Gulyaev NI, Akhmetshin IM, Gordienco AV, Yakovlev VV. 2020. Sarcopenia as the reason of hypodiagnostics of chronic kidney disease in patients with chronic heart failure. Adv Gerontol. 33(1):121–126.
- Hansen MS, Andersen A, Nielsen-Kudsk JE. 2018. Levosimendan in pulmonary hypertension and right heart failure. Pulm Circ. 8(3):2045894018790905.
- Hong T, Li LX, Han XP, Shi JL, Dan CY, Liu ZY, Wu XB. 2020. Effect of Astragalus membranaceus oral solution on lifespan and learning and memory ability of honey bees. Biomed Res Int. 2020:5745048.
- Jiang YR, Du JY, Wang DD, Yang X. 2018. miRNA-130a improves cardiac function by down-regulating TNF-α expression in a rat model of heart failure. Eur Rev Med Pharmacol Sci. 22(23):8454–8461.
- Johnson JL. 2019. Elucidating the contributory role of microRNA to cardiovascular diseases (a review). Vascul Pharmacol. 114:31–48.
- Levijoki J, Pollesello P, Kaheinen P, Haikala H. 2001. Improved survival with levosimendan after experimental myocardial infarction in rats. Eur J Pharmacol. 419(2–3):243–248.
- Li F, Zhang D, Lu X, Wang Y, Xiong Z. 2004. Enatiomeric analysis of levosimendan by CE with beta-CD as chiral selector compared with CMPA-HPLC. Biomed Chromatogr. 18(10):866–871.
- Li L, Yang D, Li J, Niu L, Chen Y, Zhao X, Oduro PK, Wei C, Xu Z, Wang Q, et al. 2020. Investigation of cardiovascular protective effect of Shenmai injection by network pharmacology and pharmacological evaluation. BMC Complement Med Ther. 20(1):112.
- Lino DOC, Freitas IA, Meneses GC, Martins AMC, Daher EF, Rocha JHC, Silva Junior GB. 2019. Interleukin-6 and adhesion molecules VCAM-1 and ICAM-1 as biomarkers of post-acute myocardial infarction heart failure. Braz J Med Biol Res. 52(12):e8658.
- Mazloumi E, Poorolajal J, Sarrafzadegan N, Roohafza HR, Faradmal J, Karami M. 2019. Avoidable burden of cardiovascular diseases in the eastern Mediterranean region: contribution of selected risk factors for cardiovascular-related deaths. High Blood Press Cardiovasc Prev. 26(3):227–237.
- Mohamed AS, Hosney M, Bassiony H, Hassanein SS, Soliman AM, Fahmy SR, Gaafar K. 2020. Sodium pentobarbital dosages for exsanguination affect biochemical, molecular and histological measurements in rats. Sci Rep. 10(1):378.
- Noale M, Limongi F, Maggi S. 2020. Epidemiology of cardiovascular diseases in the elderly. Adv Exp Med Biol. 1216:29–38.
- Papp Z, Agostoni P, Alvarez J, Bettex D, Bouchez S, Brito D, Černý V, Comin-Colet J, Crespo-Leiro MG, Delgado JF. 2020. Levosimendan efficacy and safety: 20 years of SIMDAX in clinical use. Card Fail Rev. 6:e19.
- Pu X, Chai Y, Guan L, Li W, Gao J, Jiang Z, Li Q, Wu Y, Chen Y. 2020. Astragalus improve aging bone marrow mesenchymal stem cells (BMSCs) vitality and osteogenesis through VD-FGF23-Klotho axis. Int J Clin Exp Pathol. 13(4):721–729.
- Raczkiewicz D, Bojar I, Wdowiak A, Rzeźnicki A, Krakowiak J. 2019. Stress at intellectual work and cardiovascular diseases in women at non-mobility working age. Ann Agric Environ Med. 26(3):456–461.
- Segiet OA, Piecuch A, Mielanczyk L, Michalski M, Nowalany-Kozielska E. 2019. Role of interleukins in heart failure with reduced ejection fraction. Anatol J Cardiol. 22(6):287–299.
- Shen Q, Chen W, Liu J, Liang Q. 2019. Galectin-3 aggravates pulmonary arterial hypertension via immunomodulation in congenital heart disease. Life Sci. 232:116546.
- Shi H, Wang L, Fang ZH, Ni YQ, Shen AL, Liu PP, Wang X, Huang JL. 2019. [Experimental study on effect and mechanism of Danzhi Jiangtang Capsules on diabetic myocardial injury]. Zhongguo Zhong Yao Za Zhi. 44(23):5159–5165.
- Song N, Ma J, Meng XW, Liu H, Wang H, Song SY, Chen QC, Liu HY, Zhang J, Peng K. 2020. Heat shock protein 70 protects the heart from ischemia/reperfusion injury through inhibition of p38 MAPK signaling. Oxid Med Cell Longev. 2020:3908641.
- Tulay P, Temel SG, Ergoren MC. 2019. Investigation of KCNQ1 polymorphisms as biomarkers for cardiovascular diseases in the Turkish Cypriots for establishing preventative medical measures. Int J Biol Macromol. 124:537–540.
- Wang A, Cui C, Fan Y, Zi J, Zhang J, Wang G, Wang F, Wang J, Tan Q. 2019a. Prophylactic use of levosimendan in pediatric patients undergoing cardiac surgery: a prospective randomized controlled trial. Crit Care. 23(1):428.
- Wang C, Yao D, Zhang P, Xie X, Wang B, Liu J, Zhang Z. 2019b. Clinical efficacy and safety of Shenqi Fuzheng injection for the treatment of chronic heart failure: protocol for a meta-analysis and systematic review. Medicine (Baltimore). 98(52):e18556.
- Xu T, Ding W, Ji X, Ao X, Liu Y, Yu W, Wang J. 2019. Oxidative stress in cell death and cardiovascular diseases. Oxid Med Cell Longev. 2019:9030563.
- Yang F, Zhao LN, Sun Y, Chen Z. 2019. Levosimendan as a new force in the treatment of sepsis-induced cardiomyopathy: mechanism and clinical application. J Int Med Res. 47(5):1817–1828.
- Yang L, Zhang C, Chen J, Zhang S, Pan G, Xin Y, Lin L, You Z. 2020. Shenmai injection suppresses multidrug resistance in MCF-7/ADR cells through the MAPK/NF-κB signalling pathway. Pharm Biol. 58(1):276–285.
- You JS, Huang HF, Chang YL, Lee YS. 2006. Sheng-mai-san reduces adriamycin-induced cardiomyopathy in rats. Am J Chin Med. 34(2):295–305.
- Zhang MJ, Gu Y, Wang H, Zhu PF, Liu XY, Wu J. 2016. Valsartan attenuates cardiac and renal hypertrophy in rats with experimental cardiorenal syndrome possibly through down-regulating galectin-3 signaling. Eur Rev Med Pharmacol Sci. 20(2):345–354.