 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The postharvest life of most fruit, vegetables and cereals is limited by fungal proliferation. The chemical composition of Mentha piperita, M. spicata and M. suaveolens essential oils (EO), and the antifungal activity against four pathogenic and post-harvest fungi isolated from food, were herein investigated to evaluate their potential as natural food preservatives. The EO were obtained by hydrodistillation of aerial parts leaves, stems and inflorescences (except for peppermint oil, which was purchased in a specialized store) and submitted to GC-MS and GC-FID analysis. Regarding the EO composition, carvone (41.1%) and limonene (14.1%) were the major compounds in M. spicata, menthol (47.0%) and menthone (23.1%), as well as other menthol derivatives (neomenthol -3.6%- and menthofurane -3.7%-) in M. piperita, and piperitone oxide (40.2%) and piperitenone oxide (31.4%) in M. suaveolens.
Botryotinia fuckeliana was the most sensitive fungus. The three studied EO inhibited growth by 92–100%. The highest dose of M. suaveolens EO, 400 µg/mL, produced 100% MGI in all the studied fungi, except Fusarium oxysporum with 94.21%. The M. suaveolens EO can be considered to develop a low-risk enviro-friendly botanical biofungicide.
Introduction
Lamiaceae is a family with over 232 genera that include shrubby or sufruticosas plants, or perennial or annual herbs, which are sometimes ephemeral and often aromatic. Many aromatic plants used in medicine, food and pharmaceutical industries belong to the Lamiaceae family. Mentha is a well-known genus of this family and includes numerous species that generally grow in temperate areas worldwide, particularly in Europe, Asia, Africa, Australia and North America. Mentha spp. includes plants that exhibit relevant biological activities. It has a wide morphological variability and very high chemical diversity in relation to its their essential oils (Tucker and Naczi Citation2006; Stringaro et al. Citation2018).
Mentha essential oils (EO) have been used as a popular remedy for respiratory diseases like bronchitis, sinusitis, tuberculosis and the common cold (Peixoto et al. Citation2009). The Mentha chemistry is complex and extremely variable, and each species has a characteristic main compound. Additionally, the chemical composition of essential oils can vary depending on the stage of the life cycle, plant organs, geographic location, soil, and ecological factors (Llorens-Molina et al. Citation2017; Valadares et al. Citation2018; Silva et al. Citation2019; Diasa et al. Citation2020). Among the properties of the genus Mentha the following have been reported: antiviral, antibacterial, antifungal, high antioxidant, cytotoxic, contraceptive, anti-inflammatory and antiallergic (Sharma et al. Citation2014; Chávez-González et al. Citation2016).
The taxonomy of the genus Mentha is controversial and several classifications are proposed due to frequent hybridisation occurring in both wild populations and cultivation (El-Kashoury et al. Citation2014). Tucker (Citation2006) describes 42 species, 15 hybrids and hundreds of subspecies, varieties and cultivars distributed in five sections. The genus is of much gastronomical, pharmacological, chemical and agricultural interest.
The EO of the species belonging to the genus Mentha show high chemodiversity according to their chemotypes and ecotypes (Lawrence Citation2006). Thus Mentha x piperita (peppermint oil) is characterised by its high menthol proportion and its derivatives, such as menthona, menthyl acetate, etc., and their isomers. Significant proportions of 1,8-cineole and menthofuran have sometimes been reported. Other compounds containing the menthane skeleton are the main components in other species: piperitone, piperitenone and their oxides (M. suaveolens and some chemotypes of M. spicata, M. longifolia, and others); carvone and related compounds like carveol and carvyl esters (M. spicata); pulegone, a major compound in many different Mentha specie, such as M. pulegium, M. rotundofolia, M. arvensis, M. piperita, etc. (Baser et al. Citation1998). It is worth mentioning that all these compounds can be found in a higher or lower proportion in many chemotypes of a consdierable number of species. It is also notworthy that the presence of certain monoterpenic compounds, such as limonene or 1,8-cineole, can also be found in high proportions.
Society's tendency to eliminate chemicals to treat diseases caused by different biological agents, consumer consumers and food safety agencies worldwide have led researchers to consider natural alternatives to control these diseases. Mentha sp. as antimicrobial agents contain several EO that are odorous and volatile products with a complex variable composition that is rich in biologically active molecules displaying antibacterial, antifungal, antiviral and other biological activities (Stringaro et al. Citation2018; Luković et al. Citation2019; Oliveira Filho et al. Citation2019; Karpiński Citation2020).
The postharvest life of most fruit and vegetables is limited by fungal proliferation occurring when these products are stored, and fungal appearance implies mass losses of harvest quality and yield. Fungal food contamination causes both economic and human health problems. The objectives of this study were to: determinate the composition of Mentha piperita, M. spicata and M. Suaveolens; evaluate its antifungal potential against four important phytopathogenic and post-harvest fungi Verticillium dahliae, Fusarium oxysporum, Curvularia hawaiiensis and Botryotinia fuckeliana.
Materials and methods
Essential oils
The essential oils of M. spicata and M. suaveolens were obtained from the plant material taken from the experimental field at the Universitat Politécnica de València. For this purpose, 300 g of air-dried (room temperature) aerial parts of both species collected in the full flowering stage were submitted to hydrodistillation with 3 L of distilled water using a Clevenger-type apparatus (Vidra Foc, SA) for 3 h. After drying with anhydrous sodium sulphate (Sigma-Aldrich), the essential oil was diluted to 2% (v/v) in dichloromethane (Sigma-Aldrich, capillary GC grade) and stored in glass vials at −18°C in the absence of light until the GC analysis.
The peppermint essential oil was purchased from Sigma-Aldrich (steam distillation). It was diluted and stored in the same way.
Gas chromatography (GC/FID)
Gas Chromatography (GC) was performed using a Perkin-Elmer Clarus 500GC apparatus equipped with a flame ionization detector (FID), and a Hewlett-Packard HP-1 (cross-linked methyl silicone) capillary column (30 m × 0.25 mm i.d. × 0.25 µm film thickness). The column temperature programme was 60°C for 5 min, with 3°C/min increases to 180°C, and then 20°C/min increases to 280°C, which was maintained for 10 min. The carrier gas was helium applied at a flow rate of 1 mL/min. Both the FID detector and injector port temperature were 250 and 220°C, respectively. Data were expressed as % peak normalised areas.
Gas chromatography and mass spectrometry (GC-MS)
The GC-MS analysis was carried out on a Varian Saturn 2000 equipped with a Varian C.S VA-5MS with the same capillary column. The same working conditions used for GC and split mode injection (ratio 1:25) were employed. Mass spectra were taken over the m/z 28–400 range with an ionising voltage of 70 eV. Linear retention indices were calculated using co-chromatographed standard hydrocarbons. Individual compounds were identified by MS and their identity was confirmed by comparing their LRIs to C8-C25 n-alkanes, and by comparing their mass spectra and retention times with those of authentic samples or with data already available in the NIST 2005 Mass Spectral Library and the literature (Adams Citation2007). For quantification purpose, relative peak areas percentages from GC/FID analysis were used without considering response factors.
Fungal species
The fungal strain herein employed were:
Fusarium oxysporum lycopersici (FO) CECT 2715 isolated from tomato, Botryotinia fuckeliana (BF) CECT 2100 isolated from grape wine, and Verticillium dahliae (VD) CECT 2694 isolated from olive, were supplied by the Spanish Type Culture Collection (CECT).
Curvularia hawaiiensis (CH) (CECT 20934), which was isolated in the Botany Laboratory in the Department of Agroforest Ecosystems from the rice samples collected from the ‘La Albufera’ rice-producing Mediterranean Region in Valencia (Spain). The fungal species was morphologically and molecularly identified and then deposited in the CECT.
Antifungal activity
The bioassay was performed in Petri dishes (90 × 15 mm and 150 × 20 mm) with dissolving depending on mint 200, 300 and 400 µg/mL (Tween 20, 0.1%) of EO in previously sterilised Potato Dextrose Agar (PDA-Pronodisa) growth medium flasks at 45–50°C, while the medium was still liquid to be distributed in Petri dishes. Petri dishes were inoculated with an 8 mm-diameter disk of a 7-day old colony on the PDA of each tested fungi. Plates were incubated in the dark at 25°C for 7 days. Fungal growth was evaluated by measuring the colony diameter in two perpendicular directions daily. Five replicate dishes were used for each EO, dose and fungus, it was done 3 times. The control Petri dishes contained only PDA/Tween 20 (0.1%) and the analysed fungus. Mycelial growth was evaluated by measuring the perpendicular colony diameters after 7 days. Mycelial growth inhibition was determined by the following formula (Albuquerque et al. Citation2006):
Where DC is the average of the colonies in the control dishes, DO is the average of the colonies’ diameter in the dishes with oil.
Statistical analysis
The fungal growth results were submitted to an analysis of variance (ANOVA). The HSD Tukey intervals were represented to compare species and treatment, with significant values at P < 0.05. The data analysis was performed by the Statgraphics Centurion XVII software (Stat Point, Inc., Herndon, Virginia, USA).
Results and discussion
Chemical composition of commercial essential oils
According to classes of terpenic compounds, the composition of oils from M. spicata, M. piperita and M. suaveolens were, respectively: 63.6%, 92.0% and 77.3% for oxygenated monoterpenes (the major fraction) and 25.4%, 4.0% and 7.3% for hydrocarbon monoterpenes. Sesquiterpenic compounds (both hydrocarbon and oxygenated) accounted for 7.9%, 3.1% and 6.6%, respectively (Table ).
Table 1. Chemical composition of the Mentha spicata, M. piperita and M. suaveolens essential oils (EOs).
The major compounds in M. piperita were menthol (47.0%) and its derivatives: menthone (23.1%), menthyl acetate (5.2%), menthofurane (3.7%) and neomenthol (3.6%). It is worth noting the relatively large amount of 1,8-cineole (5%) as a very similar composition has been reported by De Oliveira et al. (Citation2017) and Desam et al. (Citation2019). Peppermint oil generally exhibits a composition in which menthol isomers and their esters, as well as derivatives menthone, isomenthone and menthofurane, are major compounds. Nevertheless as reported by Desam et al. (Citation2019), some samples show noticeably different profiles in which hydrocarbon monoterpenes, such as limonene, α-terpinene or oxygenated monoterpenes like linalool, pulegone or carvone are major compounds.
Carvone (41.1%) and limonene (14.4%) were the major components in M. spicata (spearmint), together with some noticeable amounts of oxygenated monoterpenes in: terpinen-4-ol (5.7%), α-terpineol (4.8%) and (Z)-sabinene hydrate (4.7%). This profile closely coincided with that reported by Bardaweel et al. (Citation2018) with carvone (49.5%) and limonene (16.1%), but with different other oxygenated monoterpenes, namely: 1,8-cineole (8.7%) and (Z)-dihydrocarvone (3.9%). Carvone and limonene have generally been reported as major compounds in spearmint: Brahmi et al. (Citation2017): 20.8% and 48.5%; Nikšić et al. (Citation2018): 56.4% and 16.2%, of these components, respectively.
Regarding M. suaveolens, the oil herein applied showed a balanced profile between the two of the most representative compounds in this species: piperitone oxide (40.2%) and piperitenone oxide (31.4%), and small amounts of other monoterpenic compounds likes limonene (3.0%) or (Z)-sabinene hydrate (2.1%). M. suaveolens oil generally shows a profile characterised by the dominating menthane skeleton compounds oxygenated in C3: piperitone, piperitenone, and their epoxides (Bouyahya et al. Citation2019), as well as pulegone and oxygenated compounds in C2 like carvone and dihydrocarvone (El-Kashoury et al. Citation2012). Other compositions have been reported: such as (Z)-piperitol and 1,8-cineol chemotypes (Lawrence Citation2006), or oils rich in cinerone, β-caryophyllene, terpinen-4-ol and other monoterpenes (Ed-Dra et al. Citation2019).
Antifungal activity. Mycelial growth inhibition
The fungus Botryotinia fuckeliana was the most sensitive species to the effect of the three tested EO: M. piperita, M. spicata and M. suaveolens (Tables and ). B. fuckeliana was completely or practically inhibited, at the 400 µg/mL, in all trials, and also at 300 µg/mL in M. suaveolens. The three studied EO inhibited its growth with 92–100% mycelial growth inhibition (MGI).
Table 2. Mean growth (mm) and standard deviation values calculation for each fungus species grown on PDA (control), PDA-M. piperita EO, PDA-M. spicata EO and PDA-M. suaveolens EO at different concentrations.
Table 3. Mycelial Growth Inhibition (MGI) percentage for each fungus grown on PDA- M. piperita EO, PDA-M. spicata EO and PDA-M. suaveolens EO at different ferent doses.
The EO of Mentha suaveolens obtained the best antifungal activity results. The highest dose, 400 µg/mL, produced 100% MGI in all the studied fungi, except for Fusarium oxysporum with 94.21%. At 300 the µg/mL dose, effectiveness slightly decreased, but the results were still very satisfactory with 74% MGI for C. hawaiiensis, 89.94% for F. oxysporum, 91.03% for V. dahliae and up to 100% for B. fuckeliana. When lowering the dose to 200 µg/mL in the M. suaveolens EO, considerably low MGI values were obtained, with a major difference in the MGI percentages compared to the results of the immediately higher dose (300 µg/mL), except for BF that exceeded 50%. The behaviour of the V. dahliae fungus in M. piperita and M. spicata led to similar results. The Tukey HSD intervals (Figures ) showed the significance of the results for fungal growth under the tested conditions. Botryotinia fuckeliana was the most sensitive fungus to treatment with the EO of the three mints. No significant differences appeared in the growth of BF and F. oxysporum at the 400 µg/mL and 300 µg/mL doses, respectively, in the treatment with the M. suaveolens EO. This the treatment was equally effective. Significant differences in V. dahliae growth were observed at both doses.
Figure 1. Interaction plot, mean growth, species, concentration 300 and 400 µg/mL of Mentha piperita essential oil against Verticillium dahliae (VD), Fusarium oxysporum lycopersici (FO), Curvularia hawaiiensis (CH) and Botryotinia fuckeliana (BF). n (30) observations per treatment were used in the statistical analysis.
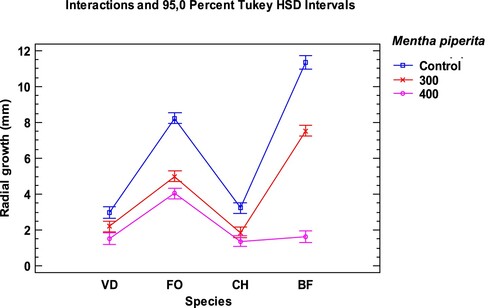
Figure 2. Interaction plot, mean growth, species, concentration 300 and 400 µg/mL of Mentha spicata essential oil against Verticillium dahliae (VD), Fusarium oxysporum lycopersici (FO), Curvularia hawaiiensis (CH) and Botryotinia fuckeliana (BF). n (30) observations per treatment were used in the statistical analysis.
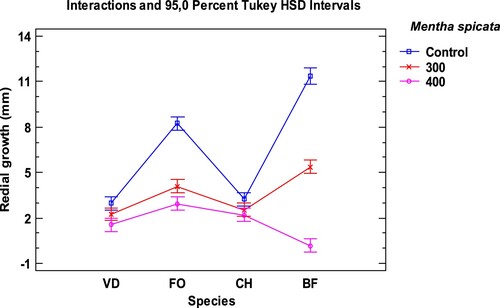
Figure 3. Interaction plot, mean growth, species, concentration 200, 300 and 400 µg/mL of Menta suaveolens essential oil against Verticillium dahliae (VD), Fusarium oxysporum lycopersici (FO), Curvularia hawaiiensis (CH) and Botryotinia fuckeliana (BF). n (30) observations per treatment were used in the statistical analysis.
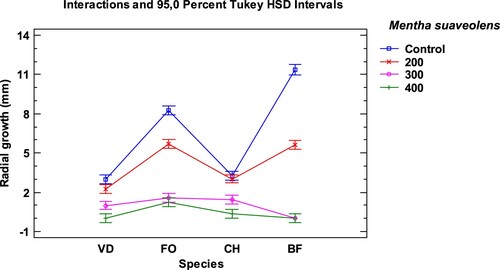
The antifungal activity of the EO from different Mentha species has been tested by many researchers (Peixinho et al. Citation2017; Stringaro et al. Citation2018; Oliveira Filho et al. Citation2019).
The results obtained in this work with the M. piperita EO against the four pathogenic fungi are similar to those obtained against other fungal species. The M. piperita mint oil was evaluated with the spore germination of Colletotrichum gloeosporioides and its mycelial growth in vitro and with papaya fruit at differents concentrations. The results revealed a strong inhibitory effect on both spore germination and mycelial growth in vitro and in vivo (Andrade and Vieira Citation2016). Fialho et al. (Citation2015) tested the same EO against Phakopsora euvitis and other pathogenic fungi and obtained good results. However, Behidj et al. (Citation2018) and Rachitha et al. (Citation2017), reported limited antifungal effectiveness for M. piperita on the species of the genus Fusarium, which also happened in our research.
In this study, the most inhibition recorded under the tested conditions was found for the fungus B. fuckeliana. Lorenzetti et al. (Citation2011) evaluated the mycelial growth, conidia production and germination of this B. fuckeliana species isolated from strawberry by incorporating oil into the culture medium (125–1.000 ppm), and obtained the different assayed EO (lemon grass, palmrose, citronella, clove, cinnamon, lavender, tangerine, eucalyptus, tea tree, rosemary, orange) with the best results for the M. piperita. In a recent study, Oliveira Filho et al. (Citation2019) demonstrated that M. spicata may be a potentially efficient and safe alternative to be used as a potential fumigant to control B. cinerea in stored fresh products. Bayan and Küsek (Citation2018) investigated the chemical composition and the antifungal and antibacterial activity of volatile oil from Mentha spicata. The main component was carvone (56.94%), followed by limonene (11.63%), sabinene hydrate (7.04%) and caryophyllene (4.06%). Antifungal activity was determined against Fusarium oxysporum f. sp. radicis-lycopersici, Rhizoctonia solani Alternaria solani and Verticillium dahliae. Volatile oil displayed marked antifungal activity against plant pathogenic fungi, and an oil concentration was used that doubled our concentration in this work. Volatile oil also exhibited remarkable activity against Xanthomonas spp. ‘Furthermore, Dias et al. (Citation2020) studied the antifungal activity of limonene against Sclerotinia sclerotium, obtaining excellent results at 300 µL. This monoterpene showed to be highly active by inhibiting 100% of fungal growth’.
M. suaveolens was the most effective EO in our study. In a study by El-Kashoury et al. (Citation2014), oil displayed strong antifungal activity on Aspergillus niger. The biological activities of M. suaveolens, after paying special attention to its most representative compound piperitenone oxide, have been extensively described by Božović et al. (Citation2015) and Spagnoletti et al. (Citation2016) for its antifungal and antioxidant activities. In addition, due to its low risk, it is used as food additive and flavouring agent.
Conclusion
Carvone and limonene were the major compounds in Mentha spicata (spearmint), menthol and menthone in M. piperita, and piperitone oxide and piperitenone oxide in M. suaveolens.
Botryotinia fuckeliana was the most sensitive of all the tested fungi, the three essential oils studied inhibited its growth. The Mentha suaveolens essential oil obtained the best antifungal activity results. The highest dose, 400 µg/mL, produced 100% MGI in all the studied fungi, except for Fusarium oxysporum with 94.21%. Mentha suaveolens might be an alternative for controlling Botryotinia fuckeliana, Curvularia hawaiiensis, Verticillium dahliae and Fusarium oxysporum in food and might, thus, extend their shelf life. Essential oil can be considered to develop a low-risk enviro-friendly botanical biofungicide.
Acknowledgements
The authors also thank the Spanish Type Culture Collection (CECT) for providing the molecular strain identification equipment. This study has been financed by MINECO, Ministerio de Economía y competitividad ‘Materiales biodegradables multicapa de alta barrera para el envasado activo de alimentos’ (AGL2016-76699-R).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability
The authors confirm that the data supporting the findings of this study are available in the Mendeley repository, Mendeley Data, (https://data.mendeley.com/datasets/98prxvbk8s/1).
Additional information
Funding
References
- Adams RP. 2007. Identification of essential oil components by gas chromatography/mass spectrometry. Carol Stream, IL, USA: Allured Publishing Corporation.
- Albuquerque CC, Camara TR, Willadino RDR, Ulises C. 2006. Antimicrobial action of essential oil of Lippia gracilis Schauer. Braz Arch Biol Technol. 49:527–535. doi:10.1590/S1516-89132006000500001.
- Andrade WP, Vieira GHC. 2016. Efeito dos óleos essenciais sobre a antracnose in vitro e em frutos de mamoeiro. Rev Bras Plantas Med. 18(1):367–372. doi:10.1590/1983-084X/15_089.
- Bardaweel SK, Bakchiche B, Al Salamat HA, Rezzoug M, Gherib A, Flamini G. 2018. Chemical composition, antioxidant, antimicrobial and antiproliferative activities of essential oil of Mentha spicata L. (Lamiaceae) from Algerian Saharan Atlas. BMC Complement Altern Med. 18:201. doi:10.1186/s12906-018-2274-x.
- Baser KHC, Kirimer N, Tümen G. 1998. Pulegone-rich essential oils of Turkey. J Essential Oil Res. 10(1):1–8. doi:10.1080/10412905.1998.9700830.
- Bayan Y, Küsek M. 2018. Chemical composition and antifungal and antibacterial activity of Mentha spicata L. volatile oil. Cien Inv Agr. 45(1):64–69. doi:10.7764/rcia.v45i1.1897.
- Behidj N, Chebouti N, Dahmene T, Daoudi R, Rahmoune E. 2018. Effect of the essential oil of Mentha piperita and Thymus numidicus on the growth of Fusarium sp. 18th International Multidisciplinary Scientific GeoConference SGboEM. 789–796. doi:10.5593/sgem2018/3.2
- Bouyahya A, Belmehdi O, Abrini J, Dakka N, Bakri Y. 2019. Chemical composition of Mentha suaveolens and Pinus halepensis essential oils and their antibacterial and antioxidant activities. Asian Pac J Trop Med. 12:117–122. doi:10.4103/1995-7645.254937.
- Božović M, Pirolli A, Ragno R. 2015. Mentha suaveolens Ehrh. (Lamiaceae) essential oil and its main constituent piperitenone oxide: biological activities and chemistry. Molecules. 20(5):8605–8633. doi:10.3390/molecules20058605.
- Brahmi F, Khodir M, Mohamed C, Pierre D. 2017. Chemical composition and biological activities of Mentha species. In: El-Shemy H, editor. Aromatic and medicinal plants. p. 47–78. doi:10.5772/67291.
- Chávez-González ML, Rodríguez-Herrera R, Aguilar CN. 2016. Essential oils: A natural alternative to combat antibiotics resistance in mechanisms and new antimicrobial approaches. In: Kateryna K, Mahendra R, editor. Antibiotic resistance. Cambridge, MA, USA: Academic Press; p. 227–237.
- De Oliveira KAR, Berger LRR, de Araújo SA, Câmara MPS, de Souza EL. 2017. Synergistic mixtures of chitosan and Mentha piperita L. essential oil to inhibit Colletotrichum species and anthracnose development in mango cultivar Tommy Atkins. Food Microbiol. 66:96–103. doi:10.1016/j.fm.2017.04.012.
- Desam NR, Al-Rajab AJ, Sharma M, Mylabathula MM, Gowkanapalli RR, Albratty M. 2019. Chemical constituents, in vitro antibacterial and antifungal activity of Mentha× piperita L. (peppermint) essential oils. J King Saud Univ-Sci. 31(4):528–533. doi:10.1016/j.jksus.2017.07.013.
- Dias ALB, Sousa WC, Batista HRF, Alves CCF, Souchie EL, Silva FG, Pereira PS, Sperandio EM, Cazal CM, Forim MR, et al. 2020. Chemical composition and in vitro inhibitory effects of essential oils from fruit peel of three Citrus species and limonene on mycelial growth of Sclerotinia sclerotiorum. Braz J Biol. 80(2):460–464. doi:10.1590/1519-6984.216848.
- Diasa ALB, Batistaa HRF, Sousaa WC, Bailaob EFL, Rochab JD, Sperandioa EM, Fernandesa CC, Souchiea EL, Mirandac MLD. 2020. Psidium myrtoides O. Berg fruit and leaves: physicochemical characteristics, antifungal activity and chemical composition of their essential oils in different seasons. Nat Prod Res. 1–5. doi:10.1080/14786419.2020.1844689.
- Ed-Dra A, Filali FR, Presti VL, Zekkori B, Nalbone L, Trabelsi N, Giarratana F. 2019. Evaluation of chemical composition, antioxidant and anti Listeria monocytogenes and Salmonella enterica activity of the essential oil of Mentha pulegium and Mentha. https://www.preprints.org/manuscript/201905.0017/v1.
- El-Kashoury ESA, El-Askary HI, Kandil ZA, Ezzat SM, Salem MA, Sleem AA. 2014. Chemical and biological study of Mentha suaveolens Ehrh. cultivated in Egypt. J Med Plants Res. 8(20):747–755. doi:10.5897/JMPR2014.5324.
- El-Kashoury ESA, El-Askary HI, Kandil ZA, Salem MA, Sleem AA. 2012. Chemical composition and biological activities of the essential oil of Mentha suaveolens Ehrh. Zeitschrift für Naturforschung C. 67(11-12):571–579. doi:10.1515/znc-2012-11-1207.
- Fialho RO, Papa MFS, Pereira DAS. 2015. Efeito fungitóxico de óleos essenciais sobre Phakopsora euvitis, agente causal da ferrugem da videira. Arq Inst Biol. 82:00211. Epub 15 de dezembro de 2015. doi:10.1590/1808-1657000702013.
- Karpiński TM. 2020. Essential oils of Lamiaceae family plantsas antifungals. Biomolecules. 10:103. doi:10.3390/biom10010103.
- Lawrence BM, editor. 2006. Mint: The Genus Mentha. Boca Raton (FL): CRC Press, Taylor & Francis Group. 598p.
- Llorens-Molina JA, Rivera Seclén CF, Vacas Gonzalez S, Boira Tortajada H. 2017. Mentha suaveolens EHRH. Chemotypes in eastern Iberian Peninsula: essential oil variation and relation with ecological factors. Chem Biodiversity. 14:1–9. doi:10.1002/cbdv.201700320.
- Lorenzetti ER, Monteiro FP, Souza PE, Souza RJ, Scalice HK, Diogo Jr R, Pires MSO. 2011. Bioatividade de óleos essenciais no controle de Botrytis cinerea isolado de morangueiro. Rev Bras Plantas Med. 13:619–627. doi:10.1590/S1516-05722011000500019.
- Luković J, Todorović B, Milijašević-Marčić S, Rekanović E, Kostić M, Đurović-Pejčev R, Potočnik I. 2019. Antifungal activity of plant essential oils against Verticillium Klebahn, the causal agent of Verticillium wilt of pepper. Pestic Phytomed (Belgrade). 34(1):39–46. doi:10.2298/PIF1901039L.
- Nikšić H, Durić K, Omeragić E, Nikšić H, Muratović S, Bečić F. 2018. Chemical characterization, antimicrobial and antioxidant properties of Mentha spicata L. (Lamiaceae) essential oil. Bull Chemi Technol Bosnia Herzegovina. 50:43–48.
- Oliveira Filho G, Nobre CCO, Silva GC, Azeredo HMC, Ferreira MD. 2019. Atividade antifúngica em fase de vapor de óleos essenciais e suas combinações contra Botrytis cinerea. São Carlos (SP): Simpósio Nacional de Instrumentação Agropecuária SIGRAO.
- Peixinho GS, Ribeiro VG, Amorim EP. 2017. Ação do óleo essencial de menta (Mentha arvensis) sobre o patógeno Lasiodiplodia theobromae em cachos de videira cv. Itália. Summa Phytopathol. 43(1):32–35. doi:10.1590/0100-5405/2204.
- Peixoto ITA, Furlanetti VF, Anibal PC, Duarte MCT, Höfling JF. 2009. Potential pharmacological and toxicological basis of the essential oil from Mentha spp. Rev Ciênc Farm Básica Apl. 30:235–239.
- Rachitha P, Krupashree K, Jayashree GV, Gopalan N, Khanum F. 2017. Growth inhibition and morphological alteration of Fusarium sporotrichioides by Mentha piperita essential oil. Phcog Res. 9:74–79. doi:10.4103/0974-8490.199771.
- Sharma V, Hussain S, Gupta M, Saxena A. 2014. In vitro anticancer activity of extracts of Mentha spp. against human cancer cells. Indian J Biochem Biophys. 51:416–419.
- Silva FFA, Alves CCF, Oliveira Filho JG, Vieira TM, Crotti AEM, Miranda MLD. 2019. Chemical constituents of essential oil from Murraya paniculata leaves and its application to in vitro biological control of the fungus Sclerotinia sclerotiorum. Food Sci Technol Campinas. 39(Suppl. 2):413–417. doi:10.1590/fst.20218.
- Spagnoletti A, Guerrini A, Tacchini M, Vinciguerra V, Leone C, Maresca I, Angiolella L. 2016. Chemical composition and bio-efficacy of essential oils from italian aromatic plants: Mentha suaveolens, Coridothymus capitatus, Origanum hirtum and Rosmarinus officinalis. Natural Prod Commun. 11(10):1517–1520. doi:10.1177/1934578X1601101023.
- Stringaro A, Colone M, Angiolella L. 2018. Antioxidant, antifungal, antibiofilm, and cytotoxic activities of Mentha spp. essential oils. Medicines. 5:112. doi:10.3390/medicinas5040112.
- Tucker AO. 2006. Mentha: economic uses. In: Lawrence BM, editor. Mint the genus Mentha. Boca Raton, FL: CRC Press, Taylor & Francis Group; p. 519–522.
- Tucker AO, Naczi RFC. 2006. Mentha: An overview of its classification and relationships. In: Lawrence BM, editor. Mint the genus Mentha. Boca Raton, FL: CRC Press, Taylor & Francis Group; p. 3–4.
- Valadares ACF, Alves CCF, Alves JM, De Deus IP, De Oliveira Filho JG, Dos Santos TCL, Dias HJ, Crotti AEM, Miranda ML. 2018. Essential oils from Piper aduncum inflorescences and leaves: chemical composition and antifungal activity against Sclerotinia sclerotiorum. Anais da Academia Brasileira de Ciências. 90(3):2691–2699. doi:10.1590/0001-3765201820180033.

