Abstract
Restenosis formation is initiated from intimal hyperplasia when arterial stretch and injury exist. In this study, we examined the role of CDKN1A-interacting zinc finger protein 1 (CIZ1) in neointimal hyperplasia in injured arteries. CIZ1 protein was up-regulated, and p21Cip1/Waf1 (p21) was down-regulated in the neointimal hyperplasia from a mouse femoral artery wire-injury model. In vitro, proliferation and migration were reduced in vascular smooth muscle cells transformed with Ciz1-shRNA lentiviral particles. In addition, p21 expression is increased, and MMP9 expression is decreased in vascular smooth muscle cells with CIZ1 knockdown. These data imply that CIZ1 might be a novel repressor of neointimal lesion formation caused by interventional therapy.
Introduction
Balloon angioplasty and stenting have been widely employed for restoring blood flow in occlusive vessels (Le et al. Citation10 Feb 2015). However, angioplasty and stent implantation usually cause arterial injuries, inflammation, and further neointimal hyperplasia, which plays a central role in the pathogenesis of restenosis (Gimbrone and Garcia-Cardena Citation2016). Thus, investigating the underlying molecular mechanisms of neointimal formation induced by arterial injury could be clinically beneficial for restenosis therapy.
Cdkn1A-interacting zinc finger protein 1 (CIZ1) is a novel interacting protein of CDK2 inhibitor p21Cip1/Waf1 (p21) (Mitsui et al. Citation22 Oct 1999). CIZ1 dysregulation has been observed in various human disorders, including Alzheimer’s disease (den Hollander et al. Citation2006; Dahmcke et al. Citation2008), cervical dystonia (Xiao et al. Citation2012), rheumatoid arthritis (Judex et al. Citation2003), hemangioma (Wang et al. Citation19 Nov 2018) and carcinomas, including breast cancer (den Hollander et al. Citation2006; Li et al. Citation2020), gallbladder cancer (Zhang et al. Citation2015; Chen et al. Citation2019), colon cancer (Wang et al. Citation2014), medulloblastoma, lung cancer and Ewing sarcoma and neuroblastoma (Higgins et al. Citation2012; Zhou et al. Citation2018; Chen et al. Citation2020). CIZ1 is necessary for DNA replication initiation and cell cycle progression during the G1-S phase transition (Copeland et al. Citation2010), which is inhibited by the knockdown of CIZ1 (Coverley et al. Citation2005). CIZ1 is also considered as a co-activator of transcription factors due to its DNA-binding activity (Warder and Keherly Citation2003).
The biological functions of CIZ1 in the development of these diseases could be closely related to its binding partners. p21 interacts with a 150-amino acids region containing the first zinc-finger motif on CIZ1 protein. Recruitment of CIZ1 increases the cytoplasmic distribution of p21 caused by nucleus-cytoplasm translocation (Mitsui et al. Citation22 Oct 1999). p21 is a well-known CDK2 and CDK1 inhibitor and functions in various biological activities through regulating cell cycle progression. Moreover, p21 also interacts with proliferating cell nuclear antigen (PCNA), impairs PCNA-dependent DNA polymerase activity, thereby disrupting DNA replication and various PCNA-dependent DNA repair processes (Abbas and Dutta Citation2009). In addition, p21 may alter the proliferative responses of VSMCs to arterial injuries (Sun et al. Citation2012). A previous study found that p21 decreases cell proliferation in animal models of arterial injury by modulating Rb phosphorylation and disrupting the p21/PCNA complex (Chang et al. Citation1995).
Although dysregulation of CIZ1 is reported in various diseases, its expression profile and functional implications in neointimal hyperplasia remain elusive. Here, we examined the role of CIZ1 in regulating VSMC migration and proliferation in neointimal hyperplasia in response to injuries. We found that CIZ1 was down-regulated in an intimal hyperplasia mouse model. Knockdown of CIZ1 attenuated VSMC proliferation and migration, which was correlated with up-regulated p21 and down-regulated MMP9. Our findings indicated that CIZ1 was a novel functional factor in the pathology of intimal hyperplasia and hyperproliferative restenosis.
Materials and methods
Murine model of femoral artery wire injury
Mice were maintained in Medical Research Center at Shandong Provincial Qianfoshan Hospital in a controlled, specific pathogen-free environment. Animal procedures were approved by the ethic committee of Shandong Provincial Qianfoshan Hospital (2016-S110) and performed according to ARRIVE guidelines and the AVMA euthanasia guidelines 2013 (for management of humane endpoints). For unilateral femoral artery wire injury, mice anesthetized by intraperitoneal injection of 10% chloral hydrate (350 mg/kg), and left femoral arteries were isolated under aseptic conditions. A branch of the femoral artery was partially transected to introduce a 0.015-inch diameter sterile wire (Cook Critical Care, Bloomington, IN). The wire was passed within the vessels thirty times and then removed. The arterial branch was ligated, and the incision was closed (Le et al. Citation10 Feb 2015).
Tissue processing
We performed carbon dioxide asphyxiation on the mice at 28 days post-surgery. Mice were placed in a new cage with corn cob bedding and immediately euthanized by air displacement with 100% carbon dioxide at a rate of 20%/minute of chamber volume until the mice lost breathing and heart beating. The femoral arteries were in situ perfused with cold phosphate-buffered saline (PBS) three times and fixed with 2% paraformaldehyde at 4°C for 1 hour. The femoral arteries were stored at −80°C.
Cell culture and lentiviral infection
Mouse VSMCs (MOVAS) were purchased from ATCC (Manassas, VA) and cultured in DMEM (Gibco, Karlsruhe, Germany) containing 10% FBS (Gibco, Karlsruhe, Germany), 100 IU/mL penicillin and 0.1 mg/mL streptomycin, under 37°C and 5% CO2 atmosphere. To establish a stable-transformation cell line with CIZ1 knocked down, VSMCs were incubated with RNAi lentiviral particles, containing a puromycin-resistant gene, for 24 hours. The medium, containing lentiviral particles, was replaced with a fresh medium, and the cells were further cultured for 2 days. Then 1 μg/mL puromycin (Sigma, St. Louis, MO) was used to screen the stably-transformed cells. The stable-transformation cell lines were maintained in a medium containing 0.1 μg/mL puromycin. 48 hours after infection, cells were harvested for protein extraction and RNA isolation.
Immunohistochemistry
For frozen sections, tissue was embedded in Tissue-Tek OCT (optimum cutting temperature) medium, frozen on dry ice, and cut into 5-μm sections. The sections were fixed in acetone for 10 minutes, and then stained according to H&E protocol. Find the hyperplastic intima under a microscope (Olympus Corporation) and apply immunohistochemical staining. To quench endogenous peroxidase activity, the slices were incubated with 3% H2O2 for 10 minutes. The sections were incubated with the anti-CIZ1 antibody (Cat# ab102013, Abcam, Cambridge, MA, RRID: AB_10711716) at 4 °C overnight. The sections were then washed with PBS three times. Positive staining was visualized using a detection kit based on the streptavidin–biotin method (Cat# SP-9001, ZSGB-Bio, Beijing, China, RRID: AB_2758396).
Western blotting
Western blotting was done according to a previous protocol (Liu et al. Citation2014). Briefly, tissues were lysed in ice-cold RIPA buffer containing 0.1% SDS. The BCA assay (Bio-Rad) determined protein concentration. 20µg of total protein was loaded and separated on 10% SDS-PAGE. Proteins were transferred onto PVDF membrane under a constant voltage of 100 volts for 2 hours. Membranes were blocked with 10% skim milk in TBS-T (TBS containing 0.1% Tween 20) and incubated with primary antibody overnight at 4°C. Primary antibodies: rabbit anti-CIZ1 antibody (ab102013, Abcam, RRID: AB_10711716), anti-p21 antibody (Cat# ab188224, Abcam, RRID: AB_2734729), anti-MMP9 antibody (Cat# ab38898, Abcam, RRID: AB_776512), and anti-β-actin antibody (A1978, Sigma-Aldrich, St. Louis, MO; RRID: AB_476692). Second antibodies conjugated with horseradish peroxidase were purchased from Santa Cruz Biotechnology, Inc (Cat# sc-2004, RRID: AB_631746). The protein bands were detected with a Bio-Rad imaging system (Bio-Rad, Hercules, CA) and quantified with enclosed software.
Quantitative RT–PCR (qRT-PCR)
Total RNA purification and the first strand of cDNA were synthesized using the RNeasy Mini kit (Qiagen) and High Capacity RNA-to-cDNA Master Mix (Applied Biosystems), respectively. Quantitative PCR was done on a ViiA7 Real-Time PCR machine (Applied Biosystems). Reaction parameters were 95°C 5 minutes, 95°C for 10 seconds, 60°C for 20 seconds and 72°C for 5 seconds, 40 cycles from step 2 to step 4. β-actin was used as the internal control. The relative primer (mice) sequences were as follows:
Ciz1 forward primer, 5′-GAGAGATGCTGGGATGTGG-3′
Ciz1 reverse primer, 5′-GTCCAGATGTGAGGGGTGTG-3′
p21 forward primer, 5′-CCTGGTGATGTCCGACCTG-3′
p21 reverse primer, 5′-CCATGAGCGCATCGAATC-3′
β-actin forward primer, 5′-GGATGCAGAAGGAGATTACTGC-3′
β-actin reverse primer, 5′-CCACCGATCCACACAGAGTA-3′
MMP9 forward primer, 5′-GGACCCGAAGCGGACATTG-3′
MMP9 reverse primer, 5′-CGTCGTCGAAATGGGCATCT-3′
Cell proliferation assay
Cell proliferation was evaluated with the MTT assay kit (Cayman Chemical, Ann Arbor, MI). Cells were seeded into a 96-well plate at a density of 5 × 10³ cells/well. 24 hours later, the medium was removed, and the cells were washed with PBS. MTT solution (10 µl of 5 mg/mL) was added to each well and incubated for 2 hours. Formazan was dissolved in 10% SDS (PH4.0). Colorimetric intensity at 570 nm was analyzed using a 96-well plate reader (Molecular Devices, Sunnyvale, CA). For the EdU assay, the cells were incubated in 50 nmol/L EdU solution (RiboBio, Guangzhou, China) for 2 hours and analyzed with a flow cytometer (BD FACSAria III, BD biosciences, Franklin Lakes, NJ).
VSMC migration assay
VSMCs were serum-starved for 24 hours and seeded into 6-well plates. Scratches were made using sterile pipette tips after cells reached confluency. Cells were washed with PBS for two times and cultured in basic DMEM without serum. Images were captured at fixed points of the wound at 24 hours after scratching. Image J software (NIH, Bethesda, MD) was used to estimate the healing area.
Statistical analysis
All the experiments were repeated at least three times. All statistical analyses were calculated through IBM SPSS Statistics 23 software (SPSS Inc., Chicago, IL, RRID: SCR_002865). Student t-test was used to evaluate the significance between groups. P < .05 was considered significant. Data were shown as means ± SEM.
Results
CIZ1 protein was up-regulated and p21 was down-regulated in intimal hyperplasia in mice following mechanical injury
We investigated the expression of CIZ1 in the newly formed intima of intimal hyperplasia mouse model caused by wire injury. H&E staining of the injured femoral artery showed that intimal hyperplasia could be obviously observed at 28 days after surgery (Figure (a)). To better understand the expression pattern of CIZ1 and p21, the hyperplastic vessels were isolated and immunostained for CIZ1. Immunohistochemical staining results showed that the CIZ1 protein level was increased by 25% in intimal hyperplasia (Figure (b)). As shown by qRT-PCR, Ciz1 mRNA level was up-regulated in hyperplasic arteries compared with normal arteries (P < .01) (Figure (a)), while p21 expression level in hyperplasia arteries was significantly down-regulated (P < .05) (Figure (b)). As for Western blotting, CIZ1 expression levels were up-regulated in hyperplasia arteries than normal arteries (1.28 fold) (P < .01) (Figure (c, d)), and p21 expression levels down-regulated in hyperplasia arteries compared with normal arteries (0.43 fold) (P < .05) (Figure (e, f)). Our results demonstrated that CIZ1 expression was increased, and p21 expression was decreased in intimal hyperplasia.
Figure 1. Representative images of the mouse artery 28 days after the procedure of femoral artery wire injury. (a) H&E staining of the arteries sections from sham and injured femoral arteries. Arrows refer to in-stent restenosis (arrow). (b) Immunohistochemical staining of CIZ1 on sections from sham and injured femoral arteries (arrow). The lower panel was the bar graph showing the IOD (sum)/area (sum) of each group. IOD, integrated optical density. n = 3, *, P < .05.
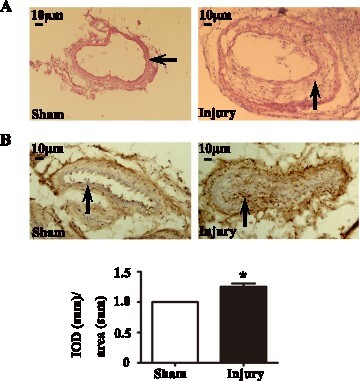
Figure 2. CIZ1 expression was up-regulated, and p21 expression was down-regulated in mouse arteries after injury with neointimal hyperplasia. The mRNA levels of Ciz1 (a) and p21 (b) in sham and injured arteries as determined by RT-PCR. n = 3, *, P < .05. Western blot analysis determines CIZ1 (c) and p21 (e) protein levels in sham and injured arteries. β-actin was used as an internal control. Densitometric analysis of the blots of CIZ1 (d) and p21 (f). n = 3, *, P < .05.
Knockdown of CIZ1 expression reduced VSMC proliferation
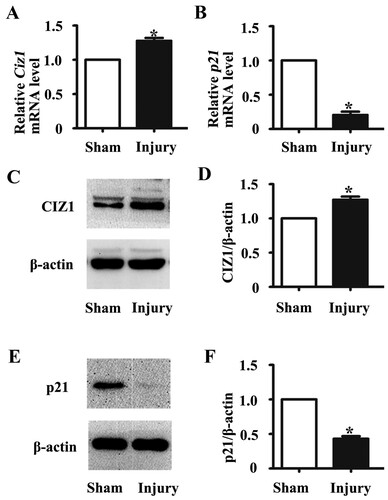
To investigate the role of CIZ1 in VSMC proliferation, we infected VSMCs with lentiviral particles to knock down the expression of CIZ1 and performed MTT and EdU assays. Reduction of CIZ1 was determined by western blotting at 48 h post-infection (Figure (a)). The Ciz1 mRNA exhibited a 40% reduction (P < .05) (Figure (b)). Down-regulation of CIZ1 reduced VSMC proliferation rate by 0.72-fold as determined by MTT in VSMCs (P < .05) (Figure (c)). In addition, EdU staining showed that knockdown of CIZ1 in VSMC significantly reduced the proliferating cells by 15.86% (P < .05) (Figure (d, e)). Our results are consistent with the previous finding that CIZ1 influences cell proliferation rate by modulating S phase entry (Coverley et al. Citation2005).
Figure 3. Knockdown of CIZ1 expression reduced VSMC proliferation. (a) Western blot of CIZ1 protein from VSMCs transformed with Ciz1-shRNA lentiviral particles. β-actin was used as an internal control. (b) RT-PCR of Ciz1 gene of VSMCs infected with Ciz1-shRNA lentiviral particles. n = 4, *, P < .05. (c) Percentage of cell number evaluated using MTT assay after 24 h of Ciz1-shRNA lentiviral infection. n = 3, *, P < .05. (d) Representative images of EDU assay after 24 h of Ciz1-shRNA lentiviral infection. (e) Bar graph of EDU assays. n = 3, *, P < .05.
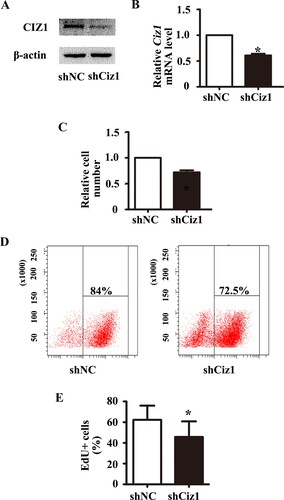
Figure 4. Knockdown of CIZ1 inhibited VSMCs’ migration. (a) Representative image of wound healing assay of VSMCs infected with Ciz1-shRNA lentiviral particles. (b) Bar graph of wound healing assay. n = 3, *, P < .05.
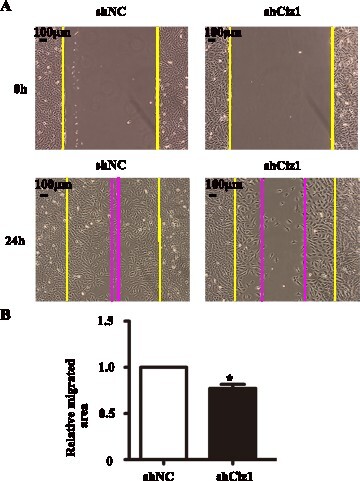
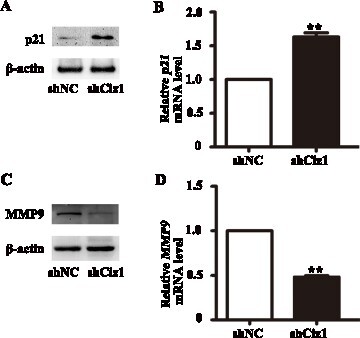
Figure 5. p21 and MMP9 expression in VSMCs infected with Ciz1-shRNA lentiviral particles. 48 hours after infection, cells were harvested for further detection. (a, c) Western blot of p21 (a) and MMP9 (c) protein of VSMCs infected with Ciz1-shRNA lentiviral particles. (b, d) The mRNA level of p21 (b) and MMP9 (d) in VSMCs infected with Ciz1-shRNA lentiviral particles was determined by RT-PCR. n = 5, **, P < .01.
Knockdown of CIZ1 expression inhibits VSMC migration
As migration of VSMCs is also necessary for the formation of intimal hyperplasia, we performed the scratch healing assay to evaluate VSMC migration. Figure (a and b) show that the healing area of VSMC with CIZ1 knockdown was significantly reduced (0.77 fold) (P < .05), implying that CIZ1 is necessary for VSMC migration.
Knockdown of CIZ1 up-regulates p21 and down-regulates MMP9
Furthermore, we detected the expression levels of p21 and MMP9 by western blotting and qRT-PCR. When CIZ1 expression was reduced, the protein level of p21 was increased (Figure (a and b)) and MMP9 was decreased (Figure (c, d)) in VSMCs compared with normal control. The mRNA expression level of p21 was increased (1.65 folds) (P < .01) (Figure (b)), while the mRNA expression level of MMP9 was decreased in VSMCs compared with the control (0.5 fold) (P < .01) (Figure (d)). These results imply that p21 and MMP9 could be involved in CIZ1-mediated VSMC proliferation and migration.
Discussion
CIZ1 participates in DNA replication initiation, DNA replicative complex formation, and S phase entry during the cell cycle (Ainscough et al. Citation2007; Liu et al. Citation2016). Several transcriptional and cell cycle regulators, including cyclin E, cyclin A, CDK2, p21 and DHX9, interact with CIZ1 to modulate cell proliferation (Pauzaite et al. Citation27 Dec 2016; Thacker et al. Citation2020). In various diseases, dysregulation or point mutations of CIZ1 were reported, suggesting that CIZ1 might be a potential therapeutic target (Liu et al. Citation2016). The present study aimed to investigate the functional roles of CIZ1 and relative mechanisms of CIZ1 in neointimal hyperplasia, a key process of restenosis. We showed that CIZ1 expression is significantly increased in vascular tissue after injury. Knockdown, the expression of CIZ1, inhibits the VSMCs’ proliferation and migration. Therefore, CIZ1 may act as a novel inducer of neointimal hyperplasia. Beyond these in vitro functional studies of CIZ1 knockdown in VSMC proliferation and migration, in vivo evidences that whether repression of CIZ1 can ameliorate intimal hyperplasia and VSMC phenotypic transformation is still lacking. Thus, the therapeutic potential of CIZ1 in intimal hyperplasia is still elusive.
In the formation of neointimal hyperplasia, VSMC is the central executive cell type and featured by increased proliferation and migration. p21 is a master negative regulator of cell cycle and proliferation through its two structural domains: the C-terminal PCNA-binding domain and the amino-terminal CDK-cyclin inhibitory domain (Luo et al. Citation1995). It competes with PCNA to bind with DNA polymerase-δ and directly inhibits DNA synthesis and arrests cell cycle at the S phase (Moldovan et al. Citation2007). p21 is closely related to neointimal hyperplasia by regulating VSMC proliferation. Studies find that the p21 protein level is decreased in the balloon-injured arteries (Sun et al. Citation2016). Induction of p21 expression could limit VSMC proliferation and prevent neointima formation after endovascular arterial injury. Our study found that p21 is dramatically decreased in neointimal hyperplasia, which was consistent with previous studies, supporting that p21 plays a negative regulator of cell cycle and neointimal hyperplasia. However, there is still no evidence indicating whether CIZ1 can regulate the expression of p21. The results of in vitro studies could not wholly simulate in vivo pathological conditions as the long-term (28 days) of neointimal formation in mice after wire injury. Thus, further efforts are needed to evaluate the biological functions of CIZ1 and p21 in mice or other in vivo models of neointimal hyperplasia.
Although CIZ1 and p21 could form complexes (Mitsui et al. Citation22 Oct 1999), they may function independently under pathological circumstances. Our study showed that CIZ1 and p21 exhibited a contrary expression profile. CIZ1 facilitates DNA replication initiation in cells with deregulated CDK activity (Coverley et al. Citation2002), and regulates CDK2 activity in S phase entry of cell cycle (den Hollander and Kumar Citation2006). p21 inhibits G1-S transition mainly by inhibiting CDK2 activity, which is required for the activity of proteins directly involved in DNA synthesis. p21 regulates gene expression by protein–protein interactions independent of CDKs and PCNA (Abbas and Dutta Citation2009; Karimian et al. Citation2016). The interaction between CIZ1 and p21 was inhibited by the overexpression of CDK2 (Mitsui et al. Citation22 Oct 1999). The interactions of CDK2 and CIZ1 regulate the levels of nuclear p21 (den Hollander and Kumar Citation2006). These results imply CIZ1 might act as a competitor of p21 to bind CDK2 during DNA replication initiation and cell cycle progression and promote VSMC proliferation and neointimal hyperplasia formation. However, the query of whether CIZ1 regulates the expression level of p21 remains to be elucidated.
Matrix metalloproteinases (MMPs) cleave and degrade extracellular matrix (ECM) components and facilitate cell migration and proliferation. Fibroblasts cells, VSMCs, and leukocytes cells can secrete MMPs. MMP-2/9 are abundantly expressed in invasive cancer cells (Kessenbrock et al. Citation2010). LAP3 (Leucine aminopeptidase 3) induces migration and invasion of breast cancer cells by activating MMP-2/9 expression (Fang et al. Citation2019). MMP9 promotes VSMC proliferation and migration (Wang and Khalil Citation2018). In our studies, decreased CIZ1 down-regulates the expression of MMP9 and reduces the VSMC proliferation and migration. The relationship between CIZ1 and MMP9 has not been reported in the current literature; thus, further investigation is necessary to address the relative molecular mechanism.
Data availability statement
The data that support the findings of this study are openly available in Harvard Dataverse at https://doi.org/10.7910/DVN/J75LUM.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abbas T, Dutta A. Jun 2009. P21 in cancer: intricate networks and multiple activities. Nat Rev Cancer. 9:400–414. Epub 2009/05/15.
- Ainscough JF, Rahman FA, Sercombe H, Sedo A, Gerlach B, Coverley D. 1 Jan 2007. C-terminal domains deliver the DNA replication factor Ciz1 to the nuclear matrix. J Cell Sci. 120:115–124. Epub 2006/12/22.
- Chang MW, Barr E, Lu MM, Barton K, Leiden JM. Nov 1995. Adenovirus-mediated over-expression of the cyclin/cyclin-dependent kinase inhibitor, p21 inhibits vascular smooth muscle cell proliferation and neointima formation in the rat carotid artery model of balloon angioplasty. J Clin Invest. 96:2260–2268. Epub 1995/11/01.
- Chen X, Wang P, Wang S, Li J, Ou T, Zeng X. 30 Nov 2019. CIZ1 knockdown suppresses the proliferation of bladder cancer cells by inducing apoptosis. Gene. 719:143946. Epub 2019/06/30.
- Chen YR, Wu YS, Wang WS, Zhang JS, Wu QG. Mar 2020. Upregulation of lncRNA DANCR functions as an oncogenic role in non-small lung cancer by regulating miR-214-5p/CIZ1 axis. Eur Rev Med Pharmacol Sci. 24:2539–2547. Epub 2020/03/21.
- Copeland NA, Sercombe HE, Ainscough JF, Coverley D. 1 Apr 2010. Ciz1 cooperates with cyclin-A-CDK2 to activate mammalian DNA replication in vitro. J Cell Sci. 123:1108–1115. Epub 2010/03/11.
- Coverley D, Laman H, Laskey RA. Jul 2002. Distinct roles for cyclins E and A during DNA replication complex assembly and activation. Nat Cell Biol. 4:523–528. Epub 2002/06/25.
- Coverley D, Marr J, Ainscough J. 1 Jan 2005. Ciz1 promotes mammalian DNA replication. J Cell Sci. 118:101–112. Epub 2004/12/09.
- Dahmcke CM, Buchmann-Moller S, Jensen NA, Mitchelmore C. Aug 2008. Altered splicing in exon 8 of the DNA replication factor CIZ1 affects subnuclear distribution and is associated with Alzheimer's disease. Mol Cell Neurosci. 38:589–594. Epub 2008/06/28.
- den Hollander P, Kumar R. 1 Jun 2006. Dynein light chain 1 contributes to cell cycle progression by increasing cyclin-dependent kinase 2 activity in estrogen-stimulated cells. Cancer Res. 66:5941–5949. Epub 2006/06/03.
- den Hollander P, Rayala SK, Coverley D, Kumar R. 15 Nov 2006. Ciz1, a Novel DNA-binding coactivator of the estrogen receptor alpha, confers hypersensitivity to estrogen action. Cancer Res. 66:11021–11029. Epub 2006/11/17.
- Fang C, Zhang J, Yang H, Peng L, Wang K, Wang Y, Zhao X, Liu H, Dou C, Shi L, et al. Mar 2019. Leucine aminopeptidase 3 promotes migration and invasion of breast cancer cells through upregulation of fascin and matrix metalloproteinases-2/9 expression. J Cell Biochem. 120:3611–3620. Epub 2018/11/13.
- Gimbrone MA Jr, Garcia-Cardena G. 19 Feb 2016. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 118:620–636. Epub 2016/02/20.
- Higgins G, Roper KM, Watson IJ, Blackhall FH, Rom WN, Pass HI, Ainscough JF, Coverley D. 6 Nov 2012. Variant Ciz1 is a circulating biomarker for early-stage lung cancer. Proc Natl Acad Sci U S A. 109:E3128–E3135. Epub 2012/10/18.
- Judex M, Neumann E, Lechner S, Dietmaier W, Ballhorn W, Grifka J, Gay S, Scholmerich J, Kullmann F, Muller-Ladner U. Jan 2003. Laser-mediated microdissection facilitates analysis of area-specific gene expression in rheumatoid synovium. Arthritis Rheum. 48:97–102. Epub 2003/01/16.
- Karimian A, Ahmadi Y, Yousefi B. Jun 2016. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair (Amst). 42:63–71. Epub 2016/05/09.
- Kessenbrock K, Plaks V, Werb Z. 2 Apr 2010. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 141:52–67. Epub 2010/04/08.
- Le V, Johnson CG, Lee JD, Baker AB. 10 Feb 2015. Murine model of femoral artery wire injury with implantation of a perivascular drug delivery patch. J Vis Exp. 96:e52403.
- Li Y, Zhou X, Liu J, Gao N, Yang R, Wang Q, Ji J, Ma L, He Q. 1 May 2020. Dihydroartemisinin inhibits the tumorigenesis and metastasis of breast cancer via downregulating CIZ1 expression associated with TGF-beta1 signaling. Life Sci. 248:117454. Epub 2020/02/24.
- Liu J, Dong F, Jeong J, Masuda T, Lobe CG. Sep 2014. Constitutively active Notch1 signaling promotes endothelialmesenchymal transition in a conditional transgenic mouse model. Int J Mol Med. 34:669–676. Epub 2014/06/28.
- Liu Q, Niu N, Wada Y, Liu J. 5 Feb 2016. The role of Cdkn1A-Interacting Zinc Finger Protein 1 (CIZ1) in DNA replication and pathophysiology. Int J Mol Sci. 17:212. Epub 2016/02/11.
- Luo Y, Hurwitz J, Massague J. 11 May 1995. Cell-cycle inhibition by independent CDK and PCNA binding domains in p21Cip1. Nature. 375:159–161. Epub 1995/05/11.
- Mitsui K, Matsumoto A, Ohtsuka S, Ohtsubo M, Yoshimura A. 22 Oct 1999. Cloning and characterization of a novel p21(Cip1/Waf1)-interacting zinc finger protein, ciz1. Biochem Biophys Res Commun. 264:457–464. Epub 1999/10/26.
- Moldovan GL, Pfander B, Jentsch S. 18 May 2007. PCNA, the maestro of the replication fork. Cell. 129:665–679. Epub 2007/05/22.
- Pauzaite T, Thacker U, Tollitt J, Copeland NA. 27 Dec 2016. Emerging roles for Ciz1 in cell cycle regulation and as a driver of tumorigenesis. Biomolecules. 7(1):1.
- Sun L, Zhao R, Zhang L, Zhang T, Xin W, Lan X, Huang C, Du G. 14 Mar 2012. Salvianolic acid A inhibits PDGF-BB induced vascular smooth muscle cell migration and proliferation while does not constrain endothelial cell proliferation and nitric oxide biosynthesis. Molecules. 17:3333–3347. Epub 2012/03/16.
- Sun L, Zhao R, Zhang L, Zhang W, He G, Yang S, Song J, Du G. 1 Mar 2016. Prevention of vascular smooth muscle cell proliferation and injury-induced neointimal hyperplasia by CREB-mediated p21 induction: An insight from a plant polyphenol. Biochem Pharmacol. 103:40–52. Epub 2016/01/26.
- Thacker U, Pauzaite T, Tollitt J, Twardowska M, Harrison C, Dowle A, Coverley D, Copeland NA. 22 Oct 2020. Identification of DHX9 as a cell cycle regulated nucleolar recruitment factor for CIZ1. Sci Rep. 10:18103. Epub 2020/10/24.
- Wang DQ, Wang K, Yan DW, Liu J, Wang B, Li MX, Wang XW, Liu J, Peng ZH, Li GX, et al. Jul 2014. Ciz1 is a novel predictor of survival in human colon cancer. Exp Biol Med (Maywood). 239:862–870. Epub 2014/06/15.
- Wang X, Khalil RA. 2018. Matrix metalloproteinases, vascular remodeling, and vascular disease. Adv Pharmacol. 81:241–330. Epub 2018/01/10.
- Wang Y, Li X, Zhang J, Liu Q, Gao P, Li D, Zhang S, Liu J. 19 Nov 2018. CIZ1 expression is upregulated in hemangioma of the tongue. Pathology Oncology Research: POR. 25(4):1653–1658.
- Warder DE, Keherly MJ. Jul-Aug 2003. Ciz1, Cip1 interacting zinc finger protein 1 binds the consensus DNA sequence ARYSR(0-2)YYAC. J Biomed Sci. 10:406–417. Epub 2003/06/26.
- Xiao J, Uitti RJ, Zhao Y, Vemula SR, Perlmutter JS, Wszolek ZK, Maraganore DM, Auburger G, Leube B, Lehnhoff K, et al. Apr 2012. Mutations in CIZ1 cause adult onset primary cervical dystonia. Ann Neurol. 71:458–469. Epub 2012/03/27.
- Zhang D, Wang Y, Dai Y, Wang J, Suo T, Pan H, Liu H, Shen S, Liu H. Apr 2015. CIZ1 promoted the growth and migration of gallbladder cancer cells. Tumour Biol. 36:2583–2591. Epub 2014/11/28.
- Zhou X, Liu Q, Wada Y, Liao L, Liu J. Jan 2018. CDKN1A-interacting zinc finger protein 1 is a novel biomarker for lung squamous cell carcinoma. Oncol Lett. 15:183–188. Epub 2017/12/30.
