Abstract
Introduction: Optic nerve gliomas (ONGs) are uncommon tumors of the central nervous system in adults. The aim of this study was to define their characteristics, prognostic factors, and the impacts of adjuvant radiotherapy (RT) and chemotherapy on outcomes.
Methods: Adult patients (age ≥18 years) with ONGs from the Surveillance, Epidemiology, and End Results (SEER) database were included. Univariate and multivariate Cox regression models were utilized to analyze the factors associated with survival. Kaplan-Meier method was used to evaluate the impacts of adjuvant therapies on overall survival (OS).
Results: A total of 179 adult patients diagnosed with ONGs were identified between 1991 and 2016, with a median follow-up period of 64.0 months. Multivariate analysis showed age at diagnosis, tumor grade, adjuvant chemotherapy were significant factors for OS. The employment of adjuvant RT or chemotherapy significantly shortened OS time in the low-grade group and could not prolong OS time in the high-grade group.
Conclusions This is the largest retrospective study of adult ONGs up to date. The overall prognosis of high-grade ONGs in adult patients is still poor despite multi-modality treatments. Adjuvant RT or chemotherapy should be applied cautiously before full clinical evaluation in adult patients with low-grade ONGs.
Introduction
Optic nerve gliomas (ONGs) are relatively rare neoplasms, accounting for only 1% of all intracranial tumors and 2% – 5% of childhood central nervous system tumors. (Farazdaghi et al. (Citation2019 Sep); Nair et al. (Citation2014 Aug); Dolecek et al. (Citation2012 Nov); Binning et al. (Citation2007)). Histopathological types vary, ranging from the most common WHO grade I pilocytic astrocytoma to uncommon grade IV high-grade glioblastoma (Louis et al. (Citation2016 Jun). Cummings et al. (Citation2000 May)). The overall incidence of ONGs is estimated at 0.73 per 100000 person-years in the populations of ages 0–19 in the United States (Peckham-Gregory et al. (Citation2018 Jun)). They preferentially occur in the pediatric population, particularly the first decade, and the majority are low-grade lesions (Shapey et al. (Citation2011 Dec)). ONGs can either be sporadic (Acharya et al. (Citation2019 Sep)), or in association with Neurofibromatosis-1 (NF-1) (Azizi et al. (Citation2021 jan Jul Citation2021 jan)), and the typical symptoms include decreased visual acuity, optic nerve atrophy, unilateral visual loss, or proptosis (Wilhelm (Citation2009 Feb); Ertiaei et al. (Citation2016)). In addition, they also interfere with normal pubertal development in childhood.
The optional treatment of ONGs remains controversial, ranging from clinical observation, surgical resection, chemotherapy, and radiotherapy (RT), due to the unpredictable course (Farazdaghi et al. (Citation2019 Sep); Thomas et al. (Citation2015 Feb)). The treatment is generally commenced when radiological tumor progression or clinical deterioration occurs, especially visual decline (Grill et al. (Citation2000 Sep)). For children, chemotherapy has been the first-line treatment over the past two decades because of fewer adverse side effects than occurs with RT (Binning et al. (Citation2007); Nicolin et al. (Citation2009 Dec Citation15); Dodgshun et al. (Citation2015 Dec Citation1)). While in adults, ONGs are rare, and the treatment for which has historically been approached in a different manner than children (Hidalgo et al. (Citation2019 jun Jun Citation2019 jun); Shofty et al. (Citation2014 Mar); Traber et al. (Citation2015 Jul)). However, the definitive efficacy of adjuvant RT or chemotherapy is still inconclusive due to the paucity of research data. Also, because ONGs are relatively uncommon in adults, it is still challenging to perform prospective studies now or soon.
In the current study, we aim to report the largest series of adult patients with ONGs based on the Surveillance, Epidemiology, and End Results (SEER) database. To better understand these rare tumors, our retrospective study investigated the epidemiology, prognostic factors, and in particular, the impact of adjuvant RT and chemotherapy on outcomes further to define the role of adjuvant therapies in adult ONGs.
Materials and methods
Study population
The data information for this study was obtained from the recent SEER database, which provides clinical incidence, treatment, and survival data on many tumors and covering nearly 36.7% of the US population according to the 2010 census, and is maintained by the National Cancer Institute, Division of Cancer Control and Population Sciences, Surveillance Research Program, Surveillance Systems Branch. We included data from the incidence SEER 18 registries custom data (with additional treatment fields).
Inclusion criteria, exclusion criteria, and data collection
Only adult patients (age ≥ 18 years) diagnosed with glioma (International Classification of Diseases for Oncology, 3rd Edition histology codes 9380 - 9442) and primary site labeled with the optic nerve (code C72.3) between January 1, 1991, and December 31, 2016, were included in the present study. Patients with more than one primary tumor were excluded from the present study. Data collected for analysis included age at diagnosis, gender, marital status, race, year of diagnosis, tumor site, tumor grade, surgery, adjuvant treatment (RT and chemotherapy), and overall survival (OS).
Data analysis and statistical methods
We categorized age as 18–29, 30–39, 40–49, 50–59, and ≥ 60 years. The race was categorized into white, others (including black and American Indian/Alaska Native or Asian/Pacific Islander), and unknown. The year of diagnosis was divided into three categorical groups: 1991–1999, 2000–2009, and 2010–2016. The tumor site was categorized into unilateral, bilateral, and unknown. The extent of surgical resection was divided into five groups, including gross total resection (GTR), subtotal resection (STR), biopsy, no surgery, and unknown on the basis of SEER surgery codes guidelines and another previous study (Mishra et al. (Citation2012 May)). For further survival analysis, patients with the unknown grade, unknown survival time, unknown surgery (code 90 and 99), and unknown radiation record were excluded, and we categorized age as ‘< 50 years’ and ‘≥ 50 years’, according to the previous literature (Mishra et al. (Citation2012 May)).
Baseline patient characteristics were summarized by standard descriptive statistics and frequency tabulation. Overall survival analysis was measured by using the Kaplan–Meier method and compared with the log-rank test. Univariate and multivariate Cox regression models were utilized to assess the effect of variables of interest on OS. All statistical analyses were carried out in SPSS software version 25.0 (IBM Corp., Armonk, NY, USA), and the statistically significant standard was P < 0.05. Ethical approval or informed consent was waivered for this study because of the de-identified information of the patients included in the SEER.
Results
Clinical characteristics
A total of 179 adult patients diagnosed with ONGs were identified between 1991 and 2016. No patients with ONGs were recorded in the SEER database prior to 1991. Of the whole population, the mean and median ages at diagnosis were 42.1 and 41.0 years (range, 18–101 years), respectively, with 55.9% of patients being female, 78.2% white people, and 43.6% of married status (Table ). 116 patients (64.8%) were located unilaterally regarding the tumor site, and 12 patients (6.7%) were bilateral. For tumor grade, 81.6% had a low-grade tumor, and 11.2% had a high-grade tumor. After excluding 18 patients with unknown information, the remaining patients included 142 (88.2%) low-grade tumors and 19 (11.8%) high-grade tumors. In the low-grade tumor group, 78.2% of patients were less than 50 years; however, 89.5% of patients were more than 50 years in the high-grade group.
Table 1. Clinical characteristics of adult patients with optic nerve gliomas.
Treatment strategy
In the present study, 15.1% of the patients underwent GTR, 6.1% of the patients underwent STR, 4.5% had a biopsy, and 72.6% had no surgery. For adjuvant therapies, RT was used in 43.6% of patients, and 26 patients (14.5%) had chemotherapy (Table ). Besides, according to the different tumor grades, 38.7% and 11.3% of the patients in the low-grade group received adjuvant RT and chemotherapy, respectively. While in the high-grade group, the proportion of the patients who received adjuvant RT and chemotherapy was 68.4% and 47.4%, respectively.
Overall survival analysis
The median OS time for the whole cohort was not reached with a median follow-up time of 64.0 months, and 44 patients (24.6%) died during follow-up. The 5- and 10-year OS rates for adult patients with ONGs were 75.4% and 73.4%, respectively. Different surgical extents and diagnosed years had no significant effect on patients’ OS (P > 0.05, Table ). Prognostic factors identified in univariate analysis were age at diagnosis, tumor grade, adjuvant RT, and chemotherapy (Table ). On further multivariate Cox regression, younger age, low-grade tumors, and no adjuvant chemotherapy were significant factors for longer OS (Table ).
Table 2. Univariate and multivariate analysis of OS.
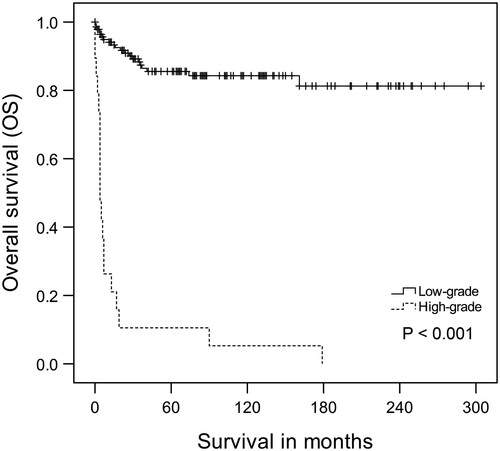
Due to the possible negative impact of the adjuvant therapies on OS, further analyses were performed in the subgroups of low-grade and high-grade tumors to clarify better the prognosis of these patients who received adjuvant therapies. For the low-grade tumor group, the 5-year OS rate was 85.5%; however, the 5-year OS rate for patients in the high-grade group was only 10.5% (P < 0.001, log-rank test; Figure ). Based on the low-grade subgroup analysis, patients with age less than 50 years, no adjuvant RT or chemotherapy had the best OS time, and the difference was significant (P < 0.05, log-rank test; Figure A–C). The results of multivariate Cox regression also showed that younger age, and no adjuvant chemotherapy were significant factors for longer OS (Table ). However, different results were found in the other subgroup of the high-grade tumor, which demonstrated that only older age resulted in a significantly shorter OS (P < 0.05, log-rank test; Figure D). Moreover, there were no significant differences in OS for high-grade patients who received the adjuvant therapies or not, according to multivariate Cox regression (Table , Figure E–F).
Figure 2. Impact of age, radiotherapy (RT), and chemotherapy on overall survival of adult optic nerve gliomas. A-C. Low-grade group. D-F. High-grade group.
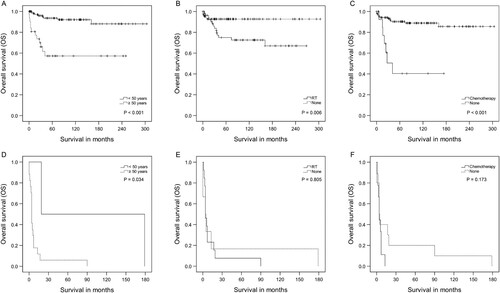
Table 3. multivariate analysis of OS in low-grade group.
Table 4. multivariate analysis of OS in high-grade group.
Discussion
Up to date, most studies of adult ONGs are individual case reports or only include a limited number of patients (Hidalgo et al. (Citation2019 jun Jun Citation2019 jun); Shofty et al. (Citation2014 Mar); Traber et al. (Citation2015 Jul); Nagaishi et al. (Citation2015 Jan); Liu et al. (Citation2013 Nov); Dario et al. (Citation1999 Nov); Wabbels et al. (Citation2004 Sep); Della Puppa et al. (Citation2014 Apr); Hoyt et al. (Citation1973)). The largest research, which included 445 patients, not only mixed pediatric and adult cases, but also focused on the analysis of tumors in the pediatric population (Mishra et al. (Citation2012 May)). Few investigations have focused explicitly on adults ONGs (Shofty et al. (Citation2014 Mar); Hoyt et al. (Citation1973)). For all we know, this study, including 179 cases on the basis of the SEER database, is the largest series of adult ONGs and is the only study to compare the effects of different treatment strategies (including surgery, adjuvant RT, and chemotherapy) on survival with a large number of adult patients. Furthermore, our data identify that both adjuvant treatments (RT and chemotherapy) have a negative impact on the survival and prognosis of adult patients with low-grade tumors.
Epidemiological and tumor characteristics
ONGs in adults are extremely uncommon and were first described by Hoyt et al. in 1973, which included 15 adult patients with malignant optic glioma (Hoyt et al. (Citation1973)). In our study, 70% of the patients were below the age of 50, especially in the low-grade tumors, and the mean age at diagnosis was 42.1 years, which is nearly consistent with the previously limited reported mean age for patients diagnosed at the adulthood of 39 years (Shofty et al. (Citation2014 Mar)). However, patients diagnosed with high-grade ONGs usually were older than those with low-grade ONGs, ranging from 57 to 66 years, (Traber et al. (Citation2015 Jul); Mishra et al. (Citation2012 May); Wabbels et al. (Citation2004 Sep)) the same with our results. In addition, although other studies showed an equal ratio of male and female in the adult population, (Shofty et al. (Citation2014 Mar); Hoyt et al. (Citation1973)) a female predominance was demonstrated in this large study.
In terms of treatment, gross total surgical resection usually was not the primary treatment option in patients with ONGs, especially for low-grade at any age (Farazdaghi et al. (Citation2019 Sep); Shofty et al. (Citation2014 Mar); Mishra et al. (Citation2012 May)). Up to 72.6% of the patients underwent no surgery in the present study. For adjuvant therapies, there were differences between low-grade and high-grade groups. The existing reported data on RT and chemotherapy in patients with low-grade ONGs are mainly limited in the pediatric population (Acharya et al. (Citation2019 Sep); Dodgshun et al. (Citation2015 Dec Citation1); Tsang et al. (Citation2017 Nov Citation1); Fisher et al. (Citation2012 Jun); Moreno et al. (Citation2010 Aug)). In our study, 38.7% and 11.3% of low-grade adult patients received RT and chemotherapy, respectively. However, for the high-grade group, surgery or biopsy, followed by adjuvant RT and/or chemotherapy, could be considered the standard of care, (Shofty et al. (Citation2014 Mar); Traber et al. (Citation2015 Jul); Nagaishi et al. (Citation2015 Jan); Dario et al. (Citation1999 Nov); Wabbels et al. (Citation2004 Sep)) and these two proportions of the adjuvant therapies in current study were 68.4% and 47.4%, respectively.
Factors associated with survival and tumor management
Gender, marital status, race, year of diagnosis, tumor site, and different surgical patterns were not critical predictors of survival in univariate and multivariate analysis. However, age at diagnosis, tumor grade, and adjuvant RT and chemotherapy were significant survival factors in adult patients with ONGs. Although age was not recognized as a prognostic factor in a previous study, (Mishra et al. (Citation2012 May)) our result showed that patients with age ≥ 50 years had a worse prognosis in both low- and high-grade groups, similar to other published series of gliomas (Carson et al. (Citation2007 Jun Citation20); Pignatti et al. (Citation2002 Apr Citation15)). It is universally acknowledged that patients with low-grade gliomas have a better survival prognosis, which had a 5-year OS rate of 85.5% compared to 10.5% for patients with high-grade gliomas in our study. Furthermore, although many reported cases of adult ONGs are high-grade, we found most of them remain low-grade tumors, inconsistent with the study by Shofty et al., in which 80% of primary adult ONGs are also low-grade (Shofty et al. (Citation2014 Mar)).
Unlike the gliomas in other sites, there is no gold standard in treating both low-grade and high-grade ONGs. The general goal of all individualized treatment strategies is to preserve the patient’s vision as long as possible. Based on this goal, surgical resection might not be a preferred option and is discouraged due to the inevitable blindness on the affected side or bilateral visual loss risks (Lee (Citation2007)). Our current results demonstrate no significant differences among various surgical options for patients with any grade ONGs regarding OS. Therefore, initial observation is frequently recommended with ophthalmological evaluation and neuroimaging surveillance to confirm clinical stability, particularly for low-grade ONGs. However, for malignant ONGs or progressive tumors with the aggressively visual decline, it might be appropriate to consider surgical excision to debulk the tumor and obtain a pathological diagnosis before commencing the following adjuvant therapy (Farazdaghi et al. (Citation2019 Sep); Nair et al. (Citation2014 Aug); Shapey et al. (Citation2011 Dec))
The role of adjuvant RT and chemotherapy in an adult population remains unclear due to its rarity. To our knowledge, there have been only nine documented cases of low-grade optic pathway gliomas in adults in the past few years (Hidalgo et al. (Citation2019 jun Jun Citation2019 jun); Shofty et al. (Citation2014 Mar)). The recent case study by Hidalgo et al. demonstrated that an adult patient with low-grade optic pathway glioma who received no adjuvant therapy after STR could survive more than 20 years without tumor progression (Hidalgo et al. (Citation2019 jun Jun Citation2019 jun)). In another study that included limited 14 children with optic chiasmatic-hypothalamic gliomas, no significant differences were observed regarding the volume change of tumors treated or not treated with chemotherapy (Calixto et al. (Citation2019 Jan)). The critical finding in our study shows that both adjuvant RT and chemotherapy may have potentially adverse effects on survival in adult patients with low-grade ONGs. The concrete reason is unknown because of the limited clinical information included in the SEER database; however, on the grounds of some previously published studies, it is most likely attribute to the systemic side effects and increased risks of malignant progression and visual, neurocognitive and hypothalamic dysfunction after such adjuvant treatments, especially RT (Farazdaghi et al. (Citation2019 Sep); Nair et al. (Citation2014 Aug); Tsang et al. (Citation2017 Nov Citation1); Perry et al. (Citation1998 Oct); Lancaster et al. (Citation2003 Jul); Sharif et al. (Citation2006 Jun Citation1)). Therefore, given the above analysis, we recommend that RT or chemotherapy should be utilized cautiously before full clinical evaluation in adult patients with low-grade ONGs.
For high-grade ONGs in adult patients, the reported average survival ranged from 1 to 2 years, despite aggressive treatment with RT and chemotherapy, similar to that of glioblastoma in other locations (Wabbels et al. (Citation2004 Sep); Cimino et al. (Citation2017 Jul/Aug); Stupp et al. (Citation2009 May)). Our results demonstrate that both adjuvant RT and chemotherapy have no positive effect on patients’ OS. Therefore, given the very limited number of reported and our cases, it can not be confirmed whether adjuvant therapies benefit the adult patient or not. Nevertheless, considering the same histopathological characteristics of high-grade gliomas located in other brain sites, a combination of adjuvant RT and chemotherapy after surgery might remain the most appropriate option that can be adopted for adult patients with ONGs at present. Indeed, large-scale collaborative multicenter prospective studies are still warranted to determine treatment consensus.
Limitations
Despite the fact that a huge amount of invaluable data for rare tumors such as ONGs can be acquired in the SEER database, there are still several important limitations to the present study. First, there is no information available on visual outcomes or tumor progression, which is important to be an endpoint for assessing the effects, particularly for the low-grade group. Second, there is a lack of details on the adjuvant treatments, such as chemotherapeutic agents, dose and type of RT, and the exact time of adjuvant treatments. Third, several critical factors, including performance status, systemic disease status, NF-1 status, or neuroendocrine morbidity, are unable to obtain from the SEER database. Finally, given the impossibly pathological and radiological review of the tumors, it is likely that some tumors may have been misdiagnosed owing to the low interobserver agreement in the diagnosis of different glioneuronal tumors. However, considering the prospective studies not available and not be expected in the near future, because of the scarcity of ONGs, a large retrospective study like this seems to be the best and most useful approach available to define the role of different adjuvant treatments for these lesions. Therefore, although several limitations mentioned above, this is the largest reported series and the best evidence available in regard to adult ONGs up to date.
Conclusion
This is the largest retrospective study of adult ONGs, including 179 cases from the SEER database. Our data reaffirms that the low-grade ONGs have a significantly better prognosis than the high-grade, and demonstrates the different role of adjuvant treatments in different grade tumors. Although both adjuvant RT and chemotherapy have no positive effect on patients’ OS in the high-grade group, they may have a potentially negative impact on patient’s survival and prognosis in the low-grade group. Therefore, adjuvant RT or chemotherapy should be applied cautiously before full clinical evaluation in adult patients with low-grade ONGs.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Acknowledgements
The authors acknowledge the SEER program that provided the data for the research project.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data in present study are available in the Surveillance, Epidemiology, and End Results (https://seer.cancer.gov).
Additional information
Funding
References
- Acharya S, Quesada S, Coca K, et al. 2019 Sep. Long-term visual acuity outcomes after radiation therapy for sporadic optic pathway glioma. J Neurooncol. 144(3):603–610.
- Azizi AA, Walker DA, Liu JF, et al. 2021 Jan 30. NF1 optic pathway glioma: analyzing risk factors for visual outcome and indications to treat. Neuro Oncol. 23(1):100–111.
- Binning MJ, Liu JK, Kestle JR, et al. 2007. Optic pathway gliomas: a review. Neurosurg Focus. 23(5):E2.
- Calixto NC, Simao GN, Dos Santos AC, et al. 2019 Jan. Monitoring optic chiasmatic-hypothalamic glioma volumetric changes by MRI in children under clinical surveillance or chemotherapy. Childs Nerv Syst. 35(1):63–72.
- Carson KA, Grossman SA, Fisher JD, et al. 2007 Jun 20. Prognostic factors for survival in adult patients with recurrent glioma enrolled onto the new approaches to brain tumor therapy CNS consortium phase I and II clinical trials. J Clin Oncol. 25(18):2601–2606.
- Cimino PJ, Sychev YV, Gonzalez-Cuyar LF, et al. 2017 Jul/Aug. Primary gliosarcoma of the optic nerve: a unique adult optic pathway glioma. Ophthalmic Plast Reconstr Surg. 33(4):e88–e92.
- Cummings TJ, Provenzale JM, Hunter SB, et al. 2000 May. Gliomas of the optic nerve: histological, immunohistochemical (MIB-1 and p53), and MRI analysis. Acta Neuropathol. 99(5):563–570.
- Dario A, Iadini A, Cerati M, et al. 1999 Nov. Malignant optic glioma of adulthood. Case report and review of the literature. Acta Neurol Scand. 100(5):350–353.
- Della Puppa A, Rustemi O, Gioffre G. 2014 Apr. The rare event of optic-chiasmatic hemorrhagic low grade glioma in adulthood. Considerations on treatment strategy. Neurol Sci. 35(4):623–625.
- Dodgshun AJ, Elder JE, Hansford JR, et al. 2015 Dec 1. Long-term visual outcome after chemotherapy for optic pathway glioma in children: Site and age are strongly predictive. Cancer. 121(23):4190–4196.
- Dolecek TA, Propp JM, Stroup NE, et al. 2012 Nov. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005-2009. Neuro Oncol. 14(Suppl 5):v1–49.
- Ertiaei A, Hanaei S, Habibi Z, et al. 2016. Optic pathway gliomas: clinical manifestation, treatment, and follow-up. Pediatr Neurosurg. 51(5):223–228.
- Farazdaghi MK, Katowitz WR, Avery RA. 2019 Sep. Current treatment of optic nerve gliomas. Curr Opin Ophthalmol. 30(5):356–363.
- Fisher MJ, Loguidice M, Gutmann DH, et al. 2012 Jun. Visual outcomes in children with neurofibromatosis type 1-associated optic pathway glioma following chemotherapy: a multicenter retrospective analysis. Neuro Oncol. 14(6):790–797.
- Grill J, Laithier V, Rodriguez D, et al. 2000 Sep. When do children with optic pathway tumours need treatment? an oncological perspective in 106 patients treated in a single centre. Eur J Pediatr. 159(9):692–696.
- Hidalgo ET, McQuinn MW, Wisoff JH. 2019 Jun. Regression after subtotal resection of an optic pathway glioma in an adult without adjuvant therapy: case report. J Neurosurg. 130:2005–2008.
- Hoyt WF, Meshel LG, Lessell S, et al. 1973. Malignant optic glioma of adulthood. Brain. 96(1):121–132.
- Lancaster DL, Hoddes JA, Michalski A. 2003 Jul. Tolerance of nitrosurea-based multiagent chemotherapy regime for low-grade pediatric gliomas. J Neurooncol. 63(3):289–294.
- Lee AG. 2007. Neuroophthalmological management of optic pathway gliomas. Neurosurg Focus. 23(5):E1.
- Liu Y, Zhang-Nunes S, Zhu X, et al. 2013 Nov. Late adult onset optic pathway astrocytoma. J Clin Neurosci. 20(11):1610–1612.
- Louis DN, Perry A, Reifenberger G, et al. 2016 Jun. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 131(6):803–820.
- Mishra MV, Andrews DW, Glass J, et al. 2012 May. Characterization and outcomes of optic nerve gliomas: a population-based analysis. J Neurooncol. 107(3):591–597.
- Moreno L, Bautista F, Ashley S, et al. 2010 Aug. Does chemotherapy affect the visual outcome in children with optic pathway glioma? a systematic review of the evidence. Eur J Cancer. 46(12):2253–2259.
- Nagaishi M, Sugiura Y, Takano I, et al. 2015 Jan. Clinicopathological and molecular features of malignant optic pathway glioma in an adult. J Clin Neurosci. 22(1):207–209.
- Nair AG, Pathak RS, Iyer VR, et al. 2014 Aug. Optic nerve glioma: an update. Int Ophthalmol. 34(4):999–1005.
- Nicolin G, Parkin P, Mabbott D, et al. 2009 Dec 15. Natural history and outcome of optic pathway gliomas in children. Pediatr Blood Cancer. 53(7):1231–1237.
- Peckham-Gregory EC, Montenegro RE, Stevenson DA, et al. 2018 Jun. Evaluation of racial disparities in pediatric optic pathway glioma incidence: results from the surveillance, epidemiology, and end results program, 2000-2014. Cancer Epidemiol. 54:90–94.
- Perry JR, Brown MT, Gockerman JP. 1998 Oct. Acute leukemia following treatment of malignant glioma. J Neurooncol. 40(1):39–46.
- Pignatti F, van den Bent M, Curran D, et al. 2002 Apr 15. Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol. 20(8):2076–2084.
- Shapey J, Danesh-Meyer HV, Kaye AH. 2011 Dec. Diagnosis and management of optic nerve glioma. J Clin Neurosci. 18(12):1585–1591.
- Sharif S, Ferner R, Birch JM, et al. 2006 Jun 1. Second primary tumors in neurofibromatosis 1 patients treated for optic glioma: substantial risks after radiotherapy. J Clin Oncol. 24(16):2570–2575.
- Shofty B, Constantini S, Bokstein F, et al. 2014 Mar. Optic pathway gliomas in adults. Neurosurgery. 74(3):273–279. discussion 279-80.
- Stupp R, Hegi ME, Mason WP, et al. 2009 May. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 10(5):459–466.
- Thomas RP, Gibbs IC, Xu LW, et al. 2015 Feb. Treatment options for optic pathway gliomas. Curr Treat Options Neurol. 17(2):333.
- Traber GL, Pangalu A, Neumann M, et al. 2015 Jul. Malignant optic glioma - the spectrum of disease in a case series. Graefes Arch Clin Exp Ophthalmol. 253(7):1187–1194.
- Tsang DS, Murphy ES, Merchant TE. 2017 Nov 1. Radiation therapy for optic pathway and hypothalamic low-grade gliomas in children. Int J Radiat Oncol Biol Phys. 99(3):642–651.
- Wabbels B, Demmler A, Seitz J, et al. 2004 Sep. Unilateral adult malignant optic nerve glioma. Graefes Arch Clin Exp Ophthalmol. 242(9):741–748.
- Wilhelm H. 2009 Feb. Primary optic nerve tumours. Curr Opin Neurol. 22(1):11–18.