Abstract
Current research endeavours are focusing to develop potent chemotherapy for oral cancer. Consistently, this study examined the anticancer effects of heptazoline on the human oral cancer cells. The findings of the present study revealed that heptazoline suppressed the growth of all the oral cancer SCC-15 oral cancer cells (IC50; 12 µM) with very low cytotoxic effects on the normal hTRET-OME cells (IC50; 85 µM). The comet and DAPI assay showed DNA damage as well other signs of apoptosis induced by heptazoline in SCC-15 cells. Annexin V/PI assay revealed that apoptotic SCC-15 cells increased from 3.5% at 0 µM to 21.27% at 24 µM of heptazoline Additionally, the expression of Bax was upregulated and that of Bcl-2 was downregulated upon heptazoline treatment of the SCC-15 cells. Heptazoline also increased the expression of caspase-3 further confirming the induction of apoptosis. Investigation of the effects of heptazoline on the PI3K/AKT signalling showed a remarkable decrease in the phosphorylation of PI3K and AKT. Taken together, the findings of the study suggest that heptazoline has potent anticancer activity and may serve as a lead molecule in oral cancer treatment.
KEYWORDS:
Introduction
Being one of the serious health issues, oral cancer together with pharyngeal cancer is ranked as the 6th most prevalent cancer type across the globe (Manikandan et al. Citation2016). Annually, 0.27 million oral cancer cases are detected throughout the world and most of these cases are reported from the developing countries (Siegel et al. Citation2018). The incidence of oral cancer differs geographically with very high frequency in South and Southeast Asia. Generally, oral cancer has been shown to be more prevalent in men than in women (Cancer Research Campaign Citation2005). The oral cancer risk increases with age and most of the oral cancers are detected in people above the age of 50 years. However, oral cancer may also be found in younger people. It has been reported that 6% of the oral cancer cases are reported in people below the age of 45 years (Conway et al. Citation2006). The 5 year survival rate for oral cancer is around 5% which is considered very poor. The patients who survive after successful treatment of the oral cancer have to face some severe consequences of the treatment such as appearance and function. They have severe issues in eating and speaking which result in depression and mal-nutrition (Banoczy and Squier Citation2004). Therefore, there is tremendous urgency to the development viable chemotherapy for the successful treatment of oral cancers. The objective of the present study was to examine the anticancer properties of heptazoline. Heptazoline is a carbazole alkaloid (Utaipan et al. Citation2017). The carbazole alkaloids have been shown to exhibit tremendous pharmacological potential, especially the anticancer effects (Shaikh et al. Citation2015). This study investigated the anticancer effects of heptazoline against the human oral cancer cells. Recent advancements in molecular biology have proved that phosphatidylinositol 3-kinases (PI3K)/AKT/mammalian target of the rapamycin (mTOR) signalling cascade has critical functions which include processes such as proliferation, metastasis and angiogenesis (Vara et al. Citation2004). The PI3K/AKT has been reported to be activated in cancer cells which in turn leads to the phosphorylation of the mTOR complex 1 (Rosen and She Citation2006). Studies have shown that this pathway may act as a therapeutic target for the treatment of different cancers. Hence, molecules that can block this pathway are considered important in cancer research (Bellacosa et al. Citation2005). Consistently, this study also investigated the effects of heptazoline on the PI3K/AKT/mTOR signalling pathway.
Materials and methods
Cell lines
The oral cancer SCC-15 (ATCC: CRL-1623™) and normal hTRET-OME cell lines were obtained from ATCC, USA and cultured in Dulbecco’s modified Eagle medium (DMEM, Gibco, USA) medium with 10% FBS under standard culturing conditions consisting of a humidified CO2 incubator at 37°C with 5% CO2/95% air concentration.
XTT cell viability assay
Cell viability was determined by 2, 3-Bis(2-methoxy-4-nitro-5-sulfophnyl)-2H-tetrazolium-5-carboxanlide inner salt (XTT) assay as described previously (Hong et al. Citation2017). Briefly, the oral cancer SCC-15 and normal htRET-OME cells were seeded in 96-well plates at the density of 5 × 103 cells/well and cultured for 24 h. The medium was replaced with a fresh media containing different concentrations of Heptazoline (0, 6, 12 and 24 µM) and incubated at 37○C for another 24 h. The cell viability was subsequently determined by XTT cell viability kit (Biological Industries). The media was again replaced, XTT was added and the cells were again incubated for 4 h at 37○C. Finally, the resulting signal was measured by Power Wave × 340-I ELISA reader (Biotek Instruments, Winooski, VT, USA).
Comet assay
The comet assay was carried out as described previously (Dong et al. Citation2014). Approximately 2.5 × 105 cells/mL oral cancer cells were subjected to 0, 6, 12 and 24 µM heptazoline and then mixed with 1.5 mL of low-melting agarose. From this, 100 µL was put onto the comet slides which were already covered with 1% agarose. The cells were then run on electrophoresis for 15 min at 25 V, stained with acridine orange and finally observed by fluorescence microscopy.
Detection of apoptosis
To understand if heptazoline induces apoptosis, DAPI (4′,6-diamidino-2-phenylindole) and annexin V/PI staining was performed (Qin Citation2002). For DAPI staining the SCC-15 cells were seeded at the density of 3 × 105 cells/well in 6-well plates with sterile coverslips and incubated for 24 h at 37°C. Subsequently, the cells were subjected to treatment with 0, 6, 12 and 24 µM of heptazoline and incubated again for 24 h at 37°C. Then cells were then fixed with formaldehyde (4%)/trition X-100 (0.1%) for 5 min and washed thrice with PBS. The cells were finally stained with DAPI (250 ng/mL) for 5 min at 25°C. The cells were then examined by inverted fluorescent microscope (Olympus BX64, Olympus, Japan) equipped with a U-MWU2 fluorescence filter.
For annexin V/PI staining, SCC-15 cells were seeded into 6-well plates and subjected to treatment with different concentrations of heptazoline for 24 h at 37°C. Subsequently, the cells were detached by 0.25% trypsin-EDTA and washed by PBS. Following the manufacturer guideline of annexin V-FITC/PI kit, SCC-15 cells were suspended in 100 µL 1× binding buffer. To this, 5 µL of FITC-conjugated annexin V was added. Thereafter, addition of 10 µL of PI (propidium iodide) staining solution was done and the SCC-1 cells were then incubated at 25°C under dark conditions for 15 min. Finally, the FITC-labeled annexin V-positive cells were measured in the FL1 channel and the PI-labeled cells were measured in the FL2 channel using FACS Calibur flow cytometry (Becton Dickinson, San Jose, CA).
ROS and MMP determination
The mitochondrial membrane potential (MMP) and reactive oxygen species (ROS) quantification was done by DiOC6 (1 µmol/l) and dihydrofluorscein diacetate (10 µM) respectively by flow cytometrically and presented in the form of bar diagrams. The procedure was repeated thrice.
Western blotting
After washing the heptazoline-treated SCC-15 cells with phosphate-buffered saline (PBS), the cells were lysed using RIPA buffered and centrifuged to collect the supernatant. BCA assay was utilised to determine protein content of the cell extracts. Subsequently, 20 µg protein was subjected to separation on SDS-PAGE and then shifted to PVDF. Next, the non-fat milk was used for blocking and then the membranes were incubated with primary antibodies Bax (cat. sc-6236), Bcl-2 (cat. sc-509), Akt (cat. sc-135829), caspase 3 (cat. sc-7272), phosphorylated (p)-AKT (cat. no. sc-7985-R) and PI3 K (cat. no.sc-136298) purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA) overnight at 4°C (dilution1:1,000), followed by incubation with horseradish peroxidase-conjugated (cat. no. 9003-99-0) for 1 h at room temperature. Finally, ECL-chemiluminescent kit was used to observe the protein bands of interest.
Statistical analysis
The experiments were performed in triplicate and expressed as mean ± SD. The P < 0.05 was considered as the statically significant difference. Student’s t test and one-way ANOVA were used for statistical analysis by employing GraphPad prism 7 software.
Results
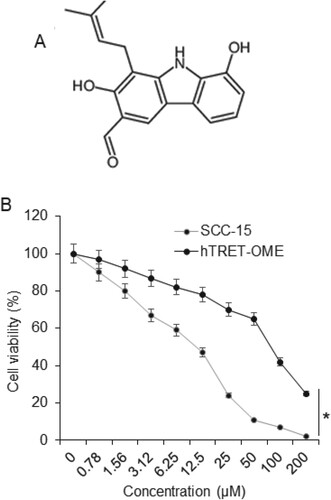
Cell viability of SCC-15 cells is decreased by heptazoline
The results of the XTT assay showed that heptazoline (Figure (A)) caused a remarkable decrease in the viability of the oral cancer cells. The IC50 of the heptazoline against the SCC-15 human oral cancer cells was found to be 12 µM. Nevertheless, the viability of the normal hTRET-OME cells was not affected that much. The IC50 of heptazoline was found to be 85 µM against the hTRET-OME cells (Figure (B)). It is noteworthy that the effects of heptazoline on the viability of both oral cancer as well as normal cells showed concentration dependence.
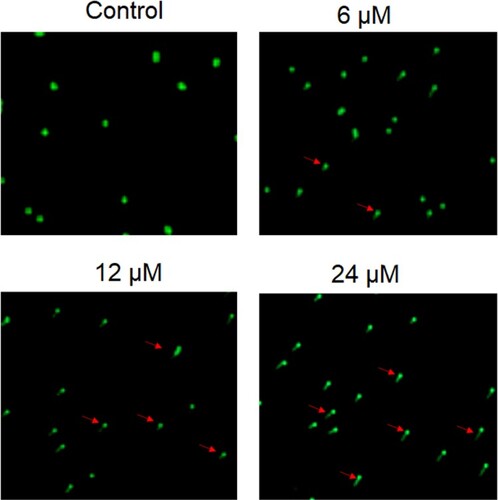
Heptazoline induces DNA damage in SCC-15 cells
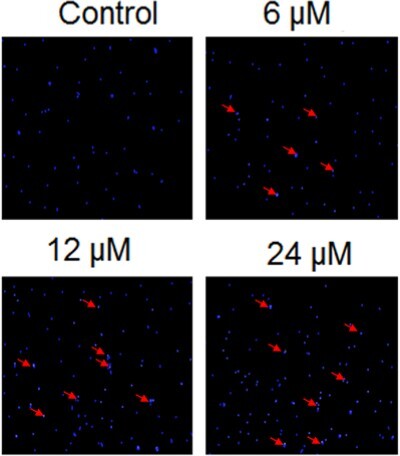
The effects of heptazoline on DNA damage of SCC-15 cells was investigated by comet assay. The SCC-15 cells were subjected to 0, 6, 12 and 24 µM doses of heptazoline and it was found that heptazoline triggered DNA damage in the SCC-15 cells as evident from the formation of tails in the cells (Figure ). Additionally, the extent of DNA damage increased with the increase in the dosage of Heptazoline.
Heptazoline induces apoptosis of SCC-15 cells
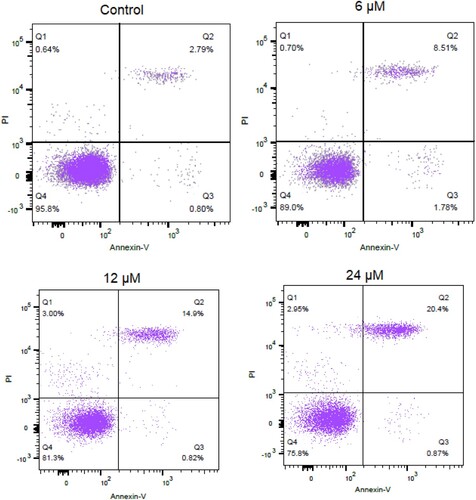
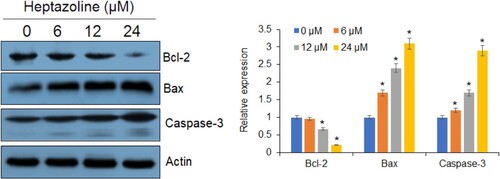
Next, the heptazoline-treated SCC-15 cells were stained with DAPI and the nuclear morphological analysis was performed by fluorescence microscopy. It was observed that heptazoline caused DNA fragment and altered the nuclear morphology of SCC-15 cells suggestive of apoptosis (Figure ). The extent of apoptosis in SCC-15 was determined by annexin V/PI assay which showed that the apoptotic SCC-15 cells increased from 3.5% at 0 µM to 21.7% at 24 µM of heptazoline (Figure ). Additionally, the expression of Bax was upregulated and that of Bcl-2 was downregulated upon heptazoline treatment of the SCC-15 cells. Moreover, the caspase-3 increased by heptazoline suggestive of the involvement of both the intrinsic and extrinsic apoptosis (Figure ).
Figure 3. Heptazine induces DNA damage in SCC-15 cells. DNA damage analysis of the heptazoline-treated SCC-15 cells by Comet assay. Experiments were done in triplicate.
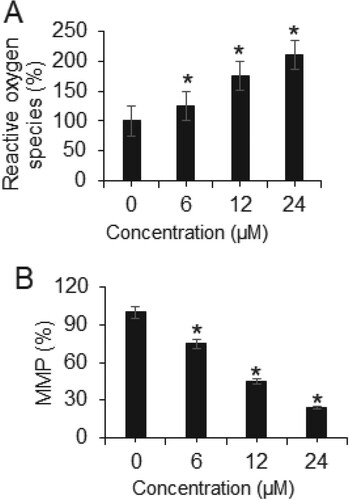
Heptazoline altered ROS and MMP production in SCC-15 cells
The ROS and MMP levels of the SCC-15 cells were also evaluated after treatment with a varied dosage of heptazoline. The results showed that ROS levels increased significantly (P < 0.05) in SCC-15 cells and the MMP levels showed significant (P < 0.05) depletion. The ROS levels were 100%, 127%, 185% and 213% at 0, 6, 12 and 24 µM of heptazoline (Figure (A)). Furthermore, the MMP levels were 100%, 76%, 45% and 26% at 0, 6, 12 and 24 µM of heptazoline (Figure (B)).
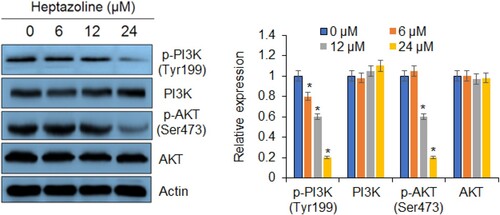
Heptazoline blocks PI3K/AKT signalling pathway
The effects of heptazoline were also investigated on the phosphorylation status of PI3K (Tyr199) and AKT (Ser473) in SCC-15 cells. It was observed that heptazoline caused a considerable and dose-dependent decrease in phosphorylation of PI3K as well as AKT in SCC-15 cells (Figure ). Nonetheless, the total PI3K and AKT concentrations remained constant and unaffected.
Discussion
Over the last few decades, carbazole alkaloids have shown remarkable pharmacological properties. Among the pharmacological properties, the anticancer effects of carbazole alkaloids are well reported (Amin et al. Citation2009). For instance, Mahanine has been shown to suppress the proliferation of the prostate cancer cells via arrest of the cell division and promotion of the programmed cell death (Sinha et al. Citation2006). Consistently, this study was undertaken to investigate the anticancer effects of heptazoline against the human oral SCC-15 cancer cells and the normal hTRET-OME cells. It was observed that heptazoline caused a remarkable decline in the viability of the SCC-15 cells. However, it was interesting to see that heptazoline could inhibit the growth of the normal cancer cells very minimally. The IC50 of heptazoline against the cancer cells was found to be 8 times higher than the normal cells. A vital mechanism that is employed by different anticancer drugs to eliminate the tumour cells is apoptosis. The activation of apoptosis also known as the programmed cell death is one of the novel strategies to treat cancer cells (Sun et al. Citation2004). Anticancer drugs such as cisplatin have been reported to suppress the proliferation of the cancer cells via stimulation of the apoptotic cell death (Youn et al. Citation2015). In the current investigation, we observed that heptazoline stimulated the programmed cell death in the SCC-15 oral cancer cells. The stimulation of programmed cell death was linked with the accumulation of Bax, suppression of Bcl-2 and activation of caspase-3. We also observed that heptazoline treatment of the SCC-15 caused remarkable accumulation of ROS which was accompanied by a decline in the MMP levels suggesting that heptazoline promotes ROS-mediated apoptosis in SCC-15 cells. This is in agreement with several previous studies, wherein several molecules have been reported to stimulate apoptosis in cancer cells by accretion of ROS. For instance, withaferin A has been shown to deplete MMP and create oxidative stress-mediated apoptosis in osteosarcoma cells (Li et al. Citation2017). PI3K/AKT signalling cascade has been shown to be an important therapeutic target in cancer cells as this pathway has been shown to be responsible for the development and progression of different cancer types (Caporali et al. Citation2016). A number of drugs such as the rapamycin analogue (rapalog) sirolimus has been used to target this pathway for the treatment of several cancers (Dienstmann et al. Citation2014). In this study, we found that heptazoline blocks this pathway and as such may prove essential in oral cancer treatment.
Conclusion
Taken together, heptazoline suppresses the growth of the human oral cancer cells via ROS-mediated programmed cell death and inhibition of the PI3 K/AKT cascade. Therefore, heptazoline may prove essential in oral cancer treatment and deserves further studies.
Acknowledgements
We acknowledge the funding support from National Natural Science Foundation of China (No: 81500857) and Natural Science Foundation of Shanxi Province of China (No: 2017JM8090).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
That data that supports the findings of this study are available at the figshare repository (https://figshare.com/) at https://figshare.com/s/1e00e5af93b0b38ad55c
Additional information
Funding
References
- Amin A, Gali-Muhtasib H, Ocker M. 2009. Schneider-Stock R: overview of major classes of plant-derived anticancer drugs. Int J Biomed Sci (IJBS). 5(1):1.
- Banoczy J, Squier C. 2004. Smoking and disease. Eur J Dent Educ. 8(4):7–10.
- Bellacosa A, Kumar CC, Di Cristofano A, Testa JR. 2005. Activation of AKT kinases in cancer: implications for therapeutic targeting. Adv Cancer Res. 94:29–86.
- Cancer Research Campaign. 2005. Cancerstats. Oral cancer – UK, 2005. London: CRC.
- Caporali S, Alvino E, Lacal PM, Levati L, Giurato G, Memoli D, Caprini E, Cappellini GCA, D’atri S. 2016. Targeting the PI3K/AKT/mTOR pathway overcomes the stimulating effect of dabrafenib on the invasive behavior of melanoma cells with acquired resistance to the BRAF inhibitor. Int J Oncol. 49(3):1164–1174.
- Conway D, Macpherson LMD, Warnakulasuriya KAAS, Ogden G, Stockton DL. 2006. Incidence of oral and oropharyngeal cancer in the United Kingdom (1990– 1999) – current status, recent trends and intercountry comparisons. Oral Oncol. 42:586–592.
- Dienstmann R, Rodon J, Serra V, Tabernero J. 2014. Picking the point of inhibition: a comparative review of PI3K/AKT/mTOR pathway inhibitors. Mol Cancer Therap. 13(5):1021–1031.
- Dong H, Xu D, Hu L, Li L, Song E, Song Y. 2014. Evaluation of N-acetyl-cysteine against tetrachlorobenzoquinone-induced genotoxicity and oxidative stress in HepG2 cells. Food Chem Toxicol. 64:291–297.
- Hong SO, Choi IK, Jeong W, Lee SR, Sung HJ, Hong SS, Seo JH. 2017. Ulmus davidiana nakai induces apoptosis and autophagy on non-small cell lung cancer cells. J Ethnopharmacol. 202:1–1.
- Li AX, Sun M, Li X. 2017. Withaferin-A induces apoptosis in osteosarcoma U2OS cell line via generation of ROS and disruption of mitochondrial membrane potential. Euro Rev Med Pharma Sci. 21:1368–1374.
- Manikandan M, Rao AK, Arunkumar G, Manickavasagam M, Rajkumar KS, Rajaraman R, Munirajan AK. 2016. Oral squamous cell carcinoma: microRNA expression profiling and integrative analyses for elucidation of tumourigenesis mechanism. Mol Cancer. 15(1):28–32.
- Qin LF. 2002. Ng IO: induction of apoptosis by cisplatin and its effect on cell cycle-related proteins and cell cycle changes in hepatoma cells. Cancer Lett. 175(1):27–38.
- Rosen N, She QB. 2006. AKT and cancer—is it all mTOR? Cancer Cell. 10:254–256.
- Shaikh M, Karpoormath R, Thapliyal N, Rane R, Palkar M, Faya AM, Patel H, Alwan W, Jain K, Hampannavar G. 2015. Current perspective of natural alkaloid carbazole and its derivatives as antitumor agents. Anti-Cancer Agents Med Chem. 15(8):1049–1065.
- Siegel RL, Miller KD, Jemal A. 2018. Cancer statistics. CA Cancer J Clin. 68(1):7–30.
- Sinha S, Pal BC, Jagadeesh S, Banerjee PP, Bandyopadhaya A, Bhattacharya S. 2006. Mahanine inhibits growth and induces apoptosis in prostate cancer cells through the deactivation of Akt and activation of caspases. Prostate. 66(12):1257–1265.
- Sun SY, Hail N, Lotan R. 2004. Apoptosis as a novel target for cancer chemoprevention. J Natl Can Inst. 96:662–672.
- Utaipan T, Athipornchai A, Suksamrarn A, Jirachotikoon C, Yuan X, Lertcanawanichakul M, Chunglok W. 2017. Carbazole alkaloids from Murraya koenigii trigger apoptosis and autophagic flux inhibition in human oral squamous cell carcinoma cells. J Nat Med. 71(1):158–169.
- Vara JAF, Casado E, de Castro J, Cejas P, Belda-Iniesta C, Gonzalez-Baron M. 2004. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 30:193–204.
- Youn CK, Kim J, Park J-H, Do NY, Cho SI. 2015. Role of autophagy in cisplatin-induced ototoxicity. Int J PediatrOtorhinolaryngol. 79(11):1814–1819.