Abstract
The cotton leafhopper (Jacobiasca lybica) is one of the polyphagous species and its control is mainly based on chemical treatments, however its bio-ecological parameters are still poorly understood especially under Moroccan conditions. In this work, the population ecology of this leafhopper and the planning of insecticide treatments based on the recorded degree-days (DD) in a citrus orchard and vineyard separated by a windbreak (WB) of Cypress and Acacia were investigated. Leafhopper adults were monitored with five yellow sticky traps for each experimental unit and removed weekly from April to August for identification and counting. The sampling indicated the presence of five peaks of adult flight during 2017 and seven during 2018. Whereas six generations were predicted each year according to DD recorded until mid-August. Leafhopper populations were present on WB plants throughout the study period, and its preference for Acacia over Cypress was observed. During 2018, insecticides used were applied one week after DD predicted new generations on WB plants, and they were also applied on grapes at second, third, fourth and fifth adult peaks. The use of DD to predict the outbreak of new generations is discussed as a promising strategy to manage leafhopper populations below economic thresholds.
1. Introduction
The cotton leafhopper Jacobiasca lybica (Bergevin & Zanon Citation1922) (Hemiptera: Cicadellidae) is a polyphagous species considered to be a major grapevines pest in Southern regions of Spain, Portugal and Italy, where it causes significant economic damage (Ocete et al. Citation1999; Delrio et al. Citation2000; Lentini et al. Citation2000; Manzella et al. Citation2001; Alma Citation2002; Tsolakis Citation2003). Another closely related species, Empoasca vitis (Göthe Citation1875), also known as a green leafhopper, is considered in Morocco as a secondary pest of several crops including citrus fruits (Mazih Citation2015), and a major grapevines pest in Europe (Baillod et al. Citation1990; Pavan et al. Citation2000). Prior studies have examined the population dynamics of the green leafhopper, that has a fast reproduction rate which leads to several overlapping generations in a short period of time (Decante and van Helden Citation2006). The cotton leafhopper overwinters on evergreen plants and migrates in spring to vineyards or relay plants where it produces at least three overlapping generations (Vidano Citation1962; Van Helden Citation2000; Del-Campo-Sanchez et al. Citation2019). During the spring, overwintering females lay between 60 and 80 eggs inside the veins or leave petioles which give rise to the first-generation larvae. There are five nymphal stages which are distinguished by their size, and the presence and shape of the wing drafts (Klerks and Van Lenteren Citation1991).
Damage caused by J. lybica may include stem discoloration resulting in the delimitation of the secondary and tertiary veins, which can develop into grills (Carle and Moutous Citation1965). On citrus fruits, the feeding stings result in irregular-shaped oleocellosis blemishes in the rind which have a diameter of 2–7 mm. On the first colored citrus fruits, the damage is initially pale, but most blemishes darken with time. Due to these discolorations, affected fruit are no longer marketable (Moore Citation2013).
Knowledge about ecology, behavior and distribution of the cotton leafhopper is very incomplete and particularly complex (Dworakowska Citation1972; Wiliam Citation2002a; William Citation2002b), with much poorly understood, especially under Moroccan conditions. Furthermore, the prediction of the leafhopper population dynamics is often hampered by seasonal migration due to changes of host plants for oviposition (Lamp and Zhao Citation1993; Taylor and Shields Citation1995; Chancellor et al. Citation1996; Holt et al. Citation1996; Emmen et al. Citation2004). Monitoring and the planning of suitable periods to spray chemicals based on degree-days (DD) can be one of tools to develop an integrated pest management (IPM) as an alternative to the use of systematic insecticides to control this pest. Therefore, this study was initiated to understand the population dynamics, as well as the effect of insecticides treatments based on DD to control J. lybica inhabiting citrus, grapevine and wind-break plants in Morocco.
2. Materials and methods
2.1. Study area and field trial
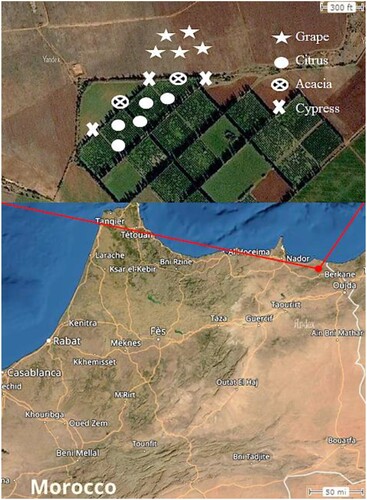
The study was conducted on a citrus orchard and a vineyard located in the Berkane Province in Eastern, Morocco (35°01'45.7"N 2°24'04.3"W – Elevation: 55 m). The 2 ha citrus orchard was planted to a 40 years old ‘Berkane’ clementine, while the 1.8 ha vineyard (Guyot system) was planted to 8 years old Vitis vinifera cv. Carignan grapevine. The two plots were contiguous and separated by a windbreak (WB) of Acacia (Acacia karroo) and Cypress (Cupresus sempervirens) trees as shown in Figure , so that they were exposed to the same environmental conditions. Spontaneous vegetation was cleared under the plants. The vineyard and citrus orchard was drip irrigated 4–6 h per day and 3–4 h per day, respectively, depending on outside ambient temperatures of each year, with a flow rate of 7.1 L per second for 1 ha land segment.
2.2. Identification and monitoring of the leafhopper
The leafhopper adults were monitored using five yellow sticky traps (15 cm × 10 cm) in each crop (citrus and grapevine) and windbreak trees (3 traps for Cypress and 2 for Acacia) from the end of March to mid-August during 2017, and from the beginning of April to the end of August during 2018. The traps were placed on grape plants (about 0.5 m high in the canopy), citrus and WB trees (about 1.5 m high), facing South-East. The traps were replaced weekly and followed by the enumeration of leafhopper adults under binocular loupe. One trap per crop and windbreak trees was collected randomly twice per year to select adults randomly for identification.
The abdomen of the inspected specimens was dissected to study the male genitalia, and identified by using published taxonomic keys (Dworakowska Citation1972, Citation1980; Della Giustina et al. Citation1989). Photographs were taken with an Olympus Q-Color 3 digital camera on a Olympus BX41 microscope (USA). Voucher specimens are deposited in the collection of Illinois Natural History Survey (University of Illinois) in Champaign (USA).
2.3. Pesticide sprays during trial
During the first year of study (2017), Lambda-cyhalothrine, at 25 cc/hl, was the only insecticide sprayed to reduce the leafhopper populations. Insecticide was applied on grapevine plants only after leaf damage was observed at the end of July, which was the normal management practice of this farm.
In 2018, different insecticides were applied for cotton leafhopper control in each week following the achieved DD in citrus (Thiametoxam and Abamectine), grapevine (Methomyl and Lambda-cyhalothrine) and WB (Acetamiprid alternated with other molecules cited previously to avoid the resistance). The insecticides timing during this year were applied on WB plants one week after DD predicted a new generation, and on grapevines at the predicted second, third, fourth and fifth generations. Synchronized treatments were applied on citrus, grapevine and WB plants for the predicted sixth and seventh generations.
2.4. Data analysis
The number of leafhopper adults was calculated weekly based on the average of specimens caught by 15 traps used per week, also the average of the 5 traps in each crop per week was calculated. The DD were calculated by cumulating daily totals obtained by subtracting minimum known developmental zero of the closely green leafhopper (9.7°C) from mean daily temperature [(temperature minimal + temperature maximal)/2] (Wuyang et al. Citation1997). The daily DD was corrected to zero if the mean daily temperature was under developmental zero of the leafhopper. The minimal theoretical DD from the egg to adult emergence (270 DD) was used to predict the emergence of the new generation (Wuyang et al. Citation1997). The weather data were recorded daily by the weather station (GP2 Data Logger, Delta-T Devices Ltd) at National Institute for Agricultural Research (Qualipole of Berkane), which was only 5 km from the study field.
The data were analyzed by IBM SPSS statistical software (2020). Analysis of variance (One-Way ANOVA) was used to determine the differences between crops on leafhopper populations.
3. Results and discussion
3.1. Identification of the green leafhopper
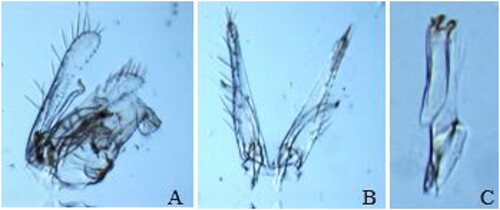
The specimens collected were identified as J. lybica (Bergevin & Zanon Citation1922). This species is morphologically similar to other closely related species in the tribe Empoascini, e.g. some species in the genus Empoasca. For example, the overall size, shape and coloration bear a striking resemblance with E. vitis, which is commonly collected in vineyards and other perennial crops in the Mediterranean region. These species can be distinguished by the examination of characters of male genitalia. Male adult (Figure ) and dissected parts of the male genitalia of J. lybica are shown in Figure , which present a pygofer distal lobe not separated from the base by a membranous cleft but with distinct internal sclerotized ridge, compared to E. vitis that has this pygofer partly separated by a membranous cleft.
3.2. Monitoring of the leafhopper
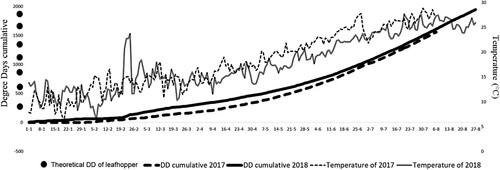
Traps monitoring for the leafhopper populations from April to August revealed that the periods of adult flight peaks differed during 2017 and 2018 (Figure ). Seven peaks of adult flight were observed and recorded during 2018, and only five peaks were observed during 2017. In comparison, six generations should have been predicted during both years according to DD obtained until mid-August.
Figure 4. Observed J. lybica leafhopper adult populations and its theoretical emergence cycles based on degree-days (DD).
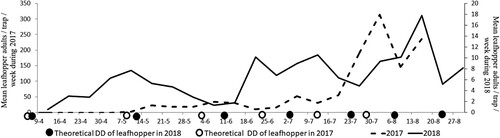
Our results indicated a maximum of 1041 and 77 adults per trap per week over the two growing seasons (2017 and 2018, respectively), during first and third week of August, respectively. According to Lentini et al. (Citation2000), three to four overlapping peaks of J. lybica were recorded in ‘Carignan’ grape located in Santadi (Southern Italy) during 1997 and 1998 and reached its maximum population level at the end of summer, with weekly captures of more than 1000 adults per trap. In Sicilia during 2002, the first appearance was at the beginning of July, with a peak of up to 270 individuals per trap found during the end of September (Mazzoni et al. Citation2003).
Due to the higher temperatures in January 2018 (by 1.29°C) than those obtained for 2017, the 270 DD required for the first leafhopper generation was reached faster in 2018 (Figure ). The difference between the two years for the development of a second generation continued to be more than a week due to high temperatures recorded for some days during the week of 19 February, 2018 (the temperature increased up to 24°C with a difference of 4°C between these 2 years for this week). This difference begins to decrease until the last week of June when the difference is only 1 or 2 days for the last generations predicted by DD due to the increased temperatures recorded in April 2017. Fornasiero et al. (Citation2012) reported that irrigation resulted in higher densities of E. vitis in vineyards, confirming findings found with other leafhoppers (Trichilo et al. Citation1990; Daane and Williams Citation2003; Costello Citation2008). Furthermore, Serra et al. (Citation2013) demonstrated over a 3-year study in Southern Sardinia that a lower restoration volume (40% of the crop evapotranspiration) allows a better water use efficiency and lower J. lybica infestations. Thus, the excessive use of irrigation in our study during summer of 2017 may have contributed to the higher densities of J. lybica, and the influence of moisture could be a parameter to be considered as a part of a management plan for controlling this insect pest in an IPM program.
Figure 5. Cumulative degree-days (DD) development with temperature during 2017 and 2018 at Qualipole of Berkane, Morocco.
From April to mid-August 2017, there were three peaks of leafhopper adults detected in citrus orchards and vineyards compared to four peaks on WB plants (Figure ). The first peak began mid-May on WB plants, with 27 leafhopper adults collected per trap that week. The maximum amount detected was 52 adults at the end of July. However, the first peak on citrus occurred during mid-June with 16 adults detected, with the highest amount of 35 adults found at the end of July. For grapes, the first peak occurred in early July with 56 adults, with the highest value of 733 adults detected at the end of the month. The average number of adults caught differed between the two crops (P = 0.000). Previous studies have shown that four to five larvae (or nymphs) per leaf or 400–500 adults per sticky trap per week are intervention thresholds for E. vitis (Redl Citation1995; Remund and Boller Citation1995; Louis Citation1996). For J. lybica, the thresholds suggested for vineyards is 4 nymphs per leaf (Prota et al. Citation1988), although Lentini et al. (Citation2000) indicated that for ‘Carignan’ grape an average of infestation of 0.5–1 leafhopper per leaf during July to August caused severe symptoms of reddish coloration in leaves, suggesting J. lybica to be more damaging than E. vitis.
Figure 6. Captures of leafhopper adults (J. lybica) in citrus, grape and windbreak plants (Cypress and Acacia) during 2017.
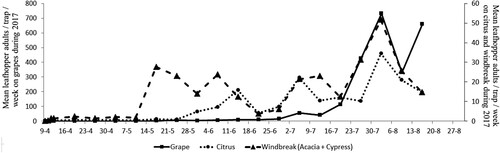
In our study, the first insecticide treatment in 2017 for leafhopper control was applied at the end of July in grapes, when injury was initially observed on leaves and 426 adults were detected from traps for that week. However, the insecticide treatments applied were not sufficient to prevent population increase and did not provide adequate control.
Leafhopper migration plays an important role in the resulting population dynamics on crops. Results from our study confirmed an earlier report by Cerutti et al. (Citation2008) who indicated that overwintering adults of E. vitis first migrated to intermediate host plants before grape bud opening. Furthermore, this was also confirmed by Decante and van Helden (Citation2006) and Van Helden et al. (Citation2003) who reported that this insect moves from overwintering plants (mainly Juniperus sp. in the Bordeaux region) during early April, and then to early budding intermediate host plants (mainly Rosaceae) before entering the vineyard in May. Our results suggest that the cotton leafhopper begins to migrate in mid-June from WB plants (Cypress + Acacia) to citrus (intermediate host plant) and then to grape in early July. Moreover, Cerutti et al. (Citation2008) also found other Gymnosperms, besides Rosaceae, as overwintering host for the adults.
J. lybica populations differed among citrus, grape and WB plantings in 2018. The first peak of leafhopper adults was observed in mid-April on WB plants with nine leafhopper adults captured per trap for that week. The highest population level on WB plants was detected in mid-May with 23 adults (Figure ). On citrus, the first peak was observed at the end of June, with a high peak of nine adults caught per trap during mid-July. On grapes, the leafhoppers were first detected in early July with 18 adults caught per trap weekly, with a high of 45 adults caught during mid-August.
Figure 7. Captures of J. lybica leafhopper adult populations in citrus, grape and windbreak plants during 2018.
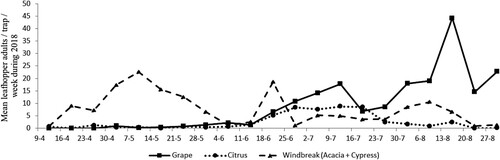
The peak of J. lybica leafhopper adult populations detected from the end of July to early August can make the most damage observed on grapes. This was the second peak of leafhopper adults on grapes, and the fifth peak overall. Controlling the first leafhopper flight peak in WBs and vineyards can be effective to maintain the population levels below the economic injury thresholds for these generations that can cause the most damage, especially in July and August on grapevines. Further studies are ongoing to suggest the importance of controlling the first leafhopper flight peak to manage and minimize damage caused by following generations. The surrounding vegetation seems to affect both the leafhopper overwintering populations and summer migrations to the preferential crop species (Cerutti et al. Citation2008). According to Delrio et al. (Citation2000), the distribution of J. lybica within the vineyard was found to be distributed in a slightly aggregated pattern, similar to E. vitis, which may be linked to the vigor of each plant. The same results were reported by Ramirez-Davila and Porcayo-Camargo (Citation2008) who found that more than one center of aggregation of this pest in grape vineyards. This difference can be also explained by the insect visual behavioral mechanism which plays an important role in leafhopper populating a vigorous host (Zhang et al. Citation2018).
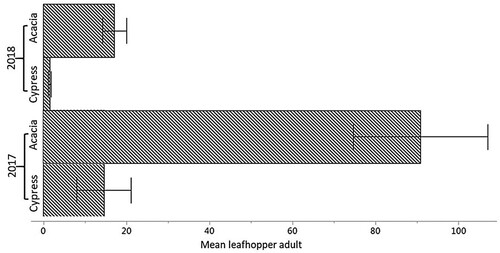
During our study, adults of J. lybica were detected on WB plants throughout the entire study period. Populations of this insect were higher on Acacia during both years compared to Cypress. During 2017, 91 leafhopper adults were found on Acacia compared to Cypress with 14 adults (Figure ). In comparison, 16 and 2 adults were detected for Acacia and Cypress, respectively, during 2018. Since our results suggest the preference of the leafhopper for Acacia over Cypress over both 2017 and 2018, the choice of WB plants can be taken into consideration for an effective integrated pest management program when trying to manage this pest. Mazzoni et al. (Citation2003) determined populations of J. lybica on Angelica sp. (Umbelliferae) and Rubus fruticosus (Rosaceae) in early winter and interrelated this to overwintering adult population.
4. Conclusion
Results from this study provided several aspects of J. lybica leafhopper ecology that was previously unknown under Moroccan conditions. The adult peaks of this insect in Moroccan environments studied herein, and the preference of this insect for Acacia over Cypress suggests that host plants in close proximity to crop production areas can be taken into consideration. Further studies still need to confirm this choice of WB that can enhance the use of an integrated pest management program for controlling this pest. The use of DD to predict the outbreak of new generations and to apply control measures at the correct time can be a promoting strategy to manage leafhopper populations below the economic thresholds. Thus, the use of DD is an important component of an integrated pest management program to not only minimize insecticide use but also to provide an effective control of this pest.
Acknowledgements
We acknowledge the Couteaux of Saidia Domains (head and technicians) for their technical support, availability and valuable advice. Dr Mohamed Sbaghi (Plant Protection Department, Scientific Division, National Institute for Agricultural Research, Rabat, Morocco) for his scientific advice and help in writing and editing the manuscript. Ms. Kseniia Koledenkova (University of Reims Champagne-Ardenne, France) for her cooperation to review the manuscript and improve the quality of pictures.
Data availability statement
The data that support the findings of this study are openly available in figshare.com at: https://figshare.com/s/09cee8c1a9f10c0a294e.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Alma A. 2002. Auchenorrhyncha as pests on grapevine. Denisia. 04:531–538.
- Baillod M, Jermini M, Schmid A. 1990. Essais de nuisibilité de la cicadelle verte, Empoasca vitis Goethe sur le cépage Merlot au Tessin et le cépage Pinot noir en Valais. IOBC/WPRS Bull. 13:158–161.
- Bergevin E, Zanon DV. 1922. Danni alla vite in cirenaica e tripolitania dovuti ad un nuovo omottero (Chlorita lybica, sp. n.). Agr Col. 16:58–64.
- Carle P, Moutous G. 1965. Observations sur le mode de nutrition sur vigne de quatre espèces de cicadelles. Ann Epiphyt. 16:333–354.
- Cerutti F, Baumgärtner J, Delucchi V. 2008. The dynamics of grape leafhopper Empoasca vitis Göthe populations in southern Switzerland and the implications for habitat management. Biocontrol Sci Technol. 1:177–194.
- Chancellor TCB, Cook AG, Heong KL. 1996. The within-field dynamics of rice tungro disease in relation to the abundance of its major leafhopper vectors. Crop Prot. 15:439–449.
- Costello MJ. 2008. Regulated deficit irrigation and density of Erythroneura spp. (Hemiptera: Cicadellidae) on grape. J Econ Entomol. 101:1287–1294.
- Daane KM, Williams LE. 2003. Manipulating vineyard irrigation amounts to reduce insect pest damage. Ecol Appl. 13:1650–1666.
- Decante D, van Helden M. 2006. Population ecology of Empoasca vitis (Göthe) and Scaphoideus titanus (Ball) in Bordeaux vineyards: influence of migration and landscape. Crop Prot. 25(7):696–704.
- Del-Campo-Sanchez AI, Ballesteros R, Hernandez-Lopez D, Fernando Ortega J, MorenoID MA. 2019. Quantifying the effect of Jacobiasca lybica pest on vineyards with UAVs by combining geometric and computer vision techniques. PLoS One. 14(4):e0215521.
- Della Giustina W, Bonfils J, Le Quesne WJ. 1989. Homoptères cicadellidae. Fed Fran Soci Sci Nat. 3:3–362.
- Delrio G, Lentini A, Serra G. 2000. Spatial distribution and sampling of Jacobiasca lybica on grapevine. IOBC/wprs Bull. 24(7):211–216.
- Dworakowska I. 1972. On some oriental and ethiopian genera of Empoascinii (Auchenorrhyncha, Cicadellidae, Typhlocybinae). Acad Pol Sci Bull Ser Sci Biol. 20:25–34.
- Dworakowska I. 1980. On some Typhlocybinae from India (Homoptera, Auchenorrhyncha, Cicadellidae). Entomologische Abhandlungen Berichte Staatlichen Museum Tierkunde Dresden. 43(8):151–201.
- Emmen DA, Fleischer SJ, Hower A. 2004. Temporal and spatial dynamics of Empoasca fabae (Harris) (Homoptera: Cicadellidae) in Alfalfa. Environ Entomol. 33:890–899.
- Fornasiero D, Duso C, Pozzebon A, Tomasi D, Gaiotti F, Pavan F. 2012. Effects of irrigation on the seasonal abundance of Empoasca vitis in North-Italian vineyards. J Econ Entomol. 105(1):176–185.
- Göthe H. 1875. Die ursachen des schwarzen Brenners an den Reben. Wien Landw Zeit. 1875:397–398.
- Holt J, Chancellor TCB, Reynolds DR, Tiongco ER. 1996. Risk assessment for rice planthopper and tungro disease outbreaks. Crop Prot. 15:359–368.
- Klerks W, Van Lenteren J. 1991. Natural enemies of Jacobiasca lybica (De Berg): a literature survey. Proc Exper Appl Entomol. 2:208–213.
- Lamp WO, Zhao L. 1993. Prediction and manipulation of movement by polyphagous, highly mobile pests!. J Agrie Entomol. 10:267–281.
- Lentini A, Delrio G, Serra G. 2000. Observations on the infestations of Jacobiasca lybica on grapevine in Sardinia. OBC/wprs Bulletin. 23(4):127–129.
- Louis F. 1996. Die grüne rebzikade—Gefahr? Dtsch Weinbau. 8:18–20.
- Manzella S, Ammavuta G, Bono G, Federico R, Spatafora F. 2001. Eccezionale infestazione di cicalina africana nei vigneti della Sicilia occidentale. L’Informatore Agrar. 57:147–148.
- Mazih A. 2015. Status of citrus IPM in the southern Mediterranean basin Morocco, North Africa. Acta Hortic. 1065:1097–1104.
- Mazzoni V, Lucchi A, Varner M, Mattedi L, Bacchi G, Bagnoli B. 2003. First remarks on the leafhopper population in a vine-growing area of South-Western Sicily. IOBC/WPRS Bull. 26(8):227–231.
- Moore SD. 2013. Leafhoppers and planthoppers. Citrus Res Int. 3:1–3.
- Ocete R, Lopez MA, Quartau J, Pèrez A. 1999. La problemática actual de los “mosquitos verdes” (“Homoptera, Cicadellidae”) en diversas zonas vitícolas españolas - Dialnet. Vitic Prof. 63:16–20.
- Pavan F, Stefanelli G, Villani A, Gasparinetti P, Colussi G, Mucignat D, Del Cont Bernard D, Mutton P. 2000. Danni da Empoasca vitis (Goethe) (Homoptera: Cicadellidae) in vignetidell ÕItalianord-orientale e soglied Õintervento. Frust Entomol. 21:109–124.
- Prota R, Delrio G, Luciano P. 1988. Prospect of integrated control of the main pests in Sardinian viticulture. International Symposium on Plant Protection Problems and Prospect Integrated Control Viticulture. p. 755–761.
- Ramirez-Davila JF, Porcayo-Camargo E. 2008. Spatial distribution and mapping of Jacobiasca lybica (Bergevin and Zanon) (Hemiptera: Cicadellidae) egg populations in irrigated sherry vineyards. Rev Chil Entomol. 34:37–55.
- Redl H. 1995. Verstärkter befall der rebenbei IP durch die grüne rebzikade. Pflanzenarzt. 48:8–9.
- Remund U, Boller E. 1995. Untersuchungenzur grünen rebzikade in der ostschweiz. Schweiz Z Obs. 8:200–203.
- Serra G, Cocco A, Mameli MG, Delrio G, Lentini A. 2013. Influence of regulated deficit irrigation and partial rootzone drying on leafhoppers infestations on grapevine. Integr Prot Prod Vitic IOBC-WPRS Bull. 85:117–120.
- Taylor PS, Shields EJ. 1995. Phenology of Empoasca fabae (Harris) (Homoptera: Cicadellidae) in its overwintering area and proposed seasonal phenology. Environ Entomol. 24:1096–1108.
- Trichilo PJ, Wilson LT, Grimes DW. 1990. Influence of irrigation management on the abundance of leafhoppers (Homoptera: Cicadellidae) on grapes. Environ Entomol. 19:1803–1809.
- Tsolakis H. 2003. La cicalina africana Jacobiasca lybica Bergevin (Homoptera, Cicadellidae) ricompare nei vigneti siciliani. Inf Fitopatol. 53:34–40.
- Van Helden M. 2000. La cicadelle verte (Empoasca vitis Goethe). Les Ravag la vigne Ed Féret, Bordeaux, Fr:121–129.
- Van Helden M, Decante D, Papura D. 2003. Possibilities for conservation biological control against grape pests in the Bordeaux region. Bull OILB/SROP. 26:191–196.
- Vidano C. 1962. La Empoasca lybica Bergevin, nuovo nemico della vite in Italia. Ital Agric. 99:329–344.
- Wiliam DG. 2002a. Les cicadelles nuisibles à l’agriculture 1e partie. Insectes. 126:3–6.
- William DG. 2002b. Les cicadelles nuisibles à l’agriculture 2e partie. Insectes. 127:25–28.
- Wuyang Z, Minxiong L, Hangu Z. 1997. Relationship between temperature and development of Empoasca vitis Gothe (Lepidoptera: Cicadelidae). J Anhui Agric Coll. 24:332–335.
- Zhang X, Pengsakul T, Tukayo M, Yu L, Fang W, Luo D. 2018. Host-location behavior of the tea green leafhopper Empoasca vitis Göthe (Hemiptera: Cicadellidae): olfactory and visual effects on their orientation. Bull Entomol Res. 108(4):423–433.