Abstract
Autophagy process coordinates lysosomal destruction of cytoplasmic materials including damaged organelles and proteins. The double-membranous ‘autophagosome’ selectively sequesters the cytosolic components, followed by ‘autolysosome’ formation for degradation. By recycling energy, autophagy preserves intracellular homeostasis. Autophagy also acts a crucial function in various cellular events including cell survival and death in response to stressed conditions. Thus, any dysfunction in autophagy causes a wide variety of pathologies including cancers. Multiple lines of evidence have decorated the importance of autophagy for the regulation of cancer development. Aberrant autophagy can govern healthy normal cells to endure the transformation of malignant cells. By comparison, cancer cells are apt to control autophagy to bloom it and to repel therapeutic challenges. Hence, the modulation of autolysosomes can assassinate malignant cells or sharpen them for therapy. Most current research is trying to manipulate autophagy appropriately for cancer therapies and a wide variety of medical objectives are turning forward to inhibit the acidification of lysosomes and autolysosomes with repurposing drugs. This review explores the lysosomotropic agents be utilized to treat cancers through their inhibiting roles in the acidification system and discuss the preclinical and clinical clues for hints the next generation of pharmacological agents for the treatment of cancers.
Introduction
Autophagy is a lysosome-oriented degrading process of potentially dented or redundant cytoplasmic entities for promoting nutrient recycling (Towers et al. Citation2020; Zada et al. Citation2021). Autophagy maintains cell homeostasis regarding several stressful states such as deprivation of nutrient, hypoxia or endoplasmic reticulum (ER) damage. Under basal level conditions, autophagy regulates metabolic and cellular homeostasis (Ceccariglia et al. Citation2020). This process is essential in all mammalian cells to generate building block molecules for supporting other cellular processes (Amaravadi et al. Citation2016; Yun and Lee Citation2018; Yazdani et al. Citation2019). This process also maintains cellular fitness by supplying energy and nutrients through both nonselective and selective degradation of macromolecules and cellular organelles in response to several stresses including hypoxia (He et al. Citation2018; Yu et al. Citation2018). Accumulating evidence has suggested that any dysfunctions in this intricate and securely regulated process such as inherited mutations in the highly conserved autophagy-related (ATG) genes have been concerned in a wide variety of physiological disorders and human diseases (Table ).
Table 1. Dysfunctional autophagy is associated with different human diseases.
Three major autophagic molecular features have been mechanistically described based on the target substrates involved in lysosomal degradation (Figure ), namely macroautophagy (simply autophagy), chaperone-mediated autophagy (CMA) and microautophagy (Galluzzi et al. Citation2017). Autophagy occurs in multi-stages such as initiation, elongation, closure, docking and fusion with the endolysosomal system with the regulation of the ATG genes (Al-Bari Citation2020; Devis-Jauregui et al. Citation2021). Autophagy begins with an isolating membrane (IM) formation, previously familiar with phagophore. Although the origin(s) of IM in autophagy has long been debated, several membranous organelles such as mitochondria, ER, Golgi apparatus, or plasma membrane (PM) and their contact sites such as ER exit sites (ERES), ER–Golgi intermediate compartment (ERGIC) have been linked up the membrane donors for the nascent IM (Hurley and Young Citation2017; Al-Bari Citation2020). The IM is sequestrated in the cytoplasmic cargoes to form autophagosomes that then dock and fuse with the lysosomes for the generation of de novo structures called ‘autolysosomes’ (Al-Bari Citation2020). Finally, the degradation of autophagic cargo is triggered by the lysosomal hydrolytic niche (low pH with acidic hydrolases) (Noda and Inagaki Citation2015; Hurley and Young Citation2017; Al-Bari Citation2020; Devis-Jauregui et al. Citation2021) to split up into several nutritional constituents into the cytoplasm for energy production and cellular repairing (Lamb et al. Citation2013).
Figure 1. Different classes of autophagy process. The autophagy is classified as (A) macroautophagy where cytosolic constituents are submerged and delivered to the lysosome; (B) microautophagy where cytosolic wastage products are straightly submerged and co-opted by lysosome via redisposition of its membrane and (C) chaperone-mediated autophagy (CMA) where KFERQ motif holding cellular proteins documented by the cytosolic HSP70 chaperone and translocated to the lysosomal membrane via interacting with LAMP2 receptor leading to their degradation. Macroautophagy (simply autophagy) proceeds the isolation membrane (IM) formation. Cellular stresses culminate in AMPK stimulation and mTORC1 suppression that in turn makes the ULK1 complex dissociated with mTORC1 and initiates autophagy. Through a series of interchain responses, this IM confiscates its cargos into an autophagosome (AP, a double-membrane vesicle) that in turn kisses and fuses with the lysosome to make autolysosome (AL). The AL cargos are degraded by an acidic niche containing hydrolytic enzymes in lysosomal lumen. The degrading products are used for energy production or biosynthesis of macromolecules. TFEB coordinates the stress responses like starvation via lysosome and controls countless cellular processes including autophagy and lysosomal biogenesis (Al-Bari and Xu Citation2020).
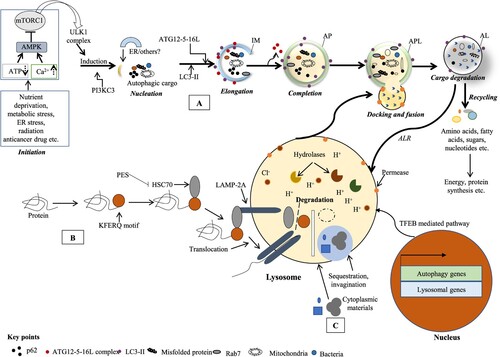
In response to various stresses (intracellular or environmental), autophagy initiation is controlled chiefly by two important cellular pathways: mTOR (mechanistic target of rapamycin)-dependent and -independent pathways (Sabatini Citation2017; Al-Bari and Xu Citation2020). In response to amino acids or growth factors, mTOR pathway is induced which in turn phosphorylated and incorporated several ATG genes within mTOR complex and inhibited autophagy. For example, when mTORC1 is active in nutrient-rich states, associates with UNC51-like kinase 1 (ULK1) complex (not free from the ULK1 complex) through Raptor and phosphorylates ULK1 at Ser758 and Ser638, and ATG13 at Ser258 resulting inhibition of autophagy (Dossou and Basu Citation2019). Conversely, during nutrient deprivation, mTOR pathway is suppressed and initiated autophagy because inactive mTOR complex releases several autophagy. The major events of mTOR pathway-dependent regulation of autophagy are represented here. The initiation stage is controlled by AMPK (AMP-activated protein kinase), ULK1 and mTOR complex 1 (mTORC1). mTOR pathway is inhibited by AMPK that induces the autophagic process (Kim et al. Citation2011; Yun and Lee Citation2018). Here, mTORC1 acts as a chief inhibitor of autophagosome development by ULK1. Under glucose deprivation, the activated AMPK suppresses mTORC1, then phosphorylates ULK1 in a straight way and leads to initiate autophagy (Kim et al. Citation2011). Nucleation stage of the IM is interceded by the complex of Beclin-1-class III phosphatidylinositol 3-kinase (PI3 K) (class III PI3 K) that comprises Beclin-1, Vps34 (class III PI3 K), p150, ATG14L and the activating molecule in Beclin-1-regulated autophagy (AMBRA-1) (Mizushima et al. Citation2011). IM elongation stage turning to completed autophagosome is controlled by two ubiquitin-like protein complexes: ATG5–ATG12–ATG16L1 and the microtubule-associated proteins 1A/1B light chain 3B (called LC3-II). Several ATGs such as ATG7 and ATG10 are required peacekeepers of the process (Ichimura et al. Citation2000; Behrends et al. Citation2010). LC3-1, the key mammalian ortholog of ATG8 is transformed to LC3-II and tarnished after the fusion of autophagosomes with lysosomes. The final degradation stage is the production of nutrient materials (Al-Bari Citation2020).
At a glance of lysosomes
Lysosomes are housekeeping organelles that play a vital role in the conservation of intracellular homeostasis by degrading cellular and extracellular constituents (Ballabio Citation2016). Over the last 10 years, innovation in lysosome biology research has recognized an extensive role of lysosomes in the pathology of diseases as well as focused on targeting the lysosome in different diseases (e.g. cancers). The magnitude of lysosomes varies from 0.1 to 1.0 µm in mammalian cells, which is suggested to be lesser than autophagosome ranges (0.5–1.5 µm) (Burman and Ktistakis Citation2010; Mizushima and Komatsu Citation2011). Anatomically, the trafficking vesicles (Figure ) may suggest as early endosomes (pH of 6.0–6.6), late endosomes (extra acidic pH of 5) or the highly acidic organelles lysosomes (pH of 4.5) (Mindell Citation2012; Nwadike et al. Citation2018; Chen et al. Citation2020). Therefore, the autolysosome, the fused form of lysosome with autophagosome would necessary to partake in a firmly practical adjustment to preserve optimal acidic pH for executing digestion of the intraluminal materials. The autolysosome retains lower pH than the lysosome based on the autophagosome. Lysosomes are surrounded by integral lipoprotein membranes called lysosome-associated membrane proteins 1 and 2 (LAMP1 and 2) (Saftig and Klumperman Citation2009) and contain an acidic interior with different soluble hydrolases (e.g. cathepsins) (Piao and Amaravadi Citation2016). LAMP1 and 2 are essential for lysosomal regeneration, maintenance lysosomal acidity as well as CMA (Saftig and Klumperman Citation2009). As a receptor of CMA, LAMP2 can be reallocated from the lumen to lysosomal membrane (Cuervo and Wong Citation2014). In addition, heat shock protein 70 (HSP70) can protect lysosomal membrane permeabilization (LMP) (Tang et al. Citation2017). Lysosomes contain at least 60 soluble hydrolases within the acidic niche (Saftig and Sandhoff Citation2013). Lysosomal acid hydrolases execute their best action at pH 4.5–5.0 within the acidic lysosome. These hydrolases are accountable for the degradation of waste materials and salvaging of essential nutrients to preserve cellular homeostasis (Saftig and Klumperman Citation2009; Stoka et al. Citation2016). The vacuolar type ATPase (v-ATPase), an ATP-driven hydrogen (H+) ion pump, uses the energy potential by hydrolyzing ATP into ADP to drive H+ ions into the intralysosomal lumen. As a result, ATP-driven proton (H+) pump maintains optimal acidic niche (pH of ∼4.5–5.0) comparatively to somewhat basic cytosol (pH 7.2) (Mindell Citation2012; Settembre et al. Citation2013) for proper activities of lysosomal hydrolases (Pamarthy et al. Citation2018). The acidic lysosomal pH is also crucial for the transport and maturation of lysosomes as well as autophagosomes (Al-Bari Citation2020). Moreover, the acidic lysosomal pH is also applied by transmembranous permeases to disseminate digestive products to the cytosol (Perera and Zoncu Citation2016).
Figure 2. The central position of lysosomes at the ALP. Activated lysosomes and autolysosomes are associated with terminal degradation stage through endocytic and autophagic pathways. Exogenous products are endocytosed by endosomes and autolysosomes act as an integral part of the autophagy for degradation. The membrane of lysosome plays as a podium for the gathering of mTORC1 complex, which plays a main role for nutrient sensing signals of lysosomes to control energy levels through downstream mediators including TFEB. Interior pHs of intracellular vesicles steadily decrease laterally from early endosomes to late endosomes to lysosomes, the mostly acidic organelles. Lysosomes create and preserve their pH homeostasis by applying v-ATPase carrier. The positive charged ions such as K+ can be dispatched by K+ ion channel or by exchanged by Cl− influx carrier via ClC-7, a Cl−/H+ antiporter.
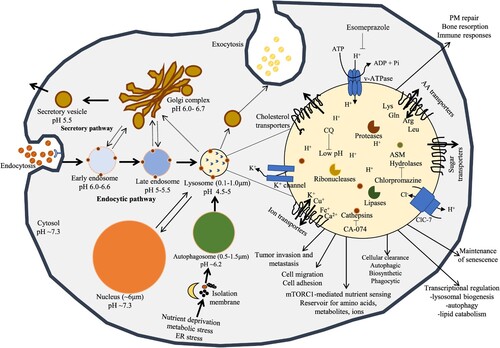
In the case of garbage disposal system in the cell, autophagosomes transport the intracellular components to the lysosomes, and endocytic and phagocytic systems transport exogenous factors to the lysosomes (Lopez-Hernandez et al. Citation2020). Here, the centrally acting lysosomes regulate autophagy in four main ways: supporting IM formation, lysophagy, autolysosomal degradation and autophagic lysosomal reformation (ALR). By interacting with special membrane contact sites of other organelles including ER, peroxisomes and mitochondria, lysosomes donate membranes for IM (Hurley and Young Citation2017; Simmen and Tagaya Citation2017; Al-Bari Citation2020). Selective autophagy controls the homeostasis of lysosomes when damaged lysosomes LMP are not repaired. The injured lysosomes are submerged by autophagosomes which in turn fuse with efficient lysosomes to eliminate them (Trivedi et al. Citation2020). The levels of lysosomes can be reestablished through ALR (autophagic lysosomal reformation) process (Chen and Yu Citation2018; Al-Bari Citation2020). The lysosomal homeostasis is then reestablished by its biogenesis.
Lysosomal repair and lysophagy
Under biological conditions, the lysosomal quality and quantity are constantly regulated by its homeostatic pathway poised between development and degradation (Maejima et al. Citation2013; Zhu et al. Citation2020). The integrity and optimum activity of lysosomes are very important for cell survival. Lysosomal membrane damage (LMD) disrupts cytosolic harmony causing the release of intralysosomal acidic components, which trigger a series of apoptotic pathways including ferroptosis (Zhu et al. Citation2020). Several signal stressors such as lysosomotropic drugs, pathogens, reactive oxygen species (ROS) and other factors may lose membrane integrity and inflict varying extent of LMP and LMD and these are potential hazards to cell fate (Zhu et al. Citation2020). For preserving lysosomal integrity and intracellular homeostatic balance, renewal of lysosomes is rapidly commenced in the LMD state, the process called lysosomal quality control (LQC) encompassing lysosomal repair, lysophagy and lysosomal regeneration (Maejima et al. Citation2013; Zhu et al. Citation2020). Indeed, the imbalance of lysosomal homeostasis is crucial for the development of several degenerative pathologies, including cancers, inflammatory diseases and infections (Zhu et al. Citation2020). Thus LQC makes a novel but potential target of lysosomal impairment and additional investigation for effective disease managements.
Interestingly, the LMD can be repaired and prevented the release of luminal hydrolases by the actions of HSP70 and ESCRT (endosomal sorting complex required for transport) machinery (Papadopoulos et al. Citation2020; Zhu et al. Citation2020). Earlier work appreciated the defensive activity of HSP70 for preserving lysosomal integrity. HSP70 binds to bis(monoacylglycerol)phosphate that leads to an increase in the activity of acid sphingomyelinase (ASM) to regulate optimum lipid configuration (Mahapatra et al. Citation2021) and prevent LMP (Papadopoulos et al. Citation2020). Recent studies showed that the ESCRT machinery comprising four complexes (ESCRT-0, -I, -II, -III) orchestrates for the recovery of LMD and cell survival through multiple mechanisms in a coordinated manner (Christ et al. Citation2017; Radulovic et al. Citation2018; Skowyra et al. Citation2018; Zhu et al. Citation2020). ESCRTs can renovate several tiny perforations in the lysosomal membrane at the initial stage of its injury (Skowyra et al. Citation2018). After LMP, the Ca2+ release from injured lysosomes into the cytosol enhances the lipid-binding capacity of ALIX (apoptosis-linked gene-2-interacting protein X), a constituent of the ESCRT machinery and activated the CHMP4B (multivesicular body protein 4B), a core factor of the ESCRT-III machinery for the regulation of the healing process. Moreover, TSG101 (tumor susceptibility gene 101 protein), a factor of the ESCRT-I machinery, is found to be vital in ALIX deficiency and helps the repairing machinery to the membranes of lysosomes (Papadopoulos et al. Citation2020; Zhu et al. Citation2020; Mahapatra et al. Citation2021). Although the exact mechanistic pathways by which ESCRT machinery heals the lysosomal membranes are ambiguous, they could be associated with promoting the development of filamentous helices and the tightening of pores in lipid bilayers on the membrane surface (Christ et al. Citation2017).
When an extensive injury of the lysosomal membrane occurs and the LMP cannot be repaired, selective autophagy of lysosomes, called lysophagy is initiated to clear the dented lysosomes (Serrano-Puebla and Boya Citation2016; Papadopoulos and Meyer Citation2017; Papadopoulos et al. Citation2020). Similar to other selective autophagy, in lysophagy the impaired lysosomes are marked with ubiquitin and ultimately fused with normal effective lysosomes through autolysosomal formation (Yao et al. Citation2021). Two ubiquitination pathways regulating lysophagy have been identified: cytosolic lectins such as galectins (Gals) and the ligase complex Skp1/CUL1/F-box (SCF) with F-box protein 27 (SCFFBXO27) (Thurston et al. Citation2012; Maejima et al. Citation2013; Yoshida et al. Citation2017; Yao et al. Citation2021). The galactose-containing lectins, Gals are usually localized in the cytosols where galactose-moiety chains are anchored at the cell surface (Li et al. Citation2021). Gals are crucial for monitoring lysophagy because LMD and its elimination can be measured by observing the levels of Gals (Thurston et al. Citation2012; Maejima et al. Citation2013; Papadopoulos and Meyer Citation2017). Gals such as Gal-1, -3, -8, and -9 play as radars for various types of LMD by interacting with β-galactosides on the luminal side of its membrane (Chauhan et al. Citation2016; Zhu et al. Citation2020). For example, LMD permits the Gal-3 recruitment to its broken membrane (Paz et al. Citation2010; Li et al. Citation2021) and induces lysophagy by activating several autophagy regulators such as ULK1, Beclin 1 and ATG16L after binding with TRIM16 (tripartite domain containing protein 16) (Chauhan et al. Citation2016). Finally, Gal-3 is adapted by a K63 ubiquitin chain and interacts with LC3 on the IM via p62, an autophagy receptor and finally forms an autolysosome (Chauhan et al. Citation2016). Likewise, Gal-8 and Gal-9 can also be engaged in dented lysosomes and promote lysophagy through various pathways (Thurston et al. Citation2012; Chauhan et al. Citation2016). For instance, Gal-8 specifically interacts with LC3 on the IM through CALCOCO2 (Ca2+-binding and coiled-coil domain-containing protein 2), an autophagy receptor, resulting in the degradation of dented lysosomes (Thurston et al. Citation2012; Zhu et al. Citation2020). For activation of lysophagy, SCFFBXO27 is also engaged in impaired lysosomes and ubiquitinates LAMP2 by interacting with LC3 on the IM surface via p62 (Yoshida et al. Citation2017; Zhu et al. Citation2020).
Two important signaling pathways can induce the biogenesis of new lysosomal components. One pathway is the ESCRT machinery-dependent restoration of dented lysosomes. The second pathway is initiated by the detachment of mTORC1 from the dented lysosomes (Jia et al. Citation2018; Papadopoulos et al. Citation2020). Since mTOR usually preserves an inactive form of the TFEB (transcription factor EB) by phosphorylation, mTORC1detachment causes TFEB dephosphorylation and nuclear translocation leading to lysosomal generation by TFEB interacting with CLEAR (coordinated lysosomal expression and regulation) (Settembre et al. Citation2013; Papadopoulos et al. Citation2020).
Lysosomal involvement in cancer
Lysosomal action and dysfunction have been shown to act vital roles in cancers; however, the mechanisms in which lysosomes impart to develop cancer and metastasis are still being explored. Quickly dividing cells like cancers are extremely reliant on efficient lysosomes to maintain homeostasis under stress conditions in the TME (Ballabio Citation2016; Ballabio and Bonifacino Citation2020). In parallel, dramatic variations in volume, composition and subcellular localization of lysosomes occur during the conversion of normal pre-malignant cells to tumorigenesis and progression (Kirkegaard and Jaattela Citation2009; Kallunki et al. Citation2013). Many cancer cells use alternative metabolic pathways that can increase ROS turning to dysregulation of lysosomes and cause LMP (Aits and Jaattela Citation2013). Moreover, in large arrays of cancers, lysosomal cathepsins are strongly expressed and delocalized during cancer progression via LMP (Kallunki et al. Citation2013). Mislocalization of these cathepsins to the extracellular matrix (ECM) stimulates tumor cell survival, angiogenesis and invasion (Gocheva et al. Citation2006). In addition, secretory lysosomal glycosidases such as β-N-acetylglucosaminidase (β-NAG) enable ECM degradation (Ramessur et al. Citation2010). Sphingosine kinase (SK) is up-regulated (Le Scolan et al. Citation2005) and ASM is downregulated (Petersen et al. Citation2013) in different types of cancer cells. These variations also affect the structural integrity of the lysosomal membrane turning to LMP. Lysosomes also act an essential function in drug resistance in cancer by confiscating weakly basic chemotherapies within the cells as well as an exocytotic mechanism (Zhitomirsky and Assaraf Citation2016). Thus cancer cells become resistant to chemotherapy and the importance of lysosomes as a therapeutic approach for cancer treatment.
It is growing evidence that the dysregulation of homeostatic dynamics in tumor cells by modifications of metabolic pathways and pH gradient reversal is a key factor for succeeding cancer development, invasion and metastases (Fais et al. Citation2007; Taylor et al. Citation2015). Cancer cells cannot endure extreme proton (H+) in their niches without appropriate removal. These cells exploit numerous routes to expel H+ ions, causing basic intracellular and acidic extracellular TME, commonly called as pH gradient reversal. In the pH gradient reversal, acidic extracellular pH (pHe) changes from normal healthy cells (∼7.4) to cancer cells (6.5–7.0) whereas preserving neutral-to-basic intracellular pH (pHi) of normal healthy cells (∼7.2) or additional basicity in it as high as value 7.6 in tumor lesions (De Milito et al. Citation2010). The acidic tumor pHe initiates cell survival and multiplications, tends to chemoresistance and provokes metastatic budding (Luciani et al. Citation2004; Rofstad et al. Citation2006) whereas the preservation of basic pHi bears resistance to cytotoxicity (Coss et al. Citation2003). Moreover, the acidic tumor pHe stimulates immune escape of tumor cells and efficient proteolytic degradation of ECM by invasive tumor cells (Brown and Murray Citation2015; Huber et al. Citation2017). The possible mechanisms conveying pH gradient reversal comprise the upregulation of multiple PM-oriented ion pumps including Na+/H+ exchanger 1 (NHE1), v-ATPase as well as carbonic anhydrases recognized as proteins for net acid bulge (White et al. Citation2017). Moreover, v-ATPase expels H+ ions from the cytoplasm into intracellular organelles including lysosomes which act as main intracellular H+ ion stores (Maxson and Grinstein Citation2014; Oot et al. Citation2017). The activity of v-ATPase is boosted in tumor cells (Sennoune et al. Citation2004) and knockdown of the c subunit of v-ATPase diminishes cell proliferation of hepatocellular carcinoma (HCC) and sensitizes breast cancers to chemotherapies (You et al. Citation2009) suggesting that dysfunctional pH gradient in cancers is well adjusted by several ion transporters (De Milito et al. Citation2010). Moreover, v-ATPase acts as a master mediator of the transcription factor, E2F1-mediated lysosomal trafficking and mTORC1 stimulation in malignancy (Meo-Evoli et al. Citation2015). Recent study suggests that STAT3 (signal transducer and activator of transcription-3) confines to the lysosomal membrane and associates with coiled-coil domain in v-ATPase. STAT3 augments lysosomal acidity by activating v-ATPase. Moreover, STAT3 transports between nuclei and lysosomes on the basis of cytosolic pH, shifting its actions as a v-ATPase activator. This action is important for preserving basic cytosol in normal state and once cancers challenge acidifying stress (Liu et al. Citation2018). Thus the pH gradient reversal is emergently associated with as a widespread trademark of cancer detected in malicious tumors (Stock and Pedersen Citation2017; White et al. Citation2017). This differential pH gradient concept becomes a potential exploitable avenue for the treatment of cancer by exploiting features of TME to destroy cancer cells.
Autophagy-lysosomal pathway and its regulation
The autophagy-lysosomal pathway (ALP) regulates a variety of signaling pathways via autophagy and endocytosis to preserve cellular homeostasis (Aits and Jaattela Citation2013; Saftig and Sandhoff Citation2013; Kroemer and Galluzzi Citation2017). In recent years, the insight of ALP has changed that the pathway participates in many other cellular processes (Ballabio Citation2016; Ballabio and Bonifacino Citation2020) such as PM restoration, exosome release, cell migration, and tumor metastasis (Figure ). However, defects of ALP have been shown to trigger different types of sporadic lysosomal storage disorders (LSDs) (Parenti et al. Citation2015; Platt et al. Citation2018). In addition, the role of dysregulated ALP has been widely studied in current years in several common human diseases including cancers (Parenti et al. Citation2015; Platt et al. Citation2018; Chang and Dong Citation2020). Thus the ALP is an important validated target of therapeutic interventions with importance on their significance for cancer therapy.
Regulation of autophagy-lysosomal pathway
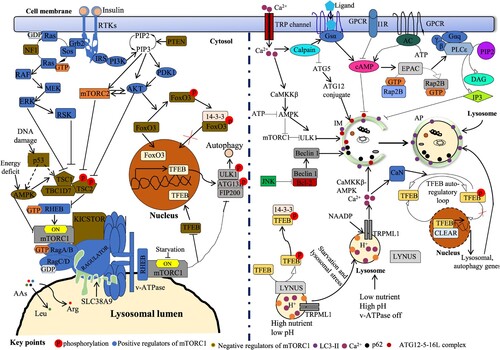
The ALP is an essential pathway for regulating intracellular homeostasis. Predictably, the ALP is co-ordinately regulated in many pathways to ensure the clearance efficacy of excessive and damaged proteins and organelles. The well-known controller of autophagy is the mTORC complex (Figure ) and based on the structural differences, this complex is subdivided into mTORC1 and mTORC2. mTORC1 replies to numerous input signals including nutrition status, growth factor levels and cellular energy demands. Amino acid-mediated cellular sensing occurs within the lumens of lysosomes, while other amino acids are sensed by cytosolic proteins (Jewell et al. Citation2013). Specifically, amino acid sensors govern the localization and activation of mTORC1. For example, glutamine (Gln), leucine (Leu), and arginine (Arg) are sensed by solute carrier family 38 member 9 (SLC38A9) and influence mTORC1 stimulation via mechanisms connecting to lysosomal v-ATPase activity and the complex ‘Ragulator’. It has been found that the localization of mTORC1 to the membrane of lysosome is activated by amino acid-mediated enrolment of GTP-binding protein RHEB and Ras-related GTP binding protein A (Rag GTPase). The v-ATPase on the lysosomal membrane is also tangled to amino acid sensing and connects with Rag GTPase and Ragulator (Al-Bari and Xu Citation2020). Amino acids stimulate RAGs with Ragulator facilitated the activation of RAGs followed by interacting with mTORC1. Thus the above mechanistic pathways are called the LYNUS (lysosome nutrient-sensing) machinery (Figure ).
Figure 3. Control of ALP by mTORC1-dependent and independent signal transductions. Amino acid (AA) signal, growth factors like insulin and cellular ATP regulate autophagy via mTORC1 mTORC1-dependent and independent pathways. Insulin stimulates both mTORC1 and mTORC2 via the traditional PI3K-AKT-TSC1/2 (tuberous sclerosis complex 1/2)-RHEB pathway. In Ras-MAPK (ERK) signal, ERK and RSK suppress TSC2 to induce mTORC1. Low nutrition status induces AMPK and TSC complex, suppressing RHEB and mTORC1. Carriage of AAs controls mTORC1 action via AA transporters like SLC3A2 in cell membrane as well as SLC38A9 in lysosomal membrane. The RAGULATOR complex ties up the RAGs to the surface of lysosome. V-ATPase is essential for the induction of mTORC1 on lysosomal surface. RAG A/B is controlled by the GATOR1 complex and GATOR2. KICSTOR complex acts as an essential action in tethering GATOR1 to the lysosomal surface, thus suppressing the activity of RAG A/B and mTORC1. Lysosomal membrane-oriented arginine (Arg) transporter, SLC38A9 binds with the RAGs and RAGULATOR to stimulate mTORC1. DNA damage inhibits mTORC1 activity by activating p53 in an AMPK- and TSC1/2-conditional way. Activated mTORC1 controls TFEB intracellular position via its phosphorylation (at Ser211) and subsequent confiscation with 14-3-3 protein. mTORC2 regulates autophagy via FOXO3 pathway. Phosphorylated FOXO3 interacts with 14-3-3 protein in cytoplasm and prevents induction of gene transcription. mTOR-independent cell signaling controlling autophagy comprises cAMP-Epac-PLCϵ-IP3, Ca2+-calpains–Gsα and inositol (Ins) signaling pathways. Intracytosolic Ca2+ status can be amplified by multiple Ca2+ channels including the TRP channel. An upsurge in cytosolic Ca2+ levels stimulates calpains and then Gsα/AC/cAMP/Epac/Rap2B/PLCϵ signal which regulates autophagy by endorsing IP3 via its receptor, IP3R activation. IP3 binds to IP3 receptor (IP3R) on ER to release stored Ca2+ that impairs AP maturation by blocking AP-lysosome fusion. Ca2+ efflux through lysosomal NAADP triggered TRP channel can be extra augmented by Ca2+ secretion from IP3R causing activation of the CaMKKβ–AMPK signal and AP formation. However, Ca2+ secretion from lysosomes can influence lysosomal pH and repeal fusion of lysosomes with APs. On the other hand, an upsurge Ca2+ concentration in cytosol next stimulates CAMKK-β, tracked by AMPK-mediated autophagy induction. In normal cases, phosphorylated TFEB by mTORC1 is sequestered with 14-3-3 protein in cytoplasm. Under fasting, lysosomal mTORC1 is inactive form and upsurge Ca2+ is secreted from lysosome via TRPML1 channel. Then Ca2+ stimulates CaN (calcineurin) that interacts and dephosphorylates TFEB. This form of TFEB is incapable to interact with 14-3-3 protein and easily moves to the nucleus (Al-Bari and Xu Citation2020).
Transcriptional regulation of autophagy-lysosomal pathway
Several stressful environments such as starvation, hypoxia and chemotherapies stimulate autophagy in cancer cells via transcriptional regulation. In tumor cells, autophagy usually supports cell growth in hypoxic sections (Singh et al. Citation2018). HIF-1α (hypoxia-inducible transcription factor 1α) transcribes several genes that endorse angiogenesis as well as reduce mitochondrial biogenesis and respiration (Wang et al. Citation2017). As a master controller, TFEB regulates the harmonization of several steps in ALP such as autophagosome, autolysosome and lysosomal biogenesis (Al-Bari Citation2020; Al-Bari and Xu Citation2020). TFEB also interacts with a promoter region for the CLEAR network element that regulates several processes such as autophagy, biogenesis of lysosomes and PM repair (Palmieri et al. Citation2011).
Under fed conditions, TFEB is provisionally engaged to lysosomes via binding to active RAG GTPase, a constituent of the mTORC1-RAG GTPase-regulator complex at the lysosomal surface (Martina et al. Citation2012; Settembre et al. Citation2012; Bahrami et al. Citation2020). This complex promotes TFEB phosphorylation at both serines, S211 via mTOR and Ser142 via extracellular signal-regulated kinase 2 (ERK2). This event provokes TFEB dislocation from the lysosomes and attaches it with YWHA, a 14-3-3 family protein that can preserve TFEB in the cytoplasm (Martina et al. Citation2012; Settembre et al. Citation2012). However, stressful situations such as caloric restriction or lysosomal stress cause inactivation of RAG GTPases as well as mTORC1 and prevent TFEB phosphorylation (Martina et al. Citation2012; Settembre et al. Citation2012). Consequently, dephosphorylated TFEB accumulates in cytosol, separates it from YWHA binding and translocates it in perinucleus (Figure ). Moreover, TFEB also auto-controls its expression via starvation-activated autoregulatory loop (Settembre et al. Citation2013). Pharmacological inhibition of mTOR can turn to TFEB dephosphorylation and relocation to the nucleus (Settembre et al. Citation2012). In the nucleus, TFEB can activate CLEAR network gene expressions (Palmieri et al. Citation2011). These genes encode for several proteins that coordinate the translocation and actions of lysosomal enzymes contributing to the autophagic degradation (Palmieri et al. Citation2011). Several pathways can be regulated by the CLEAR network genes such as peroxisome proliferator-activated receptor gamma (PPARγ) co-activator-1 (PGC1), which is associated with mitochondrial biogenesis and the AMPK signaling pathway (Palmieri et al. Citation2011). Moreover, the lysosomal Ca2+ channel, mucolipin-1 (Emanuele Citation2014; Medina et al. Citation2015) (TRPML1, gene MCOLN1) can regulate TFEB (Figure ). Thus TFEB positively controls several genes that are keys for cellular degradation and clearance through ALP (Palmieri et al. Citation2011). Under the fasting state, Ca2+ is dispersed from lysosomes through the TRPML1 channel, a TFEB transcriptional target (Medina et al. Citation2015), and a Ca2+ microdomain is twisted adjacent to lysosome (Emanuele Citation2014; Bahrami et al. Citation2020). This restricted raise in Ca2+ stimulates calcineurin which promotes TFEB dephosphorylation and translocation in the nucleus (Figure ) resulting transcription of its targeting genes (Medina et al. Citation2015).
Forkhead box O (FOXO) family member proteins, FOXO1 and FOXO3, act as important stimulators of ALP in case of starvation (Mammucari et al. Citation2007) by controlling the expressions of ATG proteins (Morel et al. Citation2017). Since mTORC1 acts as the main sensor of nutrient deprivation and growth factor signaling pathways, ALP can also be controlled by mTORC2 via mTORC2-(protein kinase B) AKT-FOXO3 pathway (Lamming et al. Citation2012). Here, mTORC2 causes AKT phosphorylation (at Ser473) that in turn results in FOXO3 phosphorylation (at Thr32). As a result, cytosolic FOXO3 is retained in the cytosol via 14-3-3 interaction (Tzivion et al. Citation2011) and therefore transcriptionally suppresses the autophagy pathway (Figure ). In the starvation environment, stimulated FOXO3 helps the transcription of genes that induce autophagy (Mammucari et al. Citation2007).
Autophagy-lysosomal pathway in cancer: the ‘double-edged sword’ role
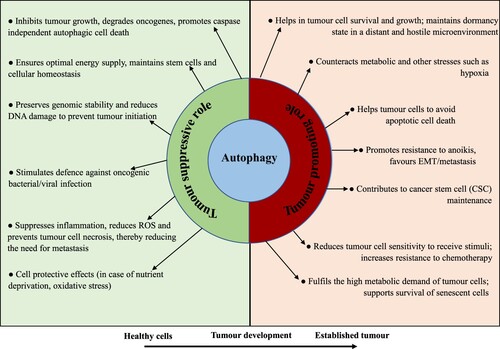
In cancers, several signaling molecules associated with autophagy have been discovered to be both overexpressed and deregulated indicating the evidence that autophagy acts ambiguous dual roles in cancer progress (Yun and Lee Citation2018; Yazdani et al. Citation2019). Autophagy functions as either tumor inhibitor or inducer based on the type, stage, and TME context (Figure ). While autophagy restricts cancer progress in the initial stages, it can assist a protumoral action in advanced stages, inducing cell growth and metastasis (Yun and Lee Citation2018). Additionally, autophagy in stem cells maintains its exclusive properties such as cell differentiation and self-renewal (Chang Citation2020). Although autophagy in normal healthy cells usually happens at low expression levels and many well-known malicious cells have high expressions of basal autophagy even in fed states (White Citation2015). Moreover, increasing evidence suggests that autophagy alters response to selective types of anticancer chemotherapy and thus represents a resistant mechanism to chemotherapy in many malignancies (Sui et al. Citation2013). Moreover, the autophagy regulation imparts to the expressions of tumor suppressive proteins or oncogenes. Tumor suppressive proteins are down-regulated by mTOR signaling pathway ensuing an initiation of autophagy and inhibition of cancer commencement (Avalos et al. Citation2014). On the other hand, oncogenes can be stimulated by mTOR pathway resulting in an inhibition of autophagy and promotion of cancer development (Yun and Lee Citation2018). While these dual intricate pathways lead autophagy an exciting target for anticancer therapies, an advanced understanding of autophagic roles in diverse stages of cancer development and precise cellular and extra-cellular context should be reserved for attention to connect autophagy in cancers.
Figure 4. The Janus-faced contradictory actions of autophagy in cancer cells. Autophagy acts as a Janus-faced play or the Yin-Yang faced function on cancer development. In healthy cells, it functions as a tumor inhibitory mechanism by ensuring optimal energy supply, preserving genomic integrity, promoting the degradation of damaged organelle and cellular oncogenes, and endorsing first-line immunity against microbial infection. In advanced tumors, however, autophagy actions can be reinstated. Autophagy functions as an oncogenic phenomenon by endorsing cancer development via declining stresses, the preservation of cancer stemness potential and metastasis; executing cells resistant to anoikis; helping the persistence of cancer cells in a dormant state and supporting the senescent cell state.
Tumor-suppressive role of autophagy-lysosomal pathway in pre-malignant cells
Fascinatingly, a complicated relation between autophagy and cancer is recognized when Beclin 1, an important promoter for IM formation is indicated to inhibit breast cancer development (Liang et al. Citation1999). The Beclin 1 deficiency results in autophagy inhibition and upsurges in growth in cancer cells, suggesting that Beclin 1 plays as a tumor inhibitor (Liang et al. Citation1999; Qu et al. Citation2003). Moreover, Beclin 1 depletion is found in various human prostate, breast and ovarian cancers (Qu et al. Citation2003) and the down-regulated Beclin 1 levels in various cancers like HCC (Cai et al. Citation2014; Zhang et al. Citation2019). However, the overexpressed Beclin 1 inhibits cell proliferation of colon cancer (Zhang et al. Citation2019) and cervical cancer (Sun et al. Citation2010). Likewise, UVRAG (UV radiation resistance-associated gene) and Bif-1 (Bax interacting factor-1) associated with Beclin 1 complex act as tumor inhibitors and certainly control autophagy (Morselli et al. Citation2009; He et al. Citation2015). More considerable data for tumor-inhibiting role of autophagy is the mouse liver adenoma progression in ATG5 and ATG7 deficiencies (Takamura et al. Citation2011). Thus the following multiple mechanisms proposed to explain the tumor-preventing role of autophagy in pre-malignant cells.
In early tumorigenesis, autophagy suppresses the initiation and expansion by eliminating the damaged proteins, dysfunctional or damaged organelles like mitochondria (called mitophagy) and the transforming active pathogens, all of which can induce genotoxic oxidative stress by producing ROS, chromatin fragments containing damaged DNA, genomic instability such as micronuclei, endogenous retrotransposons through aberrant mutations and ultimately cancer initiation and progression (Guo et al. Citation2014; Bartsch et al. Citation2017).
In stress conditions such as ER stress or starvation, tumor cells having malfunctioning and/or deficiencies in autophagy favorably accumulate damaged macromolecules (e.g. p62, an adaptor protein, ER chaperones) and injured organelles (particularly mitochondria) (Mathew et al. Citation2007; Pecoraro et al. Citation2020), subsequently inducing ROS and genomic damage and chromatin instability (Wang et al. Citation2016; Yun and Lee Citation2018). These events result in the upregulation of the new autophagy cycle and the promotion of tumor progression. However, the correct autophagy favors DNA repair by preventing nuclear p62 accumulation and coordinates mitotic fidelity (Wang et al. Citation2016).
Autophagy controls the pre-malignant cell necrosis that limits to provoke a chronic inflammatory response, which can further become a strong oncogenic driver through cytokine production (Coussens et al. Citation2013). Autophagy destroys pro-inflammatory objects like injured mitochondria that can produce ROS abundantly as well as deliver mitochondrial (mt)DNA and type I interferon (IFN) signaling components (Zitvogel et al. Citation2012).
Efficient autophagy may enable oncogene-induced senescence (OIS) (Perez-Mancera et al. Citation2014; Dou et al. Citation2015) that provokes cell cycle arrest permanently and suppresses the cancer cell proliferation (Garcia-Prat et al. Citation2016).
Autophagy-mediated apoptosis in cancers does not succumb to cell death which is caspase independent (Rybstein et al. Citation2018).
Thus integral responses of autophagy in pre-malicious cells offer vigorous protection to the cellular homeostasis of healthy tissues.
Tumor-promoting action of autophagy-lysosomal pathway in malignant cells
Cancer cells require plentiful nutrients and oxygen supply during development and invasion to retain their rapid proliferation. An increased autophagic activity of cancer cells is thought to relate to their survival in hostile TME (Morselli et al. Citation2009). Activated autophagy in tumor cells based on stress conditions, thus, it makes a pro-survival action of cancer cells (White Citation2015). Autophagy in neoplastic cells influences tumor development in an intricate way linking both basic and extrinsic mechanisms of cancer cells. The following ways where autophagy is stolen the normal cellular homeostasis for developing tumor cells.
The efficient autophagy helps the detached tumor cells from ECM to promote cell survival, circumvent apoptotic cell death and preserve latency in a detached and antagonistic niche including hypoxia and acidic TME until they form new colonies (Qureshi-Baig et al. Citation2020).
Autophagy also plays a part in tumor metastasis. Cancer cells are capable to involve in metastasis by invading and colonizing new tissues and organs. In advanced metastatic stages, autophagy performs a pro-metastatic action through the enhancement of cell viability and colony formation in inferior sites (Kenific et al. Citation2010; Sosa et al. Citation2014). For undertaking metastasis, cancer cells must be detached from ECM (Vanharanta and Massague Citation2013) and autophagy allows ECM-detached cells to circumvent anoikis (programmed cell death upon detachment) (Avivar-Valderas et al. Citation2013).
Autophagy affects the TME that comprises numerous factors such as hypoxia, cytokines chemokines and other inflammatory factors. In the case of established tumors, autophagy helps tumor cells supply energy and metabolic products for continuous proliferation and protect cytotoxicity under hostile TME (Li et al. Citation2017; Daskalaki et al. Citation2018). Under therapeutic stressful conditions, robustly induced autophagy may help the cancer cells to endure, adapt and proliferate rapidly for the promotion of tumor development (Rybstein et al. Citation2018).
RAS protein-mutated cancer cells preserve an elevated level of basal autophagy. For example, H-RAS/K-RAS mutation causes elevated autophagy which in turn augments oncogenesis and progression of several fatal cancers including colon cancers (Karnoub and Weinberg Citation2008; Goel et al. Citation2015).
Autophagy is activated during cellular senescence. Although limited proliferation, senescent cancer cells can still support detachment by inducing TME depending on activated autophagy. Interestingly, autophagy facilitates oncogene RAS-tempted senescence and suppression of autophagy postpones senescence in cancer cells (Young et al. Citation2009).
Inflammatory cytokines are highly expressed in TME suggesting that inflammatory signals participate in tumorigenesis (Franklin et al. Citation2014). Inflammation induces elevated ROS levels in cancer cells, and immune cells including macrophages which release cytokines such as IL-6 (interleukin-6), IL-10 and TNF-α (tumor necrosis factor α) into TME. Autophagy regulates these inflammatory cytokines by secreting DAMPs (degradation of damage-associated molecular patterns) such as HMGB1 (high mobility group box 1) and (mt)DNA (Pan et al. Citation2014).
In cancer biology, the EMT (epithelial–mesenchymal transition) induces metastasis ensuing in an absence of cell–cell adhesion, increase of cell motility and invasiveness (Thiery et al. Citation2009). EMT-induced cancer cells have elevated levels of autophagy for cell survival under several stress conditions (Cai et al. Citation2015). For example, autophagy in HCC has been found to accelerate metastasis by overexpressing EMT (Li et al. Citation2013).
Stimulation of protective autophagy in cancer cells is the main challenge in antineoplastic therapy because of the development of chemoresistance (Chen et al. Citation2019).
Autophagy supports natural tumor progression by protecting the structure and function of cancer stem cells (CSC) that participate in oncogenesis, resistance to chemotherapy and invasion (Liu et al. Citation2017). Numerous studies have examined the way to preserve the stemness in these cells and found that autophagy acts an essential role to regulate the homeostasis of CSCs (Pan et al. Citation2013; Sharif et al. Citation2017).
To summarize, these complex paradoxical dual functions of autophagy have directed to debate over whether or how autophagy modulation would be endeavored in effective cancer treatment. Thus these Janus-faced contradictory phenomena lead the autophagy as a challenging but auspicious target for anticancer therapies (Rubinsztein et al. Citation2012; Levy et al. Citation2017; Towers et al. Citation2020). Here, in this review implication in inhibition of autophagy by repurposing drugs may be a gorgeous novel approach to protect and treat cancers.
Drug repurposing in cancer treatment
Despite incredible sources and strategies being investigated for cancer treatment, cancer is one of the most principal reasons of morbidity worldwide (Turanli et al. Citation2018). Over the last decades, the de novo development and commercialization of a new drug is a complicated, time-consuming, expensive and often failure-prone process (Yella et al. Citation2018) despite the advances in genomics, bioinformatics, life sciences and biotechnology as well as enhanced knowledge of human diseases. Research expenses by the NIH (National Institutes of Health) USA and investments in pharmaceutical companies have steadily increased, while the proportionate number of novel anticancer drug approvals has stagnated (Pammolli et al. Citation2011; Scannell et al. Citation2012; DiMasi et al. Citation2016). Moreover, the major presently available conventional antitumor chemotherapeutic agents are extremely costly, minimal expansion in survival rate and accompanying with several toxic effects that considerably diminish the lifespan of the patients (Langedijk et al. Citation2015). Thus scientists and clinicians have approved several approaches to decrease the production cost and time associated with cancer drug discovery. One such approach is to assess the reputable non-cancer drugs that have already been permitted for non-cancer diseases, the approach is called drug repurposing.
Drug repurposing (also known as drug repositioning/ recycling/ redirecting/ retasking/ reprofiling, therapeutic/ indication switching) has increased substantial consideration over the last 5 years (Nosengo Citation2016) because of providing a rapid opportunity to convert the new therapeutic strategies into clinical trials. This approach frequently encompasses a novel formulation, route of administration or dosage form of the drug and a diverse drug target comparative to the original pharmacological indication (Urquhart Citation2018). The main merit of this approach is the availability of biopharmaceutical and toxicokinetic data of drugs and thus reducing the need for additional studies to investigate clinical trials (Waring et al. Citation2015) and can substantially reduce drug discovery cost and time with established human safety (Baker et al. Citation2018). Besides, drug repurposing may uncover new cellular pathways associated with a particular disease like carcinogenesis or reveal new molecular targets for therapy (Galluzzi et al. Citation2017). Thus drug repurposing is receiving increased interest from governments, pharmaceutical companies, nongovernmental agencies and academic researchers.
Potential drugs targeting the autolysosomes in cancers
Autolysosome, at the center stage of autophagy, emerges as an optimistic goal for the progress of innovative mediators against a wide variety of diseases including cancers (Galluzzi et al. Citation2017). Although several known compounds inhibit autophagy at different stages, regrettably real selective pharmacological suppressors of autolysosomes are not yet existing for application in cancer patients (Galluzzi et al. Citation2017). However, there are five groups of agents that can be classified that can hit the autolysosomes as well as lysosome in cancer (Table ). Most of the agents are still being examined in the preclinical trials excluding the antimalarial drug chloroquine (CQ) and its analogue hydroxychloroquine (HCQ) which are the clinically permitted autophagy inhibitor widely applied in many clinical trials in combination with several anticancer therapies. Although CQ analogues have been verified as impartial therapies, a more applied and rational strategy is to practice in their combined therapy with conventional cancer therapies. The combination anticancer drugs having synergistic effect without increasing toxicity have been reported using tumor cell lines, animal models (Table ), and cancer patients (Table ). There are some foundations sustaining to the approaches:
It is practicable that autophagy does not show a real or steady response to cancer therapies.
In general, cancer cells have an elevated level of autophagy for cell survival and adaptation under stress conditions. Autophagy in cancer cells circumvents parent autophagy inhibition (Towers and Thorburn Citation2019).
Many cancer therapies are known to stimulate autophagy and the induced autophagy converses capability of avoiding the attacks from chemotherapies, thus subsequent in chemoresistance.
Autophagy inhibitors as monotherapies alone execute a limited capacity for tumor development and unpredictable results from clinical trials.
Autophagy deficiencies are connected to many human diseases (Table ) and autophagy suppression in tumor cells is more expected to be relatively harmful to normal cellular homeostasis because autophagy routinely eliminates the damaged or dysregulated proteins.
Table 2. Selected examples of ALP inhibitors with therapeutic benefits.
Table 3. Selected examples of therapeutic benefits from ALP inhibition in animal models.
Table 4. Ongoing and completed clinical trials with ALP inhibitors.
Lysosomal lumen alkalizers: chloroquine analogues
Most of the effective drug repurposing method has chiefly been by chance discovery or clinical reflection rather than systematic, hypothesis-oriented results. One of the serendipitous discoveries for drug repurposing achievement histories connects to the (re)use of CQ analogues (Al-Bari Citation2015). These analogues are still chosen for malaria treatment as they are cheap and effective agents with a low toxicity profile in patients (Al-Bari Citation2015; Zhang et al. Citation2015). Although as an anti-cancer drug, CQ analogues particularly HCQ alone have been shown to suppress cell growth and proliferation, these analogues have been widely used in preclinical and clinical trials with auspicious outcomes (Galluzzi et al. Citation2017). CQ analogues in combination with antitumor agents impair clonogenic cancer cell growth and proliferation, improve tumor milieu and increase cell death, especially under metabolic stresses (Galluzzi et al. Citation2017). CQ analogues preferentially accumulate in and/or damage lysosomal or autolysosomal acidification system, thus abrogating lysosomal enzymatic activities (Piao and Amaravadi Citation2016). It has been found that CQ analogues are more potential sensitizer in cancer treatment mainly in cases of chemotherapy resistance (Pascolo Citation2016; Morel et al. Citation2017). In tumor treatment, CQ analogue is frequently applied in blending with chemotherapies and radiation since it is revealed to improve the cancer cell killing efficiency. These analogues have coactive actions with selective chemotherapies applied to treat several cancers such as melanoma, multiple myeloma, glioma and advanced/refractory solid tumors without causing extra adverse effects (Morel et al. Citation2017). CQ has also been found to enhance the therapeutic efficiency of antitumor drugs that cause autophagy induction (Torgersen et al. Citation2013). For example, CQ analogue causes an extra 30–40% decrease in cell survival rate treated with histone deacetylase inhibitors like valproic acid or vorinostat in acute myeloid leukemia (AML) cells (Torgersen et al. Citation2013). Interestingly, many weak basic drugs for cancer treatment gather in lysosomes without causing inhibition of autophagy (Piao and Amaravadi Citation2016). One clarification, HCQ can dislocate other mutual therapies from the lysosome to cytosolic niche by enhancing their cytosolic availability (Piao and Amaravadi Citation2016). In line with this notion, CQ analogues in combination with clinically approved chemotherapies have been revealed to suppress cancer cell growth in autophagy reliant on ways (Maes et al. Citation2014; Eng et al. Citation2016). These analogues offer significant protection against both the cancer cells and the TMEs especially when combined with standard anticancer therapies.
Current chloroquine analogues for autophagy inhibitions
The basic chemistry of CQ analogue comprises a heterocyclic fraction (7chloro-quinoline) standing on an alkaline side chain at carbon-4 position (see S. Figure 1a). Two H+ bearing sites on the side chain are accountable for its mildly alkaline property with pK's of 8.3 and 10.2 correspondingly (see S. Figure 1b) which keeps it soluble in the abdomen (Al-Bari Citation2015). Basically, HCQ varies from CQ by presenting a hydroxyl (OH–) group (see S. Figure 1a) at the terminal of the side chain. This structural alteration of HCQ causes a reduction the potential for retinal toxicity and making HCQ more satisfactory safety profile to CQ (Al-Bari Citation2015). Since retinal toxicity, a main adverse effect of CQ and incapable to arrive in tumor cells effectively due to protonation in the acidic ECM in TME of many tumors (Pellegrini et al. Citation2014; Morel et al. Citation2017), many scientists have discovered more new CQ analogues and or CQ-based hybrid compounds in challenges to progress anticancer action. Currently, several CQ analogues target the different stages of autophagy as well as lysosome in cancers including Lys05 (Amaravadi and Winkler Citation2012; McAfee et al. Citation2012), Spautin-1 (Liu et al. Citation2011; Shao et al. Citation2014), SAR405 (Ronan et al. Citation2014; Pasquier Citation2015), quinacrine, mefloquine (Wang et al. Citation2015), verteporfin (Liang et al. Citation2020; Mae et al. Citation2020), clioquinol (Khan et al. Citation2020; Mizutani et al. Citation2021), nitroxoline (Mitrovic and Kos Citation2019; Xu et al. Citation2020), EAD1 (a triazole-functionalized CQ derivative) (Nordstrom et al. Citation2015), and VR23 (a 4-piperazinylquinoline scaffold) (Piao and Amaravadi Citation2016). A ligand of REV-ERBβ (reverse of ERBα-β variant protein, a member of the REV-ERB-A family of transcription factor), ARN5187 inhibits both REV-ERB-facilitated transcriptional control and autophagy. Strangely, while ARN5187 and CQ impart the same lysosomotropic power and inhibitory action on autophagy, ARN5187 is suggestively higher cytotoxicity in BT-474 cells (De Mei et al. Citation2015). Moreover, VATG-027 (two acridine ring derivative of CQ) and VATG-032 (tetrahydroacridine ring derivative) have been identified to inhibit autophagy up to 150-fold than CQ by inhibiting acidification of lysosomes and weakening turnover of arriving vesicles like autophagosomes (Goodall et al. Citation2014). Vacquinol-1 (NSC13316, a quinolone derivative) stimulates cell death in glioblastoma multiforme. NSC23925 (a synthetic analogue to vacquinol-1) has been reported to reverse P-glycoprotein-mediated efflux of several cancer drugs in multidrug-resistant cancer cell lines (Sander et al. Citation2017). Complex derivatives such as ferroquine (ferrocene-4-aminoquinoline), platinum (II)-CQ complex, polymeric CQ (copolymerization of N-(2-hydroxypropyl) methacrylamide with methacryloylated HCQ), gold(I)-CQ complexes [Au (CQ) (PPh3)PF6] (de Souza Pereira et al. Citation2021) have also been found to inhibit ALP. Most of these analogues are still being examined in the preclinical trials excepting CQ and HCQ.
Chloroquine analogues: just act as autophagy inhibitors?
In ongoing clinical trials for cancer therapies, HCQ is frequently applied in blending with chemotherapies, radiation and immunotherapy. CQ or HCQ treatment can suppress autophagy-oriented cell survival rate and potentiate anticancer action of chemotherapies. But, current evidences suggest that CQ and its majority analogues mediated suppression of the final degradation stage of autophagy is not the only mechanistic way in case of anticancer activity; CQ analogue can also influence other mechanistic pathways such as LMP. Alike HCQ also stimulates mitochondrial membrane potential (MMP) and the proapoptotic Bcl-2 family members Bax/Bak-dependent MMP to activate caspase stimulation (Seitz et al. Citation2013). Maycotte (Maycotte et al. Citation2012) also described that CQ can hypersensitize breast cancer cells to anticancer drug independent of autophagy suppression. Besides autophagy-independent anti-tumor actions, CQ analogues may have other ways such as dropping nutrient scavenging actions, anti-inflammatory properties to sensitize chemotherapies (Maycotte et al. Citation2012; Maes et al. Citation2014; Eng et al. Citation2016). Thus the achievement of clinical trials using CQ/HCQ in combination with antitumor drugs may not only the suppressive effects CQ/HCQ on autophagy activated by chemotherapies, but also the other mechanisms that inhibit it. Therefore, a comprehensive idea of the cellular pathways of CQ/HCQ would be suggested in the ongoing clinical trials wherever CQ/HCQ are applied as suppressors of autophagy.
Caveats against efficacies of chloroquine analogues in autophagy inhibitions
Chloroquine analogues like HCQ are presently clinically accepted autophagy suppressors for cancer treatment. There are many human clinical trials discovering suppression of autophagy as a therapeutic approach applied CQ analogues because of their extended track of safety record in human patients (Table ). However, there are many highly debatable caveats as well as enquiries lingering as the analogues represent the best effective drugs for suppressing autophagy. For example,
These analogues can be necessitated in highly concentrated (even μM) solutions to obtain sufficient autophagy suppression that is contradictorily attainable in human patients than traditionally applied for malaria and rheumatoid arthritis (RA) (Pascolo Citation2016). Hence, HCQ combined with chemotherapy and/or radiation has been found to cause low efficacy in preliminary trials. Additionally, high HCQ doses applied in clinical trials yield a substantial interpatient variability of autophagy suppression. Since autophagy activation and dependency are exclusive to cancer cells, hypothetically extended autophagy suppression can offer a therapeutic index to preferentially influence cancer cells. However, the sustained period of HCQ dose for a tumor patient will inevitably influence nontransformed normal healthy cells as well.
CQ analogue fails to inhibit autophagy in acidic ECM (pH 6.5) in tumor for a decrease in cellular absorption of the agents (Pellegrini et al. Citation2014). Although CQ analogues exert their inhibitory effects on autophagy in one of the mechanisms, the analogues are possible harmful to tumor cells by fetching additional targets. Because CQ analogues cause an amplified apoptosis index, tumor relapse, and suspension in cancer reappearance in patients.
Furthermore, the biological halflives of CQ analogues are calculated as practically lengthy periods (e.g. over 3 weeks for HCQ) which can govern chronic adverse effects such as retinopathy and indigestion (Al-Bari Citation2015). Thus many clinical trials have shown dose-limiting side effects like neutropenia, thrombocytopenia, and sepsis when HCQ is used in combination with chemotherapies (Al-Bari Citation2015).
CQ analogues can have autophagy-independent anticancer actions via extra mechanisms such as suppressing nutrient scavenging activities, anti-inflammatory properties and can sensitize to other chemotherapies (Maycotte et al. Citation2012; Eng et al. Citation2016). Moreover, CQ is capable to sensitize cancer cells to chemotherapies once the earlier stages of autophagy were blocked (Maes et al. Citation2014).
CQ analogue has been applied as lysosomal suppressors but is incapable to suppress lysosomal localized mTOR pathway, thus it also has autophagy-independent effects as anti-cancer agents (Maycotte et al. Citation2012; Eng et al. Citation2016; Towers and Thorburn Citation2017). For example, CQ has known to bind DNA for many years (Kwakye-Berko and Meshnick Citation1989). However, Ravi Amaravadi and Jeffrey Winkler groups find out dimeric quinacrines as effective antitumor molecules, which simultaneously suppress mTOR signaling and autophagy (Rebecca et al. Citation2017). The quinacrine linker DQ661 inhibits PPT1 (palmitoyl-protein thioesterase 1) as a molecular goal in vitro and suppresses tumor growth of several mouse models including colorectal cancer, and thus can be securely united with chemotherapy (Rebecca et al. Citation2017).
Although pharmacokinetic data are well known, it is practically very undefined as to whether CQ/HCQ can exactly attain concentrations in the cancer cells that will efficiently suppress autophagy; in reality, it is persuaded to claim that the purpose that CQ/HCQ has confirmed to be comparatively safe to patients because the analogue is unable to truly inhibit autophagy at concentrations that are attained in standard clinical treatments.
Another important question with applying CQ/HCQ for cancer treatment is related to their immunosuppressive actions (Taherian et al. Citation2013). It is generally known that the stimulation of anticancer immunity is critical for extended therapeutic efficiency in patients influenced by major malignancies (Fridman et al. Citation2017). In this situation, the systemic management of CQ/HCQ may be harmful as it would approve the fleeing of cancer cells from immunosurveillance (Kroemer et al. Citation2015) and incapable to amend response rate and total longevity (Rojas-Puentes et al. Citation2013). Thus CQ analogue in combination with standard therapeutic schedules may consult for temporary assistances that are not duplicated by a real expansion in patient longevity. Further study would be necessary to explore more active and tolerable CQ analogues as selective inhibitors, as well as delineates the timing, interval and dose intensity that turns to maximal therapeutic actions during tumor treatment (Chude and Amaravadi Citation2017; Zeh et al. Citation2020).
Other drug repurposing lysosomal lumen alkalizers include antidepressant drugs such as nortriptyline, imipramine, clomipramine, desipramine, and siramesine. These agents have revealed efficacy in chronic lymphocytic leukemia (CLL), colon cancer and breast cancer. Antihistaminic agents like loratadine and terfenadine have also shown effectiveness by stimulating apoptosis in lung and breast cancer cells. Stilbenoid antioxidants including pterostilbene and antidepressant drugs such as thioridazine, chlorpromazine, and aripiprazole have also shown therapeutic efficiency in breast cancer cells (Levy et al. Citation2017). The particulars of these drugs are concisely discussed in Table .
V-ATPase inhibitors
Vacuolar H+-ATPase (v-ATPase), a proton pump, generates H+ ions by hydrolysis of ATP into the lysosomal lumen, thus making the lysosomes acidic. Suppression of v-ATPase can be attained by the action of several microbial compounds such as bafilomycin A1, cleistanthin A, archazolid, and manzamine A (De Milito et al. Citation2010). Although bafilomycin A1 is suggested to be the classical inhibitor of v-ATPase, its high toxicity profile has limited to use in clinical intervention for blocking autophagy in vivo (Piao and Amaravadi Citation2016). Archazolid, another v-ATPase inhibitor has shown the capability to decrease the action of the cathepsin B protease (Kubisch et al. Citation2014). The v-ATPase inhibitors manzamine A (Kallifatidis et al. Citation2013) and cleistanthin-A have also shown cytotoxic actions on several cancer cells (Zhao et al. Citation2015). The anti-melanoma effect of esomeprazole, a clinically approved proton pump inhibitor (PPI), is allied to inhibit the homeostatic mechanism of cancer cells that help their viability by eliminating H+ and acidic metabolites. Besides, esomeprazole stimulates autophagy-mediated apoptosis in gastric cancers (De Milito et al. Citation2007).
Acid sphingomyelinase modulators
Multidrug resistance is the prime obstacle to successful cancer treatment. Acid sphingomyelinase (ASM), a hydrolase within the lysosome cleaves sphingomyelin to ceramide which assists as a substrate for the production of other members of sphingolipids including S1P (sphingosine 1-phosphate) in response to cellular stresses (Petersen et al. Citation2013; Savic and Schuchman Citation2013). ASM action is important for lysosomal membrane integrity and revival of tumor cells as well as for resistance phenotypes of multidrug. Cancer cells usually exhibit lower ASM activity than in normal healthy cells, resulting in elevated sphingomyelin levels. Thus cancer cells display high level of vulnerability to ASM treatment because blocking ASM expression outcomes in higher sphingomyelin levels which restricts the normal action of lysosomal membrane. Several ASM modulators including cationic amphiphilic agents such as antidepressants (chlorpromazine, siramesine) (Petersen et al. Citation2013), CQ and antiarrhythmic drugs (amiodarone) decrease ASM action by relocating ASM from the lysosomal membrane resulting in LMP and eventual tumor cell death (Saftig and Sandhoff Citation2013). Moreover, siramesine and clomipramine also effectively inhibit autophagic flux by neutralization of the lysosomal pH effectively (Petersen et al. Citation2013).
Heat shock protein inhibitors
As chaperone molecules, HSP70 can protect the lysosome from permeabilization and maintain cellular protein homeostasis (Penke et al. Citation2018). Cancer cells in different types of origins upregulate HSPs in particular HSP70 (Granato et al. Citation2013) and HSP90 to cope with their basal stress that promotes cell metastasis deliberating a survival benefit (Mjahed et al. Citation2012). By selectively interacting with bis monoacylglycero phosphate in the lysosomal lumen, the nanomachine HSP70 stimulates ASM and supports maintaining the lysosome membrane integrity (Petersen et al. Citation2013). Elevated expressions of HSP90 and HSP70 in cancer cells appear to associate with quicker disease development and are frequently involved in amplified chemotherapy resistance and poor patient diagnosis (Kumar et al. Citation2016). Targeting HSP70 thereby inactivating ASM in cancer cells would upsurge LMP resulting in cell death (Saftig and Sandhoff Citation2013; Howe et al. Citation2014). For instance, PES (2-phenylethyenesulfonamide, also called pifithrin-μ), a specific suppressor of stress-inducible HSP70 suppresses lysosomal action and autophagy and exerts prominent anticancer effects by promoting tumor cell death (Granato et al. Citation2013; Howe et al. Citation2014). HSP90 chemical inhibitors such as 17-AAG or 17-DMAG are currently developed and 17-AAG in combination with chemotherapies is presently undergoing phase I/II trials for solid and hematologic malignancies (Workman Citation2020).
Therapeutic targeting of cathepsins in cancer
Currently, lysosomal proteases like cathepsins have grown substantial curiosity in the branch of oncology (Fuchs et al. Citation2020; Holzen et al. Citation2020). Under physiological conditions, lysosomal cathepsins maintain tissue homeostasis (Pu et al. Citation2016). However, any alterations in cathepsin expression (usually upregulation) or localization (such as in cytoplasm, nucleus, mitochondria, or ECM architecture) have been associated with cancers (Pogorzelska et al. Citation2018). For example, overexpression of cathepsins B/S has been prominently compromised the development of solid tumors (Fuchs et al. Citation2020). Also, cathepsins are involved in response to antitumor drug within TME and they can play important actions in the progression of resistance to the chemotherapies (Rudzinska et al. Citation2019). Numerous tactics have been explored to inhibit tumor-associated cathepsin action, including chemical inhibitors and antibodies (in cancer immunotherapy). Although several cathepsin inhibitors have been explored for cancer treatment (Kos et al. Citation2014), there are presently not available cathepsin inhibitors for clinical trials in cancer. However, the main effort of research on the progression of cathepsin suppressors is on osteoporosis (Drake et al. Citation2017) and arthritic diseases (Wei et al. Citation2020). Since cathepsins display ideal prognostic markers these can be investigated for manipulating improved drug delivery tactics (Wilkinson et al. Citation2015; Yan et al. Citation2017). For example, cathepsins B/L are suggested as biomarkers for cancer identification where their expressions are frequently inversely connected with patient responses (Zhang et al. Citation2014).
Other potential autophagy-lysosomal pathway targeting
The transcription factor TFEB regulates the ALP suggesting that the several TFEB-controlled genes may comprise novel targets for drug discovery in cancer treatment. Current data have also suggested that ALP can firmly control the iron, heme (Dong et al. Citation2015), and calcium (Medina et al. Citation2015) metabolisms. Thus targeting transcription factors and nonprotein residues of the lysosome including Ca2+, Fe2+, and heme components can offer an additional therapeutic approach for aiming the lysosome in cancer.
Opinions against inhibition of autophagy for cancer treatment
Numerous studies have proposed that the suppression of autophagy is not practically ideal clue an immunologic standpoint for cancer treatment as it would suppress anticancer T-cell functions (Rao et al. Citation2014). The argument behindhand, autophagy in dead cancer cells is necessary for immunological clearance, which assists in effective appreciation and stimulation of the immunity for efficient anticancer immune response (Michaud et al. Citation2011). Autophagy induces antigen cross-presentation that offers extra latent way where suppression of autophagy could hinder a vigorous anticancer immune reply (Levy et al. Citation2017; Li et al. Citation2017). In patients, suppression of autophagy is not selectively aimed at cancer cells, hence global autophagy inhibition may cause potential toxicity. For example, the global ATG7-/- adult mice are died due to lethal neuronal toxicity, interruption of glucose homeostasis, and amplified vulnerability to infection (Karsli-Uzunbas et al. Citation2014; Levy et al. Citation2017). Still, in the provision of positive clue, the extended use of HCQ for the treatment of rheumatic disorders like RA and the ailment of several cancer patients with CQ for prolonged times without producing toxicity indicates that an extended schedule strategy with autophagy suppressors is practicable (Levy et al. Citation2014).
Executive summary and perspective
Nutrient sensing property of the lysosome in ALP has provided a great influence on cellular physiological processes. Autolysosomes regulate metabolic homeostasis in cancer cells through the digestion of their cellular content. The evolving actions of autolysosomes in cancer cells may include many complex phenomena like nutrient sensing, signal transduction, apoptosis, and immunological clearance. Besides, its action as an inhibiting or promoting mechanism in cancer persists in dispute. Presently knowledge about autolysosomes and ALP is still inadequate, and several open questions are so far to be clarified.
Open questions
Autophagy acts the Yin-Yang faced functions in cancer cells which is dependent on the types and stages of cancer development. How ALP can trigger the cancer pathology and/or the carcinogenesis keeps to be elucidated.
In the early stage, autolysosomes display cancer oppressive action while in metastatic stage, these endorse cell survival by supplying nutrients under metabolic stress conditions. Whether and how autolysosomes have a role in the apoptotic pathway remain uncertain.
Stimulation of ALP obeys the ‘survival of the fittest’ rule for cancer cells. Dented proteins and organelles are eliminated by ALP which provides reserved energy and cancer cells use this energy for persistence against anticancer drugs. Thus understanding the exact changes of ALP may lead to new therapeutic strategies for a wide variety of diseases like cancers.
Moreover, the precise mechanistic pathways of lysosomal trafficking and function need to be clarified. Effectively analysis of the dynamic events occurred within the ALP vesicles due to diverse stresses needs to be clarified.
Since TFEB orchestrates as a master regulator of the ALP, transcriptional regulation of ALP activity and the precise roles of the transcribed genes associated with ALP biogenesis are still being explained.
To answer these questions, scientists should explore the genome editing roles of autolysosomes and lysosomes within the TME. Genomic and metagenomic investigation by applying high throughput screening techniques such as transcriptomics, proteomics and metabolomics to examine patient-derived cells may result in an advanced knowledge of lysosome-mediated cancer treatment.
Authors’ contributions
MAAB conceived, designed, edited, revised, and proof-read the manuscript.
Acknowledgements
The author does not take any allowance from known funding agencies in the public–private sectors, pharmaceutical companies, or not-for-profit organizations.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing does not apply to this article as no new data were created or analyzed in this study.
References
- Aits S, Jaattela M. 2013. Lysosomal cell death at a glance. J Cell Sci. 126:1905–1912.
- Al-Bari MA. 2015. Chloroquine analogues in drug discovery: new directions of uses, mechanisms of actions and toxic manifestations from malaria to multifarious diseases. J Antimicrob Chemother. 70:1608–1621.
- Al-Bari MAA. 2020. A current view of molecular dissection in autophagy machinery. J Physiol Biochem. 76:357–372.
- Al-Bari MAA, Xu P. 2020. Molecular regulation of autophagy machinery by mTOR-dependent and -independent pathways. Ann N Y Acad Sci. 1467:3–20.
- Aman Y, Schmauck-Medina T, Hansen M, Morimoto RI, Simon AK, Bjedov I, Palikaras K, Simonsen A, Johansen T, Tavernarakis N, et al. 2021. Autophagy in healthy aging and disease. Nat Aging. 1:634–650.
- Amaravadi R, Kimmelman AC, White E. 2016. Recent insights into the function of autophagy in cancer. Genes Dev. 30:1913–1930.
- Amaravadi RK, Winkler JD. 2012. Lys05: a new lysosomal autophagy inhibitor. Autophagy. 8:1383–1384.
- Angelidou I, Chrysanthopoulou A, Mitsios A, Arelaki S, Arampatzioglou A, Kambas K, Ritis D, Tsironidou V, Moschos I, Dalla V, et al. 2018. REDD1/autophagy pathway is associated with neutrophil-driven IL-1beta inflammatory response in active ulcerative colitis. J Immunol. 200:3950–3961.
- Avalos Y, Canales J, Bravo-Sagua R, Criollo A, Lavandero S, Quest AF. 2014. Tumor suppression and promotion by autophagy. Biomed Res Int. 2014:1–15.
- Avivar-Valderas A, Bobrovnikova-Marjon E, Alan Diehl J, Bardeesy N, Debnath J, Aguirre-Ghiso JA. 2013. Regulation of autophagy during ECM detachment is linked to a selective inhibition of mTORC1 by PERK. Oncogene. 32:4932–4940.
- Bahrami A, Bianconi V, Pirro M, Orafai HM, Sahebkar A. 2020. The role of TFEB in tumor cell autophagy: diagnostic and therapeutic opportunities. Life Sci. 244:117341.
- Baker NC, Ekins S, Williams AJ, Tropsha A. 2018. A bibliometric review of drug repurposing. Drug Discov Today. 23:661–672.
- Ballabio A. 2016. The awesome lysosome. EMBO Mol Med. 8:73–76.
- Ballabio A, Bonifacino JS. 2020. Lysosomes as dynamic regulators of cell and organismal homeostasis. Nat Rev Mol Cell Biol. 21:101–118.
- Barazzuol L, Giamogante F, Brini M, Cali T. 2020. PINK1/parkin mediated mitophagy, Ca(2+) signalling, and ER-mitochondria contacts in Parkinson's disease. Int J Mol Sci. 21:1772.
- Bartsch K, Knittler K, Borowski C, Rudnik S, Damme M, Aden K, Spehlmann ME, Frey N, Saftig P, Chalaris A, Rabe B. 2017. Absence of RNase H2 triggers generation of immunogenic micronuclei removed by autophagy. Hum Mol Genet. 26:3960–3972.
- Behrends C, Sowa ME, Gygi SP, Harper JW. 2010. Network organization of the human autophagy system. Nature. 466:68–76.
- Brown GT, Murray GI. 2015. Current mechanistic insights into the roles of matrix metalloproteinases in tumour invasion and metastasis. J Pathol. 237:273–281.
- Burman C, Ktistakis NT. 2010. Autophagosome formation in mammalian cells. Semin Immunopathol. 32:397–413.
- Byrne S, Jansen L, U-King-Im JM, Siddiqui A, Lidov HG, Bodi I, Smith L, Mein R, Cullup T, Dionisi-Vici C, et al. 2016. EPG5-related Vici syndrome: a paradigm of neurodevelopmental disorders with defective autophagy. Brain. 139:765–781.
- Cai M, Hu Z, Liu J, Gao J, Liu C, Liu D, Tan M, Zhang D, Lin B. 2014. Beclin 1 expression in ovarian tissues and its effects on ovarian cancer prognosis. Int J Mol Sci. 15:5292–5303.
- Cai Q, Yan L, Xu Y. 2015. Anoikis resistance is a critical feature of highly aggressive ovarian cancer cells. Oncogene. 34:3315–3324.
- Cao B, Li J, Zhou X, Juan J, Han K, Zhang Z, Kong Y, Wang J, Mao X. 2014. Clioquinol induces pro-death autophagy in leukemia and myeloma cells by disrupting the mTOR signaling pathway. Sci Rep. 4:5749.
- Ceccariglia S, Cargnoni A, Silini AR, Parolini O. 2020. Autophagy: a potential key contributor to the therapeutic action of mesenchymal stem cells. Autophagy. 16:28–37.
- Chang NC. 2020. Autophagy and stem cells: self-eating for self-renewal. Front Cell Dev Biol. 8:138.
- Chang X, Dong R. 2020. Transcriptional regulation of autophagy-lysosomal pathway in cancer. Thorac Cancer. 11:216–223.
- Chauhan S, Kumar S, Jain A, Ponpuak M, Mudd MH, Kimura T, Choi SW, Peters R, Mandell M, Bruun JA, et al. 2016. TRIMs and galectins globally cooperate and TRIM16 and galectin-3 co-direct autophagy in endomembrane damage homeostasis. Dev Cell. 39:13–27.
- Chen R, Jaattela M, Liu B. 2020. Lysosome as a central hub for rewiring PH homeostasis in tumors. Cancers (Basel). 12(9):2437.
- Chen Y, Yu L. 2018. Development of research into autophagic lysosome reformation. Mol Cells. 41:45–49.
- Chen Z, Jiang Q, Zhu P, Chen Y, Xie X, Du Z, Jiang L, Tang W. 2019. NPRL2 enhances autophagy and the resistance to Everolimus in castration-resistant prostate cancer. Prostate. 79:44–53.
- Cheung KS, Leung WK. 2019. Long-term use of proton-pump inhibitors and risk of gastric cancer: a review of the current evidence. Therap Adv Gastroenterol. 12:1756284819834511.
- Christ L, Raiborg C, Wenzel EM, Campsteijn C, Stenmark H. 2017. Cellular functions and molecular mechanisms of the ESCRT membrane-scission machinery. Trends Biochem Sci. 42:42–56.
- Chude CI, Amaravadi RK. 2017. Targeting autophagy in cancer: update on clinical trials and novel inhibitors. Int J Mol Sci. 18:1279.
- Coss RA, Storck CW, Daskalakis C, Berd D, Wahl ML. 2003. Intracellular acidification abrogates the heat shock response and compromises survival of human melanoma cells. Mol Cancer Ther. 2:383–388.
- Coussens LM, Zitvogel L, Palucka AK. 2013. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 339:286–291.
- Cuervo AM, Wong E. 2014. Chaperone-mediated autophagy: roles in disease and aging. Cell Res. 24:92–104.
- Daskalaki I, Gkikas I, Tavernarakis N. 2018. Hypoxia and selective autophagy in cancer development and therapy. Front Cell Dev Biol. 6:104.
- Davidson SM, Vander Heiden MG. 2017. Critical functions of the lysosome in cancer biology. Annu Rev Pharmacol Toxicol. 57:481–507.
- De Mei C, Ercolani L, Parodi C, Veronesi M, Lo Vecchio C, Bottegoni G, Torrente E, Scarpelli R, Marotta R, Ruffili R, et al. 2015. Dual inhibition of REV-ERBbeta and autophagy as a novel pharmacological approach to induce cytotoxicity in cancer cells. Oncogene. 34:2597–2608.
- De Milito A, Canese R, Marino ML, Borghi M, Iero M, Villa A, Venturi G, Lozupone F, Iessi E, Logozzi M, et al. 2010. pH-dependent antitumor activity of proton pump inhibitors against human melanoma is mediated by inhibition of tumor acidity. Int J Cancer. 127:207–219.
- De Milito A, Iessi E, Logozzi M, Lozupone F, Spada M, Marino ML, Federici C, Perdicchio M, Matarrese P, Lugini L, et al. 2007. Proton pump inhibitors induce apoptosis of human B-cell tumors through a caspase-independent mechanism involving reactive oxygen species. Cancer Res. 67:5408–5417.
- de Souza Pereira C, Costa Quadros H, Magalhaes Moreira DR, Castro W, De Deus Da Silva RIS, Pereira Soares MB, Fontinha D, Prudencio M, Schmitz V, Dos Santos HF, et al. 2021. A novel hybrid of chloroquine and primaquine linked by gold(I): multitarget and multiphase antiplasmodial agent. ChemMedChem. 16:662–678.
- Devis-Jauregui L, Eritja N, Davis ML, Matias-Guiu X, Llobet-Navas D. 2021. Autophagy in the physiological endometrium and cancer. Autophagy. 17(5): 1077–1095.
- DiMasi JA, Grabowski HG, Hansen RW. 2016. Innovation in the pharmaceutical industry: New estimates of R&D costs. J Health Econ. 47:20–33.
- Dong C, Zheng H, Huang S, You N, Xu J, Ye X, Zhu Q, Feng Y, You Q, Miao H, et al. 2015. Heme oxygenase-1 enhances autophagy in podocytes as a protective mechanism against high glucose-induced apoptosis. Exp Cell Res. 337:146–159.
- Donohue E, Tovey A, Vogl AW, Arns S, Sternberg E, Young RN, Roberge M. 2011. Inhibition of autophagosome formation by the benzoporphyrin derivative verteporfin. J Biol Chem. 286:7290–7300.
- Dossou AS, Basu A. 2019. The emerging roles of mTORC1 in macromanaging autophagy. Cancers (Basel). 11:1422.
- Dou Z, Xu C, Donahue G, Shimi T, Pan JA, Zhu J, Ivanov A, Capell BC, Drake AM, Shah PP, et al. 2015. Autophagy mediates degradation of nuclear lamina. Nature. 527:105–109.
- Drake MT, Clarke BL, Oursler MJ, Khosla S. 2017. Cathepsin K inhibitors for osteoporosis: biology, potential clinical utility, and lessons learned. Endocr Rev. 38:325–350.
- Emanuele E. 2014. Can trehalose prevent neurodegeneration? insights from experimental studies. Curr Drug Targets. 15:551–557.
- Eng CH, Wang Z, Tkach D, Toral-Barza L, Ugwonali S, Liu S, Fitzgerald SL, George E, Frias E, Cochran N, et al. 2016. Macroautophagy is dispensable for growth of KRAS mutant tumors and chloroquine efficacy. Proc Natl Acad Sci USA. 113:182–187.
- Fais S, De Milito A, You H, Qin W. 2007. Targeting vacuolar H+-ATPases as a new strategy against cancer. Cancer Res. 67:10627–10630.
- Franke A, Balschun T, Sina C, Ellinghaus D, Hasler R, Mayr G, Albrecht M, Wittig M, Buchert E, Nikolaus S, et al. 2010. Genome-wide association study for ulcerative colitis identifies risk loci at 7q22 and 22q13 (IL17REL). Nat Genet. 42:292–294.
- Franklin RA, Liao W, Sarkar A, Kim MV, Bivona MR, Liu K, Pamer EG, Li MO. 2014. The cellular and molecular origin of tumor-associated macrophages. Science. 344:921–925.
- Fridman WH, Zitvogel L, Sautes-Fridman C, Kroemer G. 2017. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 14:717–734.
- Fuchs N, Meta M, Schuppan D, Nuhn L, Schirmeister T. 2020. Novel opportunities for cathepsin S inhibitors in cancer immunotherapy by nanocarrier-mediated delivery. Cells. 9:2021.
- Galluzzi L, Baehrecke EH, Ballabio A, Boya P, Bravo-San Pedro JM, Cecconi F, Choi AM, Chu CT, Codogno P, Colombo MI, et al. 2017. Molecular definitions of autophagy and related processes. EMBO J. 36:1811–1836.
- Galluzzi L, Bravo-San Pedro JM, Demaria S, Formenti SC, Kroemer G. 2017. Activating autophagy to potentiate immunogenic chemotherapy and radiation therapy. Nat Rev Clin Oncol. 14:247–258.
- Galluzzi L, Bravo-San Pedro JM, Levine B, Green DR, Kroemer G. 2017. Pharmacological modulation of autophagy: therapeutic potential and persisting obstacles. Nat Rev Drug Discov. 16:487–511.
- Ganley IG, Wong PM, Gammoh N, Jiang X. 2011. Distinct autophagosomal-lysosomal fusion mechanism revealed by thapsigargin-induced autophagy arrest. Mol Cell. 42:731–743.
- Garcia-Prat L, Martinez-Vicente M, Perdiguero E, Ortet L, Rodriguez-Ubreva J, Rebollo E, Ruiz-Bonilla V, Gutarra S, Ballestar E, Serrano AL, et al. 2016. Autophagy maintains stemness by preventing senescence. Nature. 529:37–42.
- Gocheva V, Zeng W, Ke D, Klimstra D, Reinheckel T, Peters C, Hanahan D, Joyce JA. 2006. Distinct roles for cysteine cathepsin genes in multistage tumorigenesis. Genes Dev. 20:543–556.
- Goel S, Huang J, Klampfer L. 2015. K-Ras, intestinal homeostasis and colon cancer. Curr Clin Pharmacol. 10:73–81.
- Goodall ML, Wang T, Martin KR, Kortus MG, Kauffman AL, Trent JM, Gately S, MacKeigan JP. 2014. Development of potent autophagy inhibitors that sensitize oncogenic BRAF V600E mutant melanoma tumor cells to vemurafenib. Autophagy. 10:1120–1136.
- Granato M, Lacconi V, Peddis M, Lotti LV, Di Renzo L, Gonnella R, Santarelli R, Trivedi P, Frati L, D'Orazi G, et al. 2013. HSP70 inhibition by 2-phenylethynesulfonamide induces lysosomal cathepsin D release and immunogenic cell death in primary effusion lymphoma. Cell Death Dis. 4:e730.
- Groman JD, Meyer ME, Wilmott RW, Zeitlin PL, Cutting GR. 2002. Variant cystic fibrosis phenotypes in the absence of CFTR mutations. N Engl J Med. 347:401–407.
- Groth-Pedersen L, Jaattela M. 2013. Combating apoptosis and multidrug resistant cancers by targeting lysosomes. Cancer Lett. 332:265–274.
- Guo H, Chitiprolu M, Gagnon D, Meng L, Perez-Iratxeta C, Lagace D, Gibbings D. 2014. Autophagy supports genomic stability by degrading retrotransposon RNA. Nat Commun. 5:5276.
- Hamacher-Brady A, Brady NR, Gottlieb RA. 2006. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem. 281:29776–29787.
- He L, Zhang J, Zhao J, Ma N, Kim SW, Qiao S, Ma X. 2018. Autophagy: The last defense against cellular nutritional stress. Adv Nutr. 9:493–504.
- He S, Zhao Z, Yang Y, O'Connell D, Zhang X, Oh S, Ma B, Lee JH, Zhang T, Varghese B, et al. 2015. Truncating mutation in the autophagy gene UVRAG confers oncogenic properties and chemosensitivity in colorectal cancers. Nat Commun. 6:7839.
- Holzen L, Parigiani MA, Reinheckel T. 2020. Tumor cell- and microenvironment-specific roles of cysteine cathepsins in mouse models of human cancers. Biochim Biophys Acta Proteins Proteom. 1868:140423.
- Howe MK, Bodoor K, Carlson DA, Hughes PF, Alwarawrah Y, Loiselle DR, Jaeger AM, Darr DB, Jordan JL, Hunter LM, et al. 2014. Identification of an allosteric small-molecule inhibitor selective for the inducible form of heat shock protein 70. Chem Biol. 21:1648–1659.
- Huber V, Camisaschi C, Berzi A, Ferro S, Lugini L, Triulzi T, Tuccitto A, Tagliabue E, Castelli C, Rivoltini L. 2017. Cancer acidity: An ultimate frontier of tumor immune escape and a novel target of immunomodulation. Semin Cancer Biol. 43:74–89.
- Hurley JH, Young LN. 2017. Mechanisms of autophagy initiation. Annu Rev Biochem. 86:225–244.
- Ichimura Y, Kirisako T, Takao T, Satomi Y, Shimonishi Y, Ishihara N, Mizushima N, Tanida I, Kominami E, Ohsumi M, et al. 2000. A ubiquitin-like system mediates protein lipidation. Nature. 408:488–492.
- Jang JH, Baerts L, Waumans Y, De Meester I, Yamada Y, Limani P, Gil-Bazo I, Weder W, Jungraithmayr W. 2015. Suppression of lung metastases by the CD26/DPP4 inhibitor Vildagliptin in mice. Clin Exp Metastasis. 32:677–687.
- Jang JH, Janker F, De Meester I, Arni S, Borgeaud N, Yamada Y, Gil Bazo I, Weder W, Jungraithmayr W. 2019. The CD26/DPP4-inhibitor vildagliptin suppresses lung cancer growth via macrophage-mediated NK cell activity. Carcinogenesis. 40:324–334.
- Janssen JC, Beck JA, Campbell TA, Dickinson A, Fox NC, Harvey RJ, Houlden H, Rossor MN, Collinge J. 2003. Early onset familial Alzheimer's disease: mutation frequency in 31 families. Neurology. 60:235–239.
- Jewell JL, Russell RC, Guan KL. 2013. Amino acid signalling upstream of mTOR. Nat Rev Mol Cell Biol. 14:133–139.
- Jia J, Abudu YP, Claude-Taupin A, Gu Y, Kumar S, Choi SW, Peters R, Mudd MH, Allers L, Salemi M, et al. 2018. Galectins control mTOR in response to Endomembrane damage. Mol Cell. 70:120–135. e128.
- Jutten B, Keulers TG, Schaaf MB, Savelkouls K, Theys J, Span PN, Vooijs MA, Bussink J, Rouschop KM. 2013. EGFR overexpressing cells and tumors are dependent on autophagy for growth and survival. Radiother Oncol. 108:479–483.
- Kallifatidis G, Hoepfner D, Jaeg T, Guzman EA, Wright AE. 2013. The marine natural product manzamine A targets vacuolar ATPases and inhibits autophagy in pancreatic cancer cells. Mar Drugs. 11:3500–3516.
- Kallunki T, Olsen OD, Jaattela M. 2013. Cancer-associated lysosomal changes: friends or foes? Oncogene. 32:1995–2004.
- Karan D, Dubey S, Pirisi L, Nagel A, Pina I, Choo YM, Hamann MT. 2020. The marine natural product manzamine A inhibits cervical cancer by targeting the SIX1 protein. J Nat Prod. 83:286–295.
- Karnoub AE, Weinberg RA. 2008. Ras oncogenes: split personalities. Nat Rev Mol Cell Biol. 9:517–531.
- Karsli-Uzunbas G, Guo JY, Price S, Teng X, Laddha SV, Khor S, Kalaany NY, Jacks T, Chan CS, Rabinowitz JD, White E. 2014. Autophagy is required for glucose homeostasis and lung tumor maintenance. Cancer Discov. 4:914–927.
- Kenific CM, Thorburn A, Debnath J. 2010. Autophagy and metastasis: another double-edged sword. Curr Opin Cell Biol. 22:241–245.
- Khambu B, Yan S, Huda N, Liu G, Yin XM. 2018. Autophagy in non-alcoholic fatty liver disease and alcoholic liver disease. Liver Res. 2:112–119.
- Khan R, Khan H, Abdullah Y, Dou QP. 2020. Feasibility of repurposing clioquinol for cancer therapy. Recent Pat Anticancer Drug Discov. 15:14–31.
- Kim J, Kundu M, Viollet B, Guan KL. 2011. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 13:132–141.
- Kim MS, Jeong EG, Ahn CH, Kim SS, Lee SH, Yoo NJ. 2008. Frameshift mutation of UVRAG, an autophagy-related gene, in gastric carcinomas with microsatellite instability. Hum Pathol. 39:1059–1063.
- Kirkegaard T, Jaattela M. 2009. Lysosomal involvement in cell death and cancer. Biochim Biophys Acta. 1793:746–754.
- Knott SRV, Wagenblast E, Khan S, Kim SY, Soto M, Wagner M, Turgeon MO, Fish L, Erard N, Gable AL, et al. 2018. Asparagine bioavailability governs metastasis in a model of breast cancer. Nature. 554:378–381.
- Kondratskyi A, Yassine M, Slomianny C, Kondratska K, Gordienko D, Dewailly E, Lehen'kyi V, Skryma R, Prevarskaya N. 2014. Identification of ML-9 as a lysosomotropic agent targeting autophagy and cell death. Cell Death Dis. 5:e1193.
- Kos J, Mitrovic A, Mirkovic B. 2014. The current stage of cathepsin B inhibitors as potential anticancer agents. Future Med Chem. 6:1355–1371.
- Kroemer G, Galluzzi L. 2017. Lysosome-targeting agents in cancer therapy. Oncotarget. 8:112168–112169.
- Kroemer G, Senovilla L, Galluzzi L, Andre F, Zitvogel L. 2015. Natural and therapy-induced immunosurveillance in breast cancer. Nat Med. 21:1128–1138.
- Kubisch R, Frohlich T, Arnold GJ, Schreiner L, von Schwarzenberg K, Roidl A, Vollmar AM, Wagner E. 2014. V-ATPase inhibition by archazolid leads to lysosomal dysfunction resulting in impaired cathepsin B activation in vivo. Int J Cancer. 134:2478–2488.
- Kumar S, Stokes J 3rd, Singh UP, Scissum Gunn K, Acharya A, Manne U, Mishra M. 2016. Targeting hsp70: A possible therapy for cancer. Cancer Lett. 374:156–166.
- Kwakye-Berko F, Meshnick SR. 1989. Binding of chloroquine to DNA. Mol Biochem Parasitol. 35:51–55.
- Lamb CA, Yoshimori T, Tooze SA. 2013. The autophagosome: origins unknown, biogenesis complex. Nat Rev Mol Cell Biol. 14:759–774.
- Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, Davis JG, Salmon AB, Richardson A, Ahima RS, et al. 2012. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 335:1638–1643.
- Langedijk J, Mantel-Teeuwisse AK, Slijkerman DS, Schutjens MH. 2015. Drug repositioning and repurposing: terminology and definitions in literature. Drug Discov Today. 20:1027–1034.
- Lee JK, Shin JH, Lee JE, Choi EJ. 2015. Role of autophagy in the pathogenesis of amyotrophic lateral sclerosis. Biochim Biophys Acta. 1852:2517–2524.
- Le Scolan E, Pchejetski D, Banno Y, Denis N, Mayeux P, Vainchenker W, Levade T, Moreau-Gachelin F. 2005. Overexpression of sphingosine kinase 1 is an oncogenic event in erythroleukemic progression. Blood. 106:1808–1816.
- Leturiondo AL, Noronha AB, Mendonca CYR, Ferreira CO, Alvarado-Arnez LE, Manta FSN, Bezerra OCL, Carvalho EF, Moraes MO, Rodrigues FDC, Talhari C. 2020. Association of NOD2 and IFNG single nucleotide polymorphisms with leprosy in the Amazon ethnic admixed population. PLoS Negl Trop Dis. 14:e0008247.
- Levy JM, Thompson JC, Griesinger AM, Amani V, Donson AM, Birks DK, Morgan MJ, Mirsky DM, Handler MH, Foreman NK, Thorburn A. 2014. Autophagy inhibition improves chemosensitivity in BRAF(V600E) brain tumors. Cancer Discov. 4:773–780.
- Levy JMM, Towers CG, Thorburn A. 2017. Targeting autophagy in cancer. Nat Rev Cancer. 17:528–542.
- Li DL, Wang ZV, Ding G, Tan W, Luo X, Criollo A, Xie M, Jiang N, May H, Kyrychenko V, et al. 2016. Doxorubicin blocks cardiomyocyte autophagic flux by inhibiting lysosome acidification. Circulation. 133:1668–1687.
- Li J, Yang B, Zhou Q, Wu Y, Shang D, Guo Y, Song Z, Zheng Q, Xiong J. 2013. Autophagy promotes hepatocellular carcinoma cell invasion through activation of epithelial-mesenchymal transition. Carcinogenesis. 34:1343–1351.
- Li LQ, Xie WJ, Pan D, Chen H, Zhang L. 2016. Inhibition of autophagy by bafilomycin A1 promotes chemosensitivity of gastric cancer cells. Tumour Biol. 37:653–659.
- Li P, Shi M, Maique J, Shaffer J, Yan S, Moe OW, Hu MC. 2020. Beclin 1/Bcl-2 complex-dependent autophagy activity modulates renal susceptibility to ischemia-reperfusion injury and mediates renoprotection by Klotho. Am J Physiol Renal Physiol. 318:F772–F792.
- Li W, He P, Huang Y, Li YF, Lu J, Li M, Kurihara H, Luo Z, Meng T, Onishi M, et al. 2021. Selective autophagy of intracellular organelles: recent research advances. Theranostics. 11:222–256.
- Li YY, Feun LG, Thongkum A, Tu CH, Chen SM, Wangpaichitr M, Wu C, Kuo MT, Savaraj N. 2017. Autophagic mechanism in anti-cancer immunity: Its Pros and cons for cancer therapy. Int J Mol Sci. 18(6):1297.
- Liang J, Wang L, Wang C, Shen J, Su B, Marisetty AL, Fang D, Kassab C, Jeong KJ, Zhao W, et al. 2020. Verteporfin inhibits PD-L1 through autophagy and the STAT1-IRF1-TRIM28 signaling axis, exerting antitumor efficacy. Cancer Immunol Res. 8:952–965.
- Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. 1999. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 402:672–676.
- Lieberman AP, Puertollano R, Raben N, Slaugenhaupt S, Walkley SU, Ballabio A. 2012. Autophagy in lysosomal storage disorders. Autophagy. 8:719–730.
- Liu B, Palmfeldt J, Lin L, Colaco A, Clemmensen KKB, Huang J, Xu F, Liu X, Maeda K, Luo Y, Jaattela M. 2018. STAT3 associates with vacuolar H(+)-ATPase and regulates cytosolic and lysosomal pH. Cell Res. 28:996–1012.
- Liu J, Xia H, Kim M, Xu L, Li Y, Zhang L, Cai Y, Norberg HV, Zhang T, Furuya T, et al. 2011. Beclin1 controls the levels of p53 by regulating the deubiquitination activity of USP10 and USP13. Cell. 147:223–234.
- Liu K, Lee J, Kim JY, Wang L, Tian Y, Chan ST, Cho C, Machida K, Chen D, Ou JJ. 2017. Mitophagy controls the activities of tumor suppressor p53 to regulate hepatic cancer stem cells. Mol Cell. 68:281–292.e285.
- Liu S, Wang L, Ding W, Wang D, Wang X, Luo Q, Lu Y, Zhu L. 2020. Cleistanthin A inhibits the invasion of MDA-MB-231 human breast cancer cells: involvement of the beta-catenin pathway. Pharmacol Rep. 72:188–198.
- Liu W, Qi Y, Liu L, Tang Y, Wei J, Zhou L. 2016. Suppression of tumor cell proliferation by quinine via the inhibition of the tumor necrosis factor receptorassociated factor 6AKT interaction. Mol Med Rep. 14:2171–2179.
- Lopez E, Casasnovas C, Gimenez J, Santamaria R, Terrazas JM, Volpini V. 2015. Identification of two novel KIF5A mutations in hereditary spastic paraplegia associated with mild peripheral neuropathy. J Neurol Sci. 358:422–427.
- Lopez-Hernandez T, Puchkov D, Krause E, Maritzen T, Haucke V. 2020. Endocytic regulation of cellular ion homeostasis controls lysosome biogenesis. Nat Cell Biol. 22:815–827.
- Lu F, Hu Z, Sinard J, Garen A, Adelman RA. 2009. Factor VII-verteporfin for targeted photodynamic therapy in a rat model of choroidal neovascularization. Invest Ophthalmol Vis Sci. 50:3890–3896.
- Luciani A, Villella VR, Esposito S, Brunetti-Pierri N, Medina DL, Settembre C, Gavina M, Raia V, Ballabio A, Maiuri L. 2011. Cystic fibrosis: a disorder with defective autophagy. Autophagy. 7:104–106.
- Luciani F, Spada M, De Milito A, Molinari A, Rivoltini L, Montinaro A, Marra M, Lugini L, Logozzi M, Lozupone F, et al. 2004. Effect of proton pump inhibitor pretreatment on resistance of solid tumors to cytotoxic drugs. J Natl Cancer Inst. 96:1702–1713.
- Ma C, Storer CE, Chandran U, LaFramboise WA, Petrosko P, Frank M, Hartman DJ, Pantanowitz L, Haritunians T, Head RD, Liu TC. 2021. Crohn's disease-associated ATG16L1 T300A genotype is associated with improved survival in gastric cancer. EBioMedicine. 67:103347.
- Mae Y, Kanda T, Sugihara T, Takata T, Kinoshita H, Sakaguchi T, Hasegawa T, Tarumoto R, Edano M, Kurumi H, et al. 2020. Verteporfin-photodynamic therapy is effective on gastric cancer cells. Mol Clin Oncol. 13:10.
- Maejima I, Takahashi A, Omori H, Kimura T, Takabatake Y, Saitoh T, Yamamoto A, Hamasaki M, Noda T, Isaka Y, Yoshimori T. 2013. Autophagy sequesters damaged lysosomes to control lysosomal biogenesis and kidney injury. EMBO J. 32:2336–2347.
- Maes H, Kuchnio A, Peric A, Moens S, Nys K, De Bock K, Quaegebeur A, Schoors S, Georgiadou M, Wouters J, et al. 2014. Tumor vessel normalization by chloroquine independent of autophagy. Cancer Cell. 26:190–206.
- Mahapatra KK, Mishra SR, Behera BP, Patil S, Gewirtz DA, Bhutia SK. 2021. The lysosome as an imperative regulator of autophagy and cell death. Cell Mol Life Sci. 78:7435–7449.
- Mainz L, Rosenfeldt MT. 2018. Autophagy and cancer - insights from mouse models. FEBS J. 285:792–808.
- Maiuri L, Raia V, Piacentini M, Tosco A, Villella VR, Kroemer G. 2019. Cystic fibrosis transmembrane conductance regulator (CFTR) and autophagy: hereditary defects in cystic fibrosis versus gluten-mediated inhibition in celiac disease. Oncotarget. 10:4492–4500.
- Majer F, Vlaskova H, Krol L, Kalina T, Kubanek M, Stolnaya L, Dvorakova L, Elleder M, Sikora J. 2012. Danon disease: a focus on processing of the novel LAMP2 mutation and comments on the beneficial use of peripheral white blood cells in the diagnosis of LAMP2 deficiency. Gene. 498:183–195.
- Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, Burden SJ, Di Lisi R, Sandri C, Zhao J, et al. 2007. Foxo3 controls autophagy in skeletal muscle in vivo. Cell Metab. 6:458–471.
- Marciniak SJ, Lomas DA. 2010. Alpha1-antitrypsin deficiency and autophagy. N Engl J Med. 363:1863–1864.
- Margeta M. 2020. Autophagy defects in skeletal myopathies. Annu Rev Pathol. 15:261–285.
- Martin DD, Ladha S, Ehrnhoefer DE, Hayden MR. 2015. Autophagy in huntington disease and huntingtin in autophagy. Trends Neurosci. 38:26–35.
- Martin LJ, Gupta J, Jyothula SS, Butsch Kovacic M, Biagini Myers JM, Patterson TL, Ericksen MB, He H, Gibson AM, Baye TM, et al. 2012. Functional variant in the autophagy-related 5 gene promotor is associated with childhood asthma. PLoS One. 7:e33454.
- Martina JA, Chen Y, Gucek M, Puertollano R. 2012. MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy. 8:903–914.
- Mathew R, Karantza-Wadsworth V, White E. 2007. Role of autophagy in cancer. Nat Rev Cancer. 7:961–967.
- Maxson ME, Grinstein S. 2014. The vacuolar-type H(+)-ATPase at a glance – more than a proton pump. J Cell Sci. 127:4987–4993.
- Maycotte P, Aryal S, Cummings CT, Thorburn J, Morgan MJ, Thorburn A. 2012. Chloroquine sensitizes breast cancer cells to chemotherapy independent of autophagy. Autophagy. 8:200–212.
- Maynadier M, Vezenkov LL, Amblard M, Martin V, Gandreuil C, Vaillant O, Gary-Bobo M, Basile I, Hernandez JF, Garcia M, Martinez J. 2013. Dipeptide mimic oligomer transporter mediates intracellular delivery of cathepsin D inhibitors: a potential target for cancer therapy. J Control Release. 171:251–257.
- McAfee Q, Zhang Z, Samanta A, Levi SM, Ma XH, Piao S, Lynch JP, Uehara T, Sepulveda AR, Davis LE, et al. 2012. Autophagy inhibitor Lys05 has single-agent antitumor activity and reproduces the phenotype of a genetic autophagy deficiency. Proc Natl Acad Sci USA. 109:8253–8258.
- Medina DL, Di Paola S, Peluso I, Armani A, De Stefani D, Venditti R, Montefusco S, Scotto-Rosato A, Prezioso C, Forrester A, et al. 2015. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat Cell Biol. 17:288–299.
- Mehto S, Jena KK, Nath P, Chauhan S, Kolapalli SP, Das SK, Sahoo PK, Jain A, Taylor GA, Chauhan S. 2019. The Crohn's disease risk factor IRGM limits NLRP3 inflammasome activation by impeding Its assembly and by mediating Its selective autophagy. Mol Cell. 73:429–445. e427.
- Meng D, Li Z, Wang G, Ling L, Wu Y, Zhang C. 2018. Carvedilol attenuates liver fibrosis by suppressing autophagy and promoting apoptosis in hepatic stellate cells. Biomed Pharmacother. 108:1617–1627.
- Menikdiwela KR, Ramalingam L, Rasha F, Wang S, Dufour JM, Kalupahana NS, Sunahara KKS, Martins JO, Moustaid-Moussa N. 2020. Autophagy in metabolic syndrome: breaking the wheel by targeting the renin-angiotensin system. Cell Death Dis. 11:87.
- Meo-Evoli N, Almacellas E, Massucci FA, Gentilella A, Ambrosio S, Kozma SC, Thomas G, Tauler A. 2015. V-ATPase: a master effector of E2F1-mediated lysosomal trafficking, mTORC1 activation and autophagy. Oncotarget. 6:28057–28070.
- Merk H, Messer P, Ardelt MA, Lamb DC, Zahler S, Muller R, Vollmar AM, Pachmayr J. 2017. Inhibition of the V-ATPase by archazolid A: A New strategy to inhibit EMT. Mol Cancer Ther. 16:2329–2339.
- Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, Shen S, Kepp O, Scoazec M, Mignot G, et al. 2011. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 334:1573–1577.
- Min L, Choy E, Pollock RE, Tu C, Hornicek F, Duan Z. 2017. Autophagy as a potential target for sarcoma treatment. Biochim Biophys Acta Rev Cancer. 1868:40–50.
- Mindell JA. 2012. Lysosomal acidification mechanisms. Annu Rev Physiol. 74:69–86.
- Mitrovic A, Kos J. 2019. Nitroxoline: repurposing its antimicrobial to antitumor application. Acta Biochim Pol. 66:521–531.
- Mizushima N, Komatsu M. 2011. Autophagy: renovation of cells and tissues. Cell. 147:728–741.
- Mizushima N, Yoshimori T, Ohsumi Y. 2011. The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol. 27:107–132.
- Mizutani Y, Maeda T, Murate K, Ito S, Watanabe H, Mutoh T. 2021. Clioquinol kills astrocyte-derived KT-5 cells by the impairment of the autophagy-lysosome pathway. Arch Toxicol. 95:631–640.
- Mjahed H, Girodon F, Fontenay M, Garrido C. 2012. Heat shock proteins in hematopoietic malignancies. Exp Cell Res. 318:1946–1958.
- Mollereau B, Walter L. 2019. Is WDR45 the missing link for ER stress-induced autophagy in beta-propeller associated neurodegeneration? Autophagy. 15:2163–2164.
- Morel E, Mehrpour M, Botti J, Dupont N, Hamai A, Nascimbeni AC, Codogno P. 2017. Autophagy: A druggable process. Annu Rev Pharmacol Toxicol. 57:375–398.
- Moriya S, Che XF, Komatsu S, Abe A, Kawaguchi T, Gotoh A, Inazu M, Tomoda A, Miyazawa K. 2013. Macrolide antibiotics block autophagy flux and sensitize to bortezomib via endoplasmic reticulum stress-mediated CHOP induction in myeloma cells. Int J Oncol. 42:1541–1550.
- Morselli E, Galluzzi L, Kepp O, Vicencio JM, Criollo A, Maiuri MC, Kroemer G. 2009. Anti- and pro-tumor functions of autophagy. Biochim Biophys Acta. 1793:1524–1532.
- Muzykewicz DA, Sharma A, Muse V, Numis AL, Rajagopal J, Thiele EA. 2009. TSC1 and TSC2 mutations in patients with lymphangioleiomyomatosis and tuberous sclerosis complex. J Med Genet. 46:465–468.
- Nakamura M, Kikukawa Y, Takeya M, Mitsuya H, Hata H. 2010. Clarithromycin attenuates autophagy in myeloma cells. Int J Oncol. 37:815–820.
- Noda NN, Inagaki F. 2015. Mechanisms of autophagy. Annu Rev Biophys. 44:101–122.
- Nordstrom LU, Sironi J, Aranda E, Maisonet J, Perez-Soler R, Wu P, Schwartz EL. 2015. Discovery of autophagy inhibitors with antiproliferative activity in lung and pancreatic cancer cells. ACS Med Chem Lett. 6:134–139.
- Nosengo N. 2016. Can you teach old drugs new tricks? Nature. 534:314–316.
- Nowak KL, Edelstein CL. 2020. Apoptosis and autophagy in polycystic kidney disease (PKD). Cell Signal. 68:109518.
- Nwadike C, Williamson LE, Gallagher LE, Guan JL, Chan EYW. 2018. AMPK inhibits ULK1-dependent autophagosome formation and lysosomal acidification via distinct mechanisms. Mol Cell Biol. 38(10):e00023-18.
- Oot RA, Couoh-Cardel S, Sharma S, Stam NJ, Wilkens S. 2017. Breaking up and making up: The secret life of the vacuolar H(+) -ATPase. Protein Sci. 26:896–909.
- Oz-Levi D, Ben-Zeev B, Ruzzo EK, Hitomi Y, Gelman A, Pelak K, Anikster Y, Reznik-Wolf H, Bar-Joseph I, Olender T, et al. 2012. Mutation in TECPR2 reveals a role for autophagy in hereditary spastic paraparesis. Am J Hum Genet. 91:1065–1072.
- Palmieri M, Impey S, Kang H, di Ronza A, Pelz C, Sardiello M, Ballabio A. 2011. Characterization of the CLEAR network reveals an integrated control of cellular clearance pathways. Hum Mol Genet. 20:3852–3866.
- Pamarthy S, Kulshrestha A, Katara GK, Beaman KD. 2018. The curious case of vacuolar ATPase: regulation of signaling pathways. Mol Cancer. 17:41.
- Pammolli F, Magazzini L, Riccaboni M. 2011. The productivity crisis in pharmaceutical R&D. Nat Rev Drug Discov. 10:428–438.
- Pan B, Chen D, Huang J, Wang R, Feng B, Song H, Chen L. 2014. HMGB1-mediated autophagy promotes docetaxel resistance in human lung adenocarcinoma. Mol Cancer. 13:165.
- Pan H, Cai N, Li M, Liu GH, Izpisua Belmonte JC. 2013. Autophagic control of cell ‘stemness’. EMBO Mol Med. 5:327–331.
- Papadopoulos C, Kravic B, Meyer H. 2020. Repair or lysophagy: dealing with damaged lysosomes. J Mol Biol. 432:231–239.
- Papadopoulos C, Meyer H. 2017. Detection and clearance of damaged lysosomes by the endo-lysosomal damage response and lysophagy. Curr Biol. 27:R1330–R1341.
- Parenti G, Andria G, Ballabio A. 2015. Lysosomal storage diseases: from pathophysiology to therapy. Annu Rev Med. 66:471–486.
- Park JM, Tougeron D, Huang S, Okamoto K, Sinicrope FA, Ulasov I. 2014. Beclin 1 and UVRAG confer protection from radiation-induced DNA damage and maintain centrosome stability in colorectal cancer cells. PLoS One. 9:e100819.
- Pascolo S. 2016. Time to use a dose of chloroquine as an adjuvant to anti-cancer chemotherapies. Eur J Pharmacol. 771:139–144.
- Pasquier B. 2015. SAR405, a PIK3C3/Vps34 inhibitor that prevents autophagy and synergizes with MTOR inhibition in tumor cells. Autophagy. 11:725–726.
- Paz I, Sachse M, Dupont N, Mounier J, Cederfur C, Enninga J, Leffler H, Poirier F, Prevost MC, Lafont F, Sansonetti P. 2010. Galectin-3, a marker for vacuole lysis by invasive pathogens. Cell Microbiol. 12:530–544.
- Pecoraro A, Pagano M, Russo G, Russo A. 2020. Role of autophagy in cancer cell response to nucleolar and endoplasmic reticulum stress. Int J Mol Sci. 21(19):7334.
- Pellegrini P, Strambi A, Zipoli C, Hagg-Olofsson M, Buoncervello M, Linder S, De Milito A. 2014. Acidic extracellular pH neutralizes the autophagy-inhibiting activity of chloroquine: implications for cancer therapies. Autophagy. 10:562–571.
- Penke B, Bogar F, Crul T, Santha M, Toth ME, Vigh L. 2018. Heat shock proteins and autophagy pathways in neuroprotection: from molecular bases to pharmacological interventions. Int J Mol Sci. 19:325.
- Perera RM, Zoncu R. 2016. The lysosome as a regulatory Hub. Annu Rev Cell Dev Biol. 32:223–253.
- Perez-Mancera PA, Young AR, Narita M. 2014. Inside and out: the activities of senescence in cancer. Nat Rev Cancer. 14:547–558.
- Petersen NH, Olsen OD, Groth-Pedersen L, Ellegaard AM, Bilgin M, Redmer S, Ostenfeld MS, Ulanet D, Dovmark TH, Lonborg A, et al. 2013. Transformation-associated changes in sphingolipid metabolism sensitize cells to lysosomal cell death induced by inhibitors of acid sphingomyelinase. Cancer Cell. 24:379–393.
- Piao S, Amaravadi RK. 2016. Targeting the lysosome in cancer. Ann N Y Acad Sci. 1371:45–54.
- Pickrell AM, Youle RJ. 2015. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson's disease. Neuron. 85:257–273.
- Platt FM, d'Azzo A, Davidson BL, Neufeld EF, Tifft CJ. 2018. Lysosomal storage diseases. Nat Rev Dis Primers. 4:27.
- Pogorzelska A, Zolnowska B, Bartoszewski R. 2018. Cysteine cathepsins as a prospective target for anticancer therapies-current progress and prospects. Biochimie. 151:85–106.
- Poulogiannis G, McIntyre RE, Dimitriadi M, Apps JR, Wilson CH, Ichimura K, Luo F, Cantley LC, Wyllie AH, Adams DJ, Arends MJ. 2010. PARK2 deletions occur frequently in sporadic colorectal cancer and accelerate adenoma development in Apc mutant mice. Proc Natl Acad Sci USA. 107:15145–15150.
- Pu J, Guardia CM, Keren-Kaplan T, Bonifacino JS. 2016. Mechanisms and functions of lysosome positioning. J Cell Sci. 129:4329–4339.
- Qiu W, Su M, Xie F, Ai J, Ren Y, Zhang J, Guan R, He W, Gong Y, Guo Y. 2014. Tetrandrine blocks autophagic flux and induces apoptosis via energetic impairment in cancer cells. Cell Death Dis. 5:e1123.
- Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H, Troxel A, Rosen J, Eskelinen EL, Mizushima N, Ohsumi Y, et al. 2003. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest. 112:1809–1820.
- Qureshi-Baig K, Kuhn D, Viry E, Pozdeev VI, Schmitz M, Rodriguez F, Ullmann P, Koncina E, Nurmik M, Frasquilho S, et al. 2020. Hypoxia-induced autophagy drives colorectal cancer initiation and progression by activating the PRKC/PKC-EZR (ezrin) pathway. Autophagy. 16:1436–1452.
- Radulovic M, Schink KO, Wenzel EM, Nahse V, Bongiovanni A, Lafont F, Stenmark H. 2018. ESCRT-mediated lysosome repair precedes lysophagy and promotes cell survival. EMBO J. 37(21): e99753.
- Ramessur KT, Greenwell P, Nash R, Dwek MV. 2010. Breast cancer invasion is mediated by beta-N-acetylglucosaminidase (beta-NAG) and associated with a dysregulation in the secretory pathway of cancer cells. Br J Biomed Sci. 67:189–196.
- Rangwala R, Leone R, Chang YC, Fecher LA, Schuchter LM, Kramer A, Tan KS, Heitjan DF, Rodgers G, Gallagher M, et al. 2014. Phase I trial of hydroxychloroquine with dose-intense temozolomide in patients with advanced solid tumors and melanoma. Autophagy. 10:1369–1379.
- Rao S, Yang H, Penninger JM, Kroemer G. 2014. Autophagy in non-small cell lung carcinogenesis: A positive regulator of antitumor immunosurveillance. Autophagy. 10:529–531.
- Rebecca VW, Nicastri MC, McLaughlin N, Fennelly C, McAfee Q, Ronghe A, Nofal M, Lim CY, Witze E, Chude CI, et al. 2017. A unified approach to targeting the lysosome's degradative and growth signaling roles. Cancer Discov. 7:1266–1283.
- Renna M, Schaffner C, Brown K, Shang S, Tamayo MH, Hegyi K, Grimsey NJ, Cusens D, Coulter S, Cooper J, et al. 2011. Azithromycin blocks autophagy and may predispose cystic fibrosis patients to mycobacterial infection. J Clin Invest. 121:3554–3563.
- Renvoise B, Chang J, Singh R, Yonekawa S, FitzGibbon EJ, Mankodi A, Vanderver A, Schindler A, Toro C, Gahl WA, et al. 2014. Lysosomal abnormalities in hereditary spastic paraplegia types SPG15 and SPG11. Ann Clin Transl Neurol. 1:379–389.
- Rofstad EK, Mathiesen B, Kindem K, Galappathi K. 2006. Acidic extracellular pH promotes experimental metastasis of human melanoma cells in athymic nude mice. Cancer Res. 66:6699–6707.
- Rojas-Puentes LL, Gonzalez-Pinedo M, Crismatt A, Ortega-Gomez A, Gamboa-Vignolle C, Nunez-Gomez R, Dorantes-Gallareta Y, Arce-Salinas C, Arrieta O. 2013. Phase II randomized, double-blind, placebo-controlled study of whole-brain irradiation with concomitant chloroquine for brain metastases. Radiat Oncol. 8:209.
- Ronan B, Flamand O, Vescovi L, Dureuil C, Durand L, Fassy F, Bachelot MF, Lamberton A, Mathieu M, Bertrand T, et al. 2014. A highly potent and selective Vps34 inhibitor alters vesicle trafficking and autophagy. Nat Chem Biol. 10:1013–1019.
- Rubinsztein DC, Codogno P, Levine B. 2012. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discov. 11:709–730.
- Rudzinska M, Parodi A, Soond SM, Vinarov AZ, Korolev DO, Morozov AO, Daglioglu C, Tutar Y, Zamyatnin AA, Jr. 2019. The role of cysteine cathepsins in cancer progression and drug resistance. Int J Mol Sci. 20(14): 3602.
- Rybstein MD, Bravo-San Pedro JM, Kroemer G, Galluzzi L. 2018. The autophagic network and cancer. Nat Cell Biol. 20:243–251.
- Sabatini DM. 2017. Twenty-five years of mTOR: uncovering the link from nutrients to growth. Proc Natl Acad Sci USA. 114:11818–11825.
- Saftig P, Klumperman J. 2009. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat Rev Mol Cell Biol. 10:623–635.
- Saftig P, Sandhoff K. 2013. Cancer: killing from the inside. Nature. 502:312–313.
- Sander P, Mostafa H, Soboh A, Schneider JM, Pala A, Baron AK, Moepps B, Wirtz CR, Georgieff M, Schneider M. 2017. Vacquinol-1 inducible cell death in glioblastoma multiforme is counter regulated by TRPM7 activity induced by exogenous ATP. Oncotarget. 8:35124–35137.
- Savic R, Schuchman EH. 2013. Use of acid sphingomyelinase for cancer therapy. Adv Cancer Res. 117:91–115.
- Scannell JW, Blanckley A, Boldon H, Warrington B. 2012. Diagnosing the decline in pharmaceutical R&D efficiency. Nat Rev Drug Discov. 11:191–200.
- Schurigt U, Schad C, Glowa C, Baum U, Thomale K, Schnitzer JK, Schultheis M, Schaschke N, Schirmeister T, Moll H. 2010. Aziridine-2,3-dicarboxylate-based cysteine cathepsin inhibitors induce cell death in Leishmania major associated with accumulation of debris in autophagy-related lysosome-like vacuoles. Antimicrob Agents Chemother. 54:5028–5041.
- Seitz C, Hugle M, Cristofanon S, Tchoghandjian A, Fulda S. 2013. The dual PI3K/mTOR inhibitor NVP-BEZ235 and chloroquine synergize to trigger apoptosis via mitochondrial-lysosomal cross-talk. Int J Cancer. 132:2682–2693.
- Selvakumaran M, Amaravadi RK, Vasilevskaya IA, O'Dwyer PJ. 2013. Autophagy inhibition sensitizes colon cancer cells to antiangiogenic and cytotoxic therapy. Clin Cancer Res. 19:2995–3007.
- Sennoune SR, Bakunts K, Martinez GM, Chua-Tuan JL, Kebir Y, Attaya MN, Martinez-Zaguilan R. 2004. Vacuolar H+-ATPase in human breast cancer cells with distinct metastatic potential: distribution and functional activity. Am J Physiol Cell Physiol. 286:C1443–C1452.
- Serrano-Puebla A, Boya P. 2016. Lysosomal membrane permeabilization in cell death: new evidence and implications for health and disease. Ann N Y Acad Sci. 1371:30–44.
- Settembre C, De Cegli R, Mansueto G, Saha PK, Vetrini F, Visvikis O, Huynh T, Carissimo A, Palmer D, Klisch TJ, et al. 2013. TFEB controls cellular lipid metabolism through a starvation-induced autoregulatory loop. Nat Cell Biol. 15:647–658.
- Settembre C, Fraldi A, Medina DL, Ballabio A. 2013. Signals from the lysosome: a control centre for cellular clearance and energy metabolism. Nat Rev Mol Cell Biol. 14:283–296.
- Settembre C, Zoncu R, Medina DL, Vetrini F, Erdin S, Erdin S, Huynh T, Ferron M, Karsenty G, Vellard MC, et al. 2012. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J. 31:1095–1108.
- Shao BZ, Yao Y, Zhai JS, Zhu JH, Li JP, Wu K. 2021. The role of autophagy in inflammatory bowel disease. Front Physiol. 12:621132.
- Shao S, Li S, Qin Y, Wang X, Yang Y, Bai H, Zhou L, Zhao C, Wang C. 2014. Spautin-1, a novel autophagy inhibitor, enhances imatinib-induced apoptosis in chronic myeloid leukemia. Int J Oncol. 44:1661–1668.
- Sharif T, Martell E, Dai C, Kennedy BE, Murphy P, Clements DR, Kim Y, Lee PW, Gujar SA. 2017. Autophagic homeostasis is required for the pluripotency of cancer stem cells. Autophagy. 13:264–284.
- Sharma N, Thomas S, Golden EB, Hofman FM, Chen TC, Petasis NA, Schonthal AH, Louie SG. 2012. Inhibition of autophagy and induction of breast cancer cell death by mefloquine, an antimalarial agent. Cancer Lett. 326:143–154.
- Sheng JQ, Wang MR, Fang D, Liu L, Huang WJ, Tian DA, He XX, Li PY. 2021. LncRNA NBR2 inhibits tumorigenesis by regulating autophagy in hepatocellular carcinoma. Biomed Pharmacother. 133:111023.
- Simmen T, Tagaya M. 2017. Organelle communication at membrane contact sites (MCS): from curiosity to center stage in cell biology and biomedical research. Adv Exp Med Biol. 997:1–12.
- Singh SS, Vats S, Chia AY, Tan TZ, Deng S, Ong MS, Arfuso F, Yap CT, Goh BC, Sethi G, et al. 2018. Dual role of autophagy in hallmarks of cancer. Oncogene. 37:1142–1158.
- Sironi J, Aranda E, Nordstrom LU, Schwartz EL. 2019. Lysosome membrane permeabilization and disruption of the molecular target of rapamycin (mTOR)-lysosome interaction Are associated with the inhibition of lung cancer cell proliferation by a chloroquinoline analog. Mol Pharmacol. 95:127–138.
- Skowyra ML, Schlesinger PH, Naismith TV, Hanson PI. 2018. Triggered recruitment of ESCRT machinery promotes endolysosomal repair. Science. 360(6384): eaar5078.
- Sosa MS, Bragado P, Aguirre-Ghiso JA. 2014. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer. 14:611–622.
- Stock C, Pedersen SF. 2017. Roles of pH and the Na(+)/H(+) exchanger NHE1 in cancer: from cell biology and animal models to an emerging translational perspective? Semin Cancer Biol. 43:5–16.
- Stoka V, Turk V, Turk B. 2016. Lysosomal cathepsins and their regulation in aging and neurodegeneration. Ageing Res Rev. 32:22–37.
- Sui X, Chen R, Wang Z, Huang Z, Kong N, Zhang M, Han W, Lou F, Yang J, Zhang Q, et al. 2013. Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment. Cell Death Dis. 4:e838.
- Sun Y, Liu JH, Jin L, Lin SM, Yang Y, Sui YX, Shi H. 2010. Over-expression of the Beclin1 gene upregulates chemosensitivity to anti-cancer drugs by enhancing therapy-induced apoptosis in cervix squamous carcinoma CaSki cells. Cancer Lett. 294:204–210.
- Taherian E, Rao A, Malemud CJ, Askari AD. 2013. The biological and clinical activity of anti-malarial drugs in autoimmune disorders. Curr Rheumatol Rev. 9:45–62.
- Takamura A, Komatsu M, Hara T, Sakamoto A, Kishi C, Waguri S, Eishi Y, Hino O, Tanaka K, Mizushima N. 2011. Autophagy-deficient mice develop multiple liver tumors. Genes Dev. 25:795–800.
- Tang Y, Wang XW, Liu ZH, Sun YM, Tang YX, Zhou DH. 2017. Chaperone-mediated autophagy substrate proteins in cancer. Oncotarget. 8:51970–51985.
- Taylor S, Spugnini EP, Assaraf YG, Azzarito T, Rauch C, Fais S. 2015. Microenvironment acidity as a major determinant of tumor chemoresistance: proton pump inhibitors (PPIs) as a novel therapeutic approach. Drug Resist Updat. 23:69–78.
- Thiery JP, Acloque H, Huang RY, Nieto MA. 2009. Epithelial-mesenchymal transitions in development and disease. Cell. 139:871–890.
- Thurston TL, Wandel MP, von Muhlinen N, Foeglein A, Randow F. 2012. Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion. Nature. 482:414–418.
- Torgersen ML, Engedal N, Boe SO, Hokland P, Simonsen A. 2013. Targeting autophagy potentiates the apoptotic effect of histone deacetylase inhibitors in t(8;21) AML cells. Blood. 122:2467–2476.
- Torrente E, Parodi C, Ercolani L, De Mei C, Ferrari A, Scarpelli R, Grimaldi B. 2015. Synthesis and in vitro anticancer activity of the first class of dual inhibitors of REV-ERBbeta and autophagy. J Med Chem. 58:5900–5915.
- Towers CG, Thorburn A. 2017. Targeting the lysosome for cancer therapy. Cancer Discov. 7:1218–1220.
- Towers CG, Thorburn A. 2019. Circumventing autophagy inhibition. Cell Cycle. 18:3421–3431.
- Towers CG, Wodetzki D, Thorburn A. 2020. Autophagy and cancer: modulation of cell death pathways and cancer cell adaptations. J Cell Biol. 219(1): e201909033.
- Tran M, Reddy PH. 2020. Defective autophagy and mitophagy in aging and Alzheimer's disease. Front Neurosci. 14:612757.
- Trivedi PC, Bartlett JJ, Pulinilkunnil T. 2020. Lysosomal biology and function: modern view of cellular debris Bin. Cells. 9:1131.
- Turanli B, Grotli M, Boren J, Nielsen J, Uhlen M, Arga KY, Mardinoglu A. 2018. Drug repositioning for effective prostate cancer treatment. Front Physiol. 9:500.
- Tzivion G, Dobson M, Ramakrishnan G. 2011. Foxo transcription factors; regulation by AKT and 14-3-3 proteins. Biochim Biophys Acta. 1813:1938–1945.
- Urquhart L. 2018. Market watch: Top drugs and companies by sales in 2017. Nat Rev Drug Discov. 17:232.
- Usategui-Martin R, Gestoso-Uzal N, Calero-Paniagua I, De Pereda JM, Del Pino-Montes J, Gonzalez-Sarmiento R. 2020. A mutation in p62 protein (p. R321C), associated to Paget's disease of bone, causes a blockade of autophagy and an activation of NF-kB pathway. Bone. 133:115265.
- Vanharanta S, Massague J. 2013. Origins of metastatic traits. Cancer Cell. 24:410–421.
- Veeriah S, Taylor BS, Meng S, Fang F, Yilmaz E, Vivanco I, Janakiraman M, Schultz N, Hanrahan AJ, Pao W, et al. 2010. Somatic mutations of the Parkinson's disease-associated gene PARK2 in glioblastoma and other human malignancies. Nat Genet. 42:77–82.
- Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ. 1993. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 75:229–240.
- Verbaanderd C, Maes H, Schaaf MB, Sukhatme VP, Pantziarka P, Sukhatme V, Agostinis P, Bouche G. 2017. Repurposing drugs in Oncology (ReDO)-chloroquine and hydroxychloroquine as anti-cancer agents. Ecancermedicalscience. 11:781.
- Wang P, Long M, Zhang S, Cheng Z, Zhao X, He F, Liu H, Ming L. 2017. Hypoxia inducible factor-1alpha regulates autophagy via the p27-E2F1 signaling pathway. Mol Med Rep. 16:2107–2112.
- Wang T, Goodall ML, Gonzales P, Sepulveda M, Martin KR, Gately S, MacKeigan JP. 2015. Synthesis of improved lysomotropic autophagy inhibitors. J Med Chem. 58:3025–3035.
- Wang X, Shen C, Liu Z, Peng F, Chen X, Yang G, Zhang D, Yin Z, Ma J, Zheng Z, et al. 2018. Nitazoxanide, an antiprotozoal drug, inhibits late-stage autophagy and promotes ING1-induced cell cycle arrest in glioblastoma. Cell Death Dis. 9:1032.
- Wang XJ, Yang YS, Shen K, Wang J, Han F, Wu GF, Li Y, Bai XZ, Luo L, Hu DH. 2020. [Effects and mechanism of pyrroloquinoline quinine on mitochondrial function and cell survival of rat bone marrow mesenchymal stem cells under oxidative stress]. Zhonghua Shao Shang Za Zhi. 36:378–387.
- Wang Y, Zhang N, Zhang L, Li R, Fu W, Ma K, Li X, Wang L, Wang J, Zhang H, et al. 2016. Autophagy regulates chromatin ubiquitination in DNA damage response through elimination of SQSTM1/p62. Mol Cell. 63:34–48.
- Wang Z, Zhang J, Wang Y, Xing R, Yi C, Zhu H, Chen X, Guo J, Guo W, Li W, et al. 2013. Matrine, a novel autophagy inhibitor, blocks trafficking and the proteolytic activation of lysosomal proteases. Carcinogenesis. 34:128–138.
- Waring MJ, Arrowsmith J, Leach AR, Leeson PD, Mandrell S, Owen RM, Pairaudeau G, Pennie WD, Pickett SD, Wang J, et al. 2015. An analysis of the attrition of drug candidates from four major pharmaceutical companies. Nat Rev Drug Discov. 14:475–486.
- Wei W, Ren J, Yin W, Ding H, Lu Q, Tan L, Deng S, Liu J, Yang Q, Wang J, et al. 2020. Inhibition of ctsk modulates periodontitis with arthritis via downregulation of TLR9 and autophagy. Cell Prolif. 53:e12722.
- Weihl CC, Iyadurai S, Baloh RH, Pittman SK, Schmidt RE, Lopate G, Pestronk A, Harms MB. 2015. Autophagic vacuolar pathology in desminopathies. Neuromuscul Disord. 25:199–206.
- White E. 2015. The role for autophagy in cancer. J Clin Invest. 125:42–46.
- White KA, Grillo-Hill BK, Barber DL. 2017. Cancer cell behaviors mediated by dysregulated pH dynamics at a glance. J Cell Sci. 130:663–669.
- Wilkinson RD, Williams R, Scott CJ, Burden RE. 2015. Cathepsin S: therapeutic, diagnostic, and prognostic potential. Biol Chem. 396:867–882.
- Wong VKW, Zeng W, Chen J, Yao XJ, Leung ELH, Wang QQ, Chiu P, Ko BCB, Law BYK. 2017. Tetrandrine, an activator of autophagy, induces autophagic cell death via PKC-alpha inhibition and mTOR-dependent mechanisms. Front Pharmacol. 8:351.
- Workman P. 2020. Reflections and outlook on targeting HSP90, HSP70 and HSF1 in cancer: A personal perspective. Adv Exp Med Biol. 1243:163–179.
- Xie H, Li C, Zhang M, Zhong N, Chen L. 2017. Association between IRGM polymorphisms and tuberculosis risk: A meta-analysis. Medicine (Baltimore). 96:e8189.
- Xiong D, Wang Y, Kupert E, Simpson C, Pinney SM, Gaba CR, Mandal D, Schwartz AG, Yang P, de Andrade M, et al. 2015. A recurrent mutation in PARK2 is associated with familial lung cancer. Am J Hum Genet. 96:301–308.
- Xu N, Lin W, Sun J, Sadahira T, Xu A, Watanabe M, Guo K, Araki M, Li G, Liu C, et al. 2020. Nitroxoline inhibits bladder cancer progression by reversing EMT process and enhancing anti-tumor immunity. J Cancer. 11:6633–6641.
- Yamamoto S, Ma X. 2009. Role of Nod2 in the development of Crohn's disease. Microbes Infect. 11:912–918.
- Yan Y, Zhou K, Wang L, Wang F, Chen X, Fan Q. 2017. Clinical significance of serum cathepsin B and cystatin C levels and their ratio in the prognosis of patients with esophageal cancer. Onco Targets Ther. 10:1947–1954.
- Yang B, Xu QY, Guo CY, Huang JW, Wang SM, Li YM, Tu Y, He L, Bi ZG, Ji C, Cheng B. 2017. MHY1485 ameliorates UV-induced skin cell damages via activating mTOR-Nrf2 signaling. Oncotarget. 8:12775–12783.
- Yang PW, Hsieh MS, Chang YH, Huang PM, Lee JM. 2017. Genetic polymorphisms of ATG5 predict survival and recurrence in patients with early-stage esophageal squamous cell carcinoma. Oncotarget. 8:91494–91504.
- Yao D, GangYi Y, QiNan W. 2021. Autophagic dysfunction of beta cell dysfunction in type 2 diabetes, a double-edged sword. Genes Dis. 8:438–447.
- Yao RQ, Ren C, Xia ZF, Yao YM. 2021. Organelle-specific autophagy in inflammatory diseases: a potential therapeutic target underlying the quality control of multiple organelles. Autophagy. 17:385–401.
- Yazdani HO, Huang H, Tsung A. 2019. Autophagy: dual response in the development of hepatocellular carcinoma. Cells. 8(2): 91.
- Yella JK, Yaddanapudi S, Wang Y, Jegga AG. 2018. Changing Trends in computational drug repositioning. Pharmaceuticals (Basel). 11:57.
- Yoshida Y, Yasuda S, Fujita T, Hamasaki M, Murakami A, Kawawaki J, Iwai K, Saeki Y, Yoshimori T, Matsuda N, Tanaka K. 2017. Ubiquitination of exposed glycoproteins by SCF(FBXO27) directs damaged lysosomes for autophagy. Proc Natl Acad Sci USA. 114:8574–8579.
- You H, Jin J, Shu H, Yu B, De Milito A, Lozupone F, Deng Y, Tang N, Yao G, Fais S, et al. 2009. Small interfering RNA targeting the subunit ATP6L of proton pump V-ATPase overcomes chemoresistance of breast cancer cells. Cancer Lett. 280:110–119.
- Young AR, Narita M, Ferreira M, Kirschner K, Sadaie M, Darot JF, Tavare S, Arakawa S, Shimizu S, Watt FM, Narita M. 2009. Autophagy mediates the mitotic senescence transition. Genes Dev. 23:798–803.
- Yu L, Chen Y, Tooze SA. 2018. Autophagy pathway: cellular and molecular mechanisms. Autophagy. 14:207–215.
- Yun CW, Lee SH. 2018. The roles of autophagy in cancer. Int J Mol Sci. 19(11): 3466.
- Zada S, Hwang JS, Ahmed M, Lai TH, Pham TM, Elashkar O, Kim DR. 2021. Cross talk between autophagy and oncogenic signaling pathways and implications for cancer therapy. Biochim Biophys Acta Rev Cancer. 1876:188565.
- Zeh HJ, Bahary N, Boone BA, Singhi AD, Miller-Ocuin JL, Normolle DP, Zureikat AH, Hogg ME, Bartlett DL, Lee KK, et al. 2020. A randomized phase II preoperative study of autophagy inhibition with high-dose hydroxychloroquine and gemcitabine/Nab-Paclitaxel in pancreatic cancer patients. Clin Cancer Res. 26:3126–3134.
- Zhang H, Chen L, Sun X, Yang Q, Wan L, Guo C. 2020. Matrine: A promising natural product With various pharmacological activities. Front Pharmacol. 11:588.
- Zhang MY, Wang LY, Zhao S, Guo XC, Xu YQ, Zheng ZH, Lu H, Zheng HC. 2019. Effects of beclin 1 overexpression on aggressive phenotypes of colon cancer cells. Oncol Lett. 17:2441–2450.
- Zhang W, Wang S, Wang Q, Yang Z, Pan Z, Li L. 2014. Overexpression of cysteine cathepsin L is a marker of invasion and metastasis in ovarian cancer. Oncol Rep. 31:1334–1342.
- Zhang Y, Liao Z, Zhang LJ, Xiao HT. 2015. The utility of chloroquine in cancer therapy. Curr Med Res Opin. 31:1009–1013.
- Zhang Y, Sowers JR, Ren J. 2018. Targeting autophagy in obesity: from pathophysiology to management. Nat Rev Endocrinol. 14:356–376.
- Zhao X, Ayer RE, Davis SL, Ames SJ, Florence B, Torchinsky C, Liou JS, Shen L, Spanjaard RA. 2005. Apoptosis factor EI24/PIG8 is a novel endoplasmic reticulum-localized Bcl-2-binding protein which is associated with suppression of breast cancer invasiveness. Cancer Res. 65:2125–2129.
- Zhao X, Fang Y, Yang Y, Qin Y, Wu P, Wang T, Lai H, Meng L, Wang D, Zheng Z, et al. 2015. Elaiophylin, a novel autophagy inhibitor, exerts antitumor activity as a single agent in ovarian cancer cells. Autophagy. 11:1849–1863.
- Zhao Y, Lu Y, Ma J, Zhu L. 2015. Synthesis and evaluation of cleistanthin A derivatives as potent vacuolar H(+) -ATPase inhibitors. Chem Biol Drug Des. 86:691–696.
- Zhitomirsky B, Assaraf YG. 2016. Lysosomes as mediators of drug resistance in cancer. Drug Resist Updat. 24:23–33.
- Zhou J, Li G, Zheng Y, Shen HM, Hu X, Ming QL, Huang C, Li P, Gao N. 2015. A novel autophagy/mitophagy inhibitor liensinine sensitizes breast cancer cells to chemotherapy through DNM1L-mediated mitochondrial fission. Autophagy. 11:1259–1279.
- Zhou XJ, Lu XL, Lv JC, Yang HZ, Qin LX, Zhao MH, Su Y, Li ZG, Zhang H. 2011. Genetic association of PRDM1-ATG5 intergenic region and autophagy with systemic lupus erythematosus in a Chinese population. Ann Rheum Dis. 70:1330–1337.
- Zhou Y, Ma J, Zhang J, He L, Gong J, Long C. 2017. Pifithrin-mu is efficacious against non-small cell lung cancer via inhibition of heat shock protein 70. Oncol Rep. 37:313–322.
- Zhu L, Wang H, Wu Y, He Z, Qin Y, Shen Q. 2017. The autophagy level Is increased in the synovial tissues of patients with active rheumatoid arthritis and Is correlated with disease severity. Mediators Inflamm. 2017:7623145.
- Zhu Q, Zhang Q, Gu M, Zhang K, Xia T, Zhang S, Chen W, Yin H, Yao H, Fan Y, et al. 2021. MIR106A-5p upregulation suppresses autophagy and accelerates malignant phenotype in nasopharyngeal carcinoma. Autophagy. 17:1667–1683.
- Zhu SY, Yao RQ, Li YX, Zhao PY, Ren C, Du XH, Yao YM. 2020. Lysosomal quality control of cell fate: a novel therapeutic target for human diseases. Cell Death Dis. 11:817.
- Zitvogel L, Kepp O, Galluzzi L, Kroemer G. 2012. Inflammasomes in carcinogenesis and anticancer immune responses. Nat Immunol. 13:343–351.
