Abstract
The alarming liability of breast cancer, mainly among younger Saudi women, has been attributed to diverse factors requiring a manageable valuation. Therefore, this study aimed to assess the types of tumors and breast cancer presentation stages in Northern Saudi Arabia. In the present study, retrieved data regarding breast biopsies were received from the Department of Pathology at King Salman Hospital, Hail, Northern Saudi Arabia. Included data referring to breast cancer biopsies patients (including 131 females and two males) diagnosed during the period from November 2019 to November 2020. Out of the 133 patients, breast cancer was diagnosed in 49/133(36.8%) patients included 45/49(91.8%) invasive ductal carcinoma (IDC), 2/49(4.1%) invasive lobular carcinoma (ILC), and 2/49(4.1%) papillary carcinoma. The remaining 84 benign breast lesions included 40/84(47.6%) fibroadenoma, 11/84(13%) ductal hyperplasia, 9/84(10.7%) fibrocystic disease, 8/84(9.5%) adenosis, 4/84(4.8%) chronic mastitis, 3/84(3.6%) breast abscess & intraductal papilloma, 2/84(2.4%) duct ectasia & phylloides tumor, and 1/84(1.2%) lactating adenoma & lipoma. Breast cancer is highly prevalent in Northern Saudi Arabia with predominantly invasive ductal carcinoma. There is an increase in the incidence of younger patients with advanced stages of initial presentation. Fibroadenoma is the commonest benign breast lesion, followed by fibrocystic diseases in Northern Saudi Arabia.
Introduction
Breast cancer is a leading cause of mortality in Saudi Arabia, and its incidence is rising in recent years, mimicking that occurring in western countries. The alarming burden of breast cancer, particularly among younger Saudi women, has been attributed to myriad factors that need close assessment (Almutlaq et al. Citation2017; Babiker et al. Citation2020). The low level of population awareness and absence of sustainable screening programs represent the significant drawbacks and obstacles in the control of breast cancer in Saudi Arabia (Elasbali et al. Citation2019; Alqahtani et al. Citation2020).
As breast cancer, education, and early detection campaigns can improve the ultimate breast cancer patient outcomes, implementing sustainable breast-self-examination, mammography, and clinical breast examination can reduce the disease's burden (Albeshan et al. Citation2020; Asiri et al. Citation2020).
High proportions of breast cancer cases with advanced stages of the disease with an increasing incidence among relatively younger patients are continuously being reported from Saudi Arabia (Ahmed et al. Citation2017). These may be attributed to the low utilization of open breast cancer screening services among wide-ranging Saudi women (Al-Hanawi et al. Citation2020).
Nevertheless, there is still a lack of data regarding breast cancer's pathogenesis in Saudi Arabia, in terms of histopathological patterns and other diagnostic measures concerning the patient's age. Thus, our objective in this investigation was to assess the types of tumors and breast cancer presentation stages in Northern Saudi Arabia.
Materials and methods
In the present study, retrieved data regarding breast biopsies were received from the Department of Pathology at King Salman Hospital, Hail, Northern Saudi Arabia. Included data referring to breast cancer biopsies patients (including 131 females and two males) diagnosed during the period from November 2019 to November 2020. The diagnosis of breast lesions was confirmed by conventional histopathology. The re-evaluation of the histopathological diagnosis of the tissue samples was done to verify the prior diagnosis and categorize the lesion's classification into benign and malignant types.
Statistical analysis
Retrieved information collections were entered through a computer software; Statistical Package for Social Sciences (SPSS version 16; SPSS Inc, Chicago, IL). Chi squire test was employed for statistical significance (P < 0.05 was considered significant).
Ethical consent
The protocol of this study was established agreeing with the 2013 Declaration of Helsinki and this study was approved by the ethics committee of the College of Medicine, University of Hail, Saudi Arabia. Ethical Committee approval number: EC00069.
Results
Within the period from October 2019 to October 2020, 133 patients attended our hospital with breast lesions. The patients aged 16–99 with a mean age ± Standard Deviation (Std) of 40.23 ± 15.35 years. The mean age for patients with breast cancer was 48.3 ± 13.3 years.
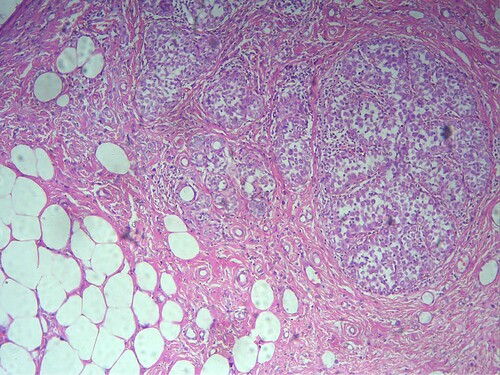
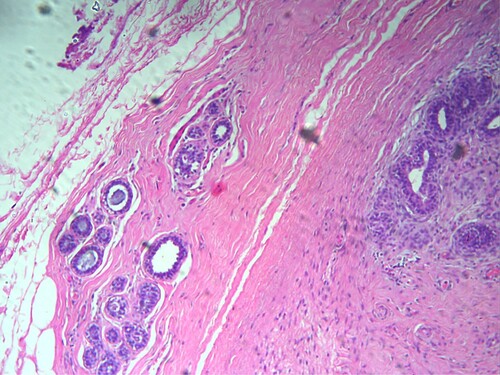
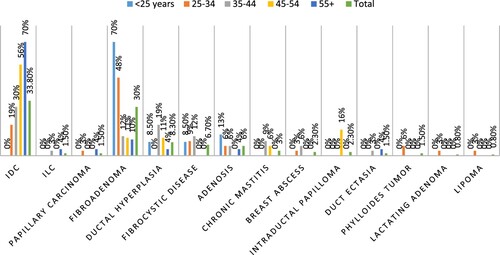
Out of the 133 patients, breast cancer was diagnosed in 49/133(36.8%) patients included 45/49(91.8%) invasive ductal carcinoma (IDC), 2/49(4.1%) invasive lobular carcinoma (ILC), and 2/49(4.1%) papillary carcinoma (see Figure ). The remaining 84 benign breast lesions included 40/84(47.6%) fibroadenoma, (see Figure )11/84(13%) ductal hyperplasia, 9/84(10.7%) fibrocystic disease, 8/84(9.5%) adenosis, 4/84(4.8%) chronic mastitis, 3/84(3.6%) breast abscess & intraductal papilloma, 2/84(2.4%) duct ectasia & phylloides tumor, and 1/84(1.2%) lactating adenoma & lipoma, as indicated in Table , Figure . IDC cases were found in 6/32(19%) of the age group 25–34, and 10/33(%30.3) of the age group 35–44. The majority of fibroadenoma cases were seen in age <34 years 31/55(56.4%). Ductal hyperplasia, fibrocystic disease, and chronic mastitis were found predominantly in the age group 35–44 years, constituting 6/33(18%), 4/33(12%), and 3/33(9%), respectively. We saw all Intraductal Papilloma cases in the age group of 45–54 years 3/18(17%). All Phylloides tumor cases were seen in the age group of 25–34 years 2/32(6%), as shown in Table , Figure .
Table 1. Distribution of the patients by diagnosis and age.
About 28/45(62.2%) of the IDC presented on the left side. All of the papillary carcinoma presented on the left side of 2/2(100%). Around 16/49(32.7%) presented with lesion size >2 cm. For quadrant diagnosis, upper outer, retro-areolar, lower outer, lower inner, and upper inner were identified in 16(46%), 8(23%), 4(11%), 3(9%), and 4(11%) of IDC, in that order. According to the Bi-Rad score, 21/33(63.7%) were scored 4, and 11/33(33.3%) were scored 5, and only 1/33(3%) score 1, as indicated in Table .
Table 2. Distribution of breast cancer by clinical presentation measures.
Positive lymph nodes were identified in 24/49(50%) cases, including 22/45(49%) IDC and 2/2(100%) ILC. The majority of patients were identified in grade 2, representing 41/47(87%). The most frequent subtypes were luminal A, followed by luminal B, Triple-negative, and Her2-enriched, constituting 14/32(44%), 7/32(22%), 6/32(19%), and 5/32(15%), correspondingly, as indicated in Table .
Table 3. Distribution of breast cancer by histopathological measures.
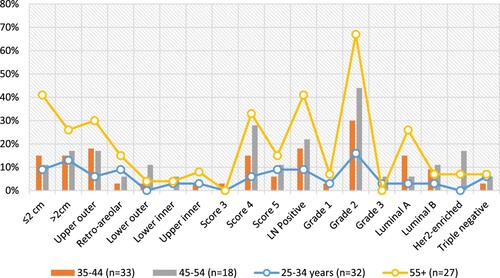
Table , Figure summarized the distribution of the patients’ diagnostic parameters by age. About 19 patients presented with lesion size >2 cm, 7/19(37%), 5/19(26%), and 4/19(21%) were in the age ranges 55+, 35-44, and 25–34 years, respectively. For quadrant, the upper outer is more frequent in 55+ years 8/19(42%), and 35–44 years 6/19(32%). Retro-areolar were seen in 55+ years 4/9(44.4%), and 25–34 years 3/9(33.3%).
Table 4. Distribution of the patients’ diagnostic parameters by age.
For the Bi-Rad score, the most frequent score was 3, seen in 55+ years 9/21(43%), and 35–54 years 10/21(47.6%). Positive lymph nodes were frequently seen in 55+ years 11/24(46%), and 35–44 years 6/24(25%). Provisional Grade, Grade 2, the most common frequently seen in 55+ years 18/41(44%), 35–44 years 10/41(24%), and 45–54 years 8/41(20%). For subtypes, Luminal A was frequently seen in 55+ years 7/14(50%), and 35–44 years 5/14(36%). Luminal B was seen in 35–44 years 3/7(43%). Her2-enriched was seen in 45–54 years 3/5(60%). Triple-negative (TN) is frequently seen in 55+ years & 25–34 years, representing 2/6(33.3%). About 4/6(67%) TN were associated with BI-RAD 4.
Discussion
In this study, we reviewed one year series of patients who were admitted to our hospital with breast tumors. The overall incidence rate of all breast cancers among breast tumors was 36.8% (35.3% in females and 1.5% in males), including 33.8% for invasive ductal carcinoma, 1.5% for invasive lobular, and 1.5% for papillary carcinoma. These findings showed higher incidence rates than the early reported incidence rate of 27.4% in females (Saggu et al. Citation2015). During the period from 1990 to 2016, breast cancer incidence increased 10-fold (Althubiti and Nour Eldein Citation2018). However, the overall reported incidence of breast cancer in Saudi Arabia (in 2018) was 14.8%, with a mortality rate of 8.5% (Saudi Arabia. Most common cancer cases Citation2018). The incidence rates of IDC, ILC, and papillary carcinoma of all female breast cancer were 91.4%, 4.3%, and 4.3%, respectively. Relatively slight lower incidence rates were previously reported in Saudi Arabia, including 81.8% for ductal carcinoma and 3.4% for lobular carcinoma (Asiri et al. Citation2020).
In the present study, the mean age for patients with breast cancer was 48.3 ± 13.3 years. This is relatively lower than all of the previous reports from Saudi Arabia (Alabdulkarim et al. Citation2018; Asiri et al. Citation2020), which indicates the increased incidence of breast cancer among somewhat younger females.
Most patients attended with left side lesions 63.3% in the present study, and upper outer quadrant 38.8%. It was reported that breast cancer is more likely to occur on the left side, except for specific histopathological patterns (Cheng et al. Citation2018). Radiotherapy for the left side breast was reported to influence the heart and increase fatality (Hashiguchi et al. Citation2020). The predominant of the upper outer quadrant supports most of the studies in this context (Burrah et al. Citation2020).
In the current study, the Bi-RAD score4 was the most frequent, 63.6%, followed by Score5, constituting 33.3% of the cases. BI-RAD scores 4& 5 are potentially valuable for the prediction of breast cancer (Luo et al. Citation2019). However, it was reported that patients with BI-RAD 4 are more likely to have triple-negative carcinoma than BI-RADS 5 (Oktay et al. Citation2014), which was consistent with our findings in the present study (67%).
Approximately 49% of the patients in this series were identified with a positive lymph node. Positive lymph nodes, which indicate breast cancer metastasis and late diagnosis, usually affect the overall patient’s management in terms of staging, treatment, and subsequent survival (Marino et al. Citation2020). The high proportions of patients presenting positive lymph nodes designate the lack of early detection programs resulting in patients presenting with advanced disease stages. Moreover, 87.2% of the patients in this study showed stage II breast cancer, and 4.3% with stage III. Better survival is usually associated with a lower stage of presentation, which reverses our findings, indicating later detection (Yang et al. Citation2019) inconsistent with Arabian countries’ reports, including Saudi Arabia (Al-Thoubaity Citation2020; Tanner and Cheung Citation2020).
In the present study, the most common breast cancer subtype was Luminal A (43.8%), followed by Luminal B (21.9%), Triple-negative (18.8%), and Her2 (15.6%). A recent study from Saudi Arabia with relatively few varying proportions included 58.5% for Luminal A, 14% for Luminal B, 16% for Triple-negative, and 11.4% for Her2 (Al-Thoubaity Citation2020).
On the other hand, several benign breast lesions were identified in the present series of patients with the most frequent fibroadenoma (30%&47.6%) of (all breast lesions & benign breast lesions) followed by Ductal hyperplasia (8.3%&13%) and fibrocystic disease (6.8%&10.7%), in that order. It was reported that fibroadenoma represents 44–94% of the breast biopsied lesions and 68% of all breast masses (Lee and Soltanian Citation2015). A study from Saudi Arabia reported incidence rates of 44.3% for fibroadenoma and 23.4% for fibrocystic disease (Albasri Citation2014). Another study from Northern Saudi Arabia has reported a prevalence of 64.6% for fibroadenoma (Ahmed et al. Citation2017). However, diverse epidemiological values have been reported from Saudi Arabia with different benign and malignant lesions (Bosaeed et al. Citation2018).
Nevertheless, several modifiable risk factors may play roles in the etiology of breast cancer in Saudi Arabia. In recent years there were increasing incidence rates of obesity and type II diabetes mellitus and thyroid disease in Saudi Arabia (Ahmed et al. Citation2014; Alzahrani et al. Citation2017; Al-Zahrani et al. Citation2019), due to fast changing in the lifestyle. Other factors such as BRCA1 and BRCA2 gene mutations are still unknown. However, there is ongoing Saudi human genome project, which is including the study of several breast cancer related genes including BRCA genes.
Conclusion
Breast cancer is highly prevalent in Northern Saudi Arabia with predominantly invasive ductal carcinoma. There is an increase in the incidence of younger patients with advanced stages of initial presentation. Fibroadenoma is the most joint benign breast lesion, followed by fibrocystic diseases in Northern Saudi Arabia.
Acknowledgements
The authors would like to thank people at the Department of Pathology, King Salama Hospital Ha'il, for their assistance in sample collection.
Authors contribution
HGA: Conception, administration, analysis, drafting, and approval of the final version; KKA: Conception, design, data acquisition, practical part, approval of the final version; HMA: Conception, analysis, drafting, practical part, approval of the final version; ABE: Conception, design, data acquisition, approval of the final version; AMAA: Conception, analysis, drafting, and approval of the final version; AOH: Conception, analysis, drafting, and approval of the final version; IAG: Conception, analysis, drafting, and approval of the final version; AME: Consultation, analysis, drafting, and approval of the final version.; HS: Conception, analysis, drafting, and approval of the final version.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author, [HGA]. The data are not publicly available due to [restrictions e.g. their containing information that could compromise the privacy of research participants].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ahmed HG, Al-Shammeri KJ, Alrashidi AG, et al. 2017. Histopathologic patterns of breast lesions in Northern Saudi Arabia. Int J Med Res Health Sci. 6(10):54–64.
- Ahmed HG, Ginawi IA, Elasbali AM, Ashankyty IM, Al-Hazimi AM. 2014. Prevalence of obesity in Hail region, KSA: in a comprehensive survey. J Obes. 2014:1–5. doi:10.1155/2014/961861.
- Alabdulkarim B, Hassanain M, Bokhari A, AlSaif A, Alkarji H. 2018. Age distribution and outcomes in patients undergoing breast cancer resection in Saudi Arabia. A single-institute study. Saudi Med J. 39(5):464–469. doi:10.15537/smj.2018.5.21993.
- Albasri AM. 2014. Profile of benign breast diseases in western Saudi Arabia. An 8-year histopathological review of 603 cases. Saudi Med J. 35(12):1517–1520.
- Albeshan SM, Hossain SZ, Mackey MG, Brennan PC. 2020. Can breast self-examination and clinical breast examination along with increasing breast awareness facilitate earlier detection of breast cancer in populations with advanced stages at diagnosis? Clin Breast Cancer. 20(3):194–200. doi:10.1016/j.clbc.2020.02.001.
- Al-Hanawi MK, Hashmi R, Almubark S, Qattan AMN, Pulok MH. 2020. Socioeconomic inequalities in uptake of breast cancer screening among Saudi women: a cross-sectional analysis of a national survey. Int J Environ Res Public Health. 17(6):2056. doi:10.3390/ijerph17062056.
- Almutlaq BA, Almuazzi RF, Almuhayfir AA, Alfouzan AM, Alshammari BT, AlAnzi HS, Ahmed HG. 2017. Breast cancer in Saudi Arabia and its possible risk factors. J Cancer Policy. 12:83–89.
- Alqahtani WS, Almufareh NA, Domiaty DM, Albasher G, Alduwish MA, Alkhalaf H, Almuzzaini B, Al-Marshidy SS, Alfraihi R, Elasbali AM, et al. 2020. Epidemiology of cancer in Saudi Arabia thru 2010–2019: a systematic review with constrained meta-analysis. AIMS Public Health. 7(3):679–696. doi:10.3934/publichealth.2020053.
- Al-Thoubaity FK. 2020. Molecular classification of breast cancer: a retrospective cohort study. Ann Med Surg (Lond). 49:44–48. doi:10.1016/j.amsu.2019.11.021.
- Althubiti MA, Nour Eldein MM. 2018. Trends in the incidence and mortality of cancer in Saudi Arabia. Saudi Med J. 39(12):1259–1262. doi:10.15537/smj.2018.12.23348.
- Alzahrani AS, Alomar H, Alzahrani N. 2017. Thyroid cancer in Saudi Arabia: A histopathological and outcome study. Int J Endocrinol. 2017:1–7. doi:10.1155/2017/8423147.
- Al-Zahrani JM, Aldiab A, Aldossari KK, Al-Ghamdi S, Batais MA, Javad S, Nooruddin S, Zahid N, Razzak HA, El-Metwally A. 2019. Prevalence of prediabetes, diabetes and its predictors among females in Alkharj, Saudi Arabia: a cross-sectional study. Ann Glob Health. 85(1):109. doi:10.5334/aogh.2467.
- Asiri S, Asiri A, Ulahannan S, Alanazi M, Humran A, Hummadi A. 2020. Incidence rates of breast cancer by age and tumor characteristics among Saudi women: recent trends. Cureus. 12(1):e6664. doi:10.7759/cureus.6664.
- Babiker S, Nasir O, Alotaibi SH, Marzogi A, Bogari M, Alghamdi T. 2020. Prospective breast cancer risk factors prediction in Saudi women. Saudi J Biol Sci. 27(6):1624–1631. doi:10.1016/j.sjbs.2020.02.012.
- Bosaeed KM, Talha KA, Asadullah M, Mitkis MA, Al-Ghamdi RY, Al-Gthami AS, Al-Thubaiti AK, Alshahrani SM. 2018. Cross-sectional descriptive study on the epidemiology of operated female breast tumor cases in a tertiary hospital of Saudi Arabia. Saudi Surg J. 6:71–74.
- Burrah R, James K, Lund J, Vinayagam R. 2020. Breast conservation surgery by round block mammoplasty. Eur J Surg Oncol. 46(2):240–244. doi:10.1016/j.ejso.2019.10.034.
- Cheng SA, Liang LZ, Liang QL, Huang ZY, Peng XX, Hong XC, Luo XB, Yuan GL, Zhang HJ, Jiang L. 2018. Breast cancer laterality and molecular subtype likely share a common risk factor. Cancer Manag Res. 10:6549–6554. doi:10.2147/CMAR.S182254.
- Elasbali AM, Alonzi Z, Mohammed EM, et al. 2019. Insight of Saudi women towards breast self-examination in Qurayyat, Northern Saudi Arabia. Int J Med Res Health Sci. 8(10):111–119.
- Hashiguchi N, Schenker N, Rottner L, Reißmann B, Rillig A, Maurer T, Lemes C, Kuck KH, Ouyang F, Mathew S. 2020. Absence of detectable effect of radiotherapy and chemotherapy for breast cancer on the presence of low voltage areas in patients receiving left atrial catheter ablation. Acta Cardiol. 1–8. doi:10.1080/00015385.2020.1812892.
- Lee M, Soltanian HT. 2015. Breast fibroadenomas in adolescents: current perspectives. Adolesc Health Med Ther. 6:159–163. Published 2015 Sep 2. doi:10.2147/AHMT.S55833.
- Luo W, Huang Q, Huang X, Hu H-T, Zeng F-Q, Wang W, et al. 2019. Predicting breast cancer in breast imaging reporting and data system (BI-RADS) ultrasound category 4 or 5 lesions: a nomogram combining radiomics and BI-RADS. Sci Rep. 9:11921. doi:10.1038/s41598-019-48488-4.
- Marino MA, Avendano D, Zapata P, Riedl CC, Pinker K. 2020. Lymph node imaging in patients with primary breast cancer: concurrent diagnostic tools. Oncologist. 25(2):e231–e242. doi:10.1634/theoncologist.2019-0427.
- Oktay M, Oktay NA, Besir FH, et al. 2014. Relation between radiographic BI-RADS scores and triple negativity in patients with ductal carcinomas. Int J Clin Exp Med. 7(8):2334–2338.
- Saggu S, Rehman H, Abbas ZK, Ansari AA. 2015. Recent incidence and descriptive epidemiological survey of breast cancer in Saudi Arabia. Saudi Med J. 36(10):1176–1180. doi:10.15537/smj.2015.10.12268.
- Saudi Arabia. Most common cancer cases. 2018. Cancer country profile 2020. [accessed 2020 Dec 1]. https://www.who.int/cancer/country-profiles/SAU_2020.pdf.
- Tanner LTA, Cheung KL. 2020. Correlation between breast cancer and lifestyle within the Gulf Cooperation Council countries: a systematic review. World J Clin Oncol. 12(4):217–242. doi:10.5306/wjco.v11.i4.217.
- Yang K, Msami K, Calixte R, Mwaiselage J, Dorn J, Soliman AS. 2019. Educational opportunities for down-staging breast cancer in low-income countries: an example from Tanzania. J Cancer Educ. 34(6):1225–1230. doi:10.1007/s13187-019-01587-2.