?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Maize Lethal Necrosis (MLN) is a disease of maize reported in Kenya in 2012 that results in yield losses of up to 100%. The epidemiology of MLN is complex as the disease is caused by the synergistic interaction of 2 viruses (Maize chlorotic mottle virus (MCMV) and a potyvirus). In addition, multiple reservoirs and transmission pathways exist for the spread of MLN. The current study was conducted to understand farmers’ maize production practices, their understanding of MLN, and the status of MLN in Kenya. Therefore, a survey of 406 randomly selected farmers was conducted in Bomet, Narok, Kirinyaga, Embu, and Nakuru. To confirm the presence of MLN, maize leaf samples were collected from 18 fields and tested for MCMV and SCMV by molecular techniques. MLN Symptoms observed included chlorotic mottle on leaves, necrosis, and premature plant death. MCMV and SCMV were detected in all the maize growing regions at varying levels of incidence, and severity. Sequence analysis of the partial coat protein genes of randomly selected positive samples of the two viruses showed little variability within the studied isolates and those retrieved from the GenBank. The results indicated that MLN is still prevalent in Kenya with farmers’ planting susceptible varieties.
Introduction
Maize (Zea mays L.) is one of the most important cereal crops in Africa, covering 40 million hectares which are mainly in smallholder settings and producing about 81 million tonnes (FAOSTAT Citation2019). In Kenya, maize is not only a significant contributor to food security and nutrition, it is also a source of employment and income to millions of subsistence farmers. Currently, maize is cultivated on 2.196 million hectares of land in the country, engaging more than 3 million smallholder farmers and with an annual production of 3.897 million tonnes (FAOSTAT Citation2019).
However, maize production in Kenya is around 1.77 t/ha (FAOSTAT Citation2019), this is far below the achievable potential of 6 t/ha when maize is cultivated under good agronomic and management conditions, use of the right quality of fertilizers and use of improved maize hybrids adaptable to the agro-ecological zones (Odendo et al. Citation2001).
Maize Lethal Necrosis (MLN), caused by the synergistic interaction between Maize chlorotic mottle virus (MCMV) and Sugarcane Mosaic Virus (SCMV) is one of the major biotic constraints that severely affects maize production in Kenya (Miano Citation2014). Maize yield losses to MLN have been reported to range from 30%-100% depending on the variety, stage of disease infection and prevailing environmental conditions (Mahuku et al. Citation2015). MLN infected plants (Figure ) show a wide range of symptoms which include chlorotic mottle on the leaves, mild to severe mottling, dwarfing, premature aging of the plants, necrosis developing from leaf margins to midrib, necrosis of young leaves in the whorl leading to a ‘dead heart’ symptom and drying up of whole plant (Miano Citation2014).
Figure 1. Maize plants infected with MLN, showing the various symptoms observed. Photo taken by Faith Njeru at farmers’ field in Kenya.

MLN is transmitted from one field or plant to another via vectors where MCMV has been shown to be transmitted by chrysomelid beetles (Nault et al. Citation1978) and thrips (Cabanas et al. Citation2013) while aphids have been reported to spread SCMV (Louie Citation1980). MLN causing viruses can also be spread between cropping cycles by farmers planting contaminated maize seeds (Jensen et al. Citation1991), or through infected maize debris (Kinyungu et al. Citation2019). Continuous maize production has been associated with MLN outbreaks (Redinbaugh and Stewart Citation2018). Crop rotation has been reported to significantly reduce the incidence of MCMV, contributing to higher maize yields (Hutchens Citation1978). Results from epidemiological studies on MLN suggest that farmer’s maize production practices have a significant effect on the incidence of MLN.
To develop MLN management practices that are effective, efficient and easily adaptable to the small-scale farmer, there is a need to understand farmers’ knowledge in relation to the different aspects of the disease. Understanding the role of knowledge in farmers’ practices is also an important starting point for developing a management strategy that fits the context of the smallholder farmer maize production practices.
In Kenya, studies have been done to understand the viruses associated with MLN (Wamaitha et al. Citation2018) and their geographical distribution (Mwatuni et al. Citation2020). However, no studies have been conducted to get insight into farmers’ knowledge and their role in MLN control. Hence, the present study focuses on understanding farmers’ know-how on scientific knowledge and recommended management practices on MLN and also on their local knowledge of the disease management. The study will also look at the limiting factors for the adoption of recommended practices and farmers’ perception on the use of mobile phones for disease monitoring and information sharing. The findings of the study are relevant to the design of MLN disease management practices in the context of smallholder farmer in Kenya.
Materials and methods
Study area
The study was conducted covering 3 agro-ecological zones: Highland tropics (HT), Moist transitional (MT), and Moist Mid attitude (MM). Five counties were purposively selected from these zones targeting those with a high incidence of MLN (40–70%) from previous survey (Mwatuni et al. Citation2020). The selection of the counties also considered that the areas have high maize production. Sampled counties were Bomet,Nakuru,Kirinyaga, Embu, and Narok.
Selection of respondents
The study population comprised maize farmers in the target counties, from which a representative sample was obtained. The sample size was obtained using Cochran’s sample size formula, the desired confidence level was set at 95% and a desired precision of 5%. The desired proportion of attributes was set at 50% (maximum variability). This gave a sample of 385 respondents from the population, which was adjusted to 482 to mitigate for 20% non-response rate. The probability proportion to size method based on the total number of maize farmers in the counties was used to determine the distribution of the sample size across the counties. Within each target sub-county, the respondents were randomly selected based on a sampling frame developed with the help of the Agricultural extension officers based in the region.
Sample size calculation:
n0 is the sample size, Z2 is the abscissa of the normal curve that cuts off an area a at the tails (1 – a equals the desired confidence level, e.g. 95%) 1, e is the desired level of precision, p is the estimated proportion of an attribute that is present in the population, and q is 1-p. Final sample size = effective sample size/ (1- non-response rate anticipated).
Data collection
In the present study, a semi-structured questionnaire was developed and pre-tested in Mutithi sub-county, Kirinyaga to improve the questions set as per the study objectives. The questions set aimed at understanding farmers’ knowledge on MLN, their perceptions and the role of knowledge in MLN control practices. The questions asked included but not limited to, personal and household characteristics of the farmer; maize production practices(varieties grown, yield obtained, external inputs used, key diseases and management practices, and chemical use); farmer knowledge on MLN and the methods practised to control the disease. In addition, farmers were questioned on the type of information they accessed regarding maize farming and the media platform they mostly used to access agricultural information.
The questionnaire was administered through face to face interviews conducted in the local language of the community during the period from March–May 2021. The respondents targeted were either the household head, spouse or the member responsible for making farming decisions. Informed consent was sought from the respondents and the data handled in accordance with the General Data Protection Regulation (GDPR).
Farmers were also provided with coloured photographs (taken at MLN CIMMYT Naivasha screening facility), at different stages of MLN severity to determine their ability to diagnose the disease.
MLN test sample collection
Maize leaf samples (84) were randomly sampled from the farmers’ fieldswhich were close either to the main or rural roads in Kirinyaga, Bomet and Narok counties. The collected maize leaf samples were preserved in silica gel and transported to Kenya Agricultural and Livestock Research Organization (KALRO) Njoro for molecular analysis. In each selected field, maize plants were selected following the staggered ‘X’ pattern and evaluated for disease severity. MLN severity was scored on a rating scale of 1–9 where 1 clean plant with no symptoms and 9 severely affected plant (https://mln.cimmyt.org/mln-scoring/mln-hybrid-scoring-scale/).
Data analysis
The survey questionnaire collected data on farmers’ maize production systems and their perceptions on MLN. The farmers’ response to the questions was recorded in the Kobo Toolbox platform which enables the data to be received in real time, where data can be easily managed and checked for any mistakes. On completion of the field survey, the final datasets were downloaded from the server as Excel (XLS) files and used for further analysis. The survey data were analysed using descriptive statistics (percentage, frequency and mean) to present findings in summaries and tables after the data were encoded. Pearson Chi-square test was used to determine whether there was significant difference in maize production practices and farmers’ knowledge of MLN management among farmers of different categories, and study counties. Disease incidence and disease prevalence were determined by the percentage of the plants showing MLN symptoms in individual farms and the percentage of farms in a county with MLN symptoms, respectively.
Total nucleic acid extraction
Total nucleic acid (deoxyribonucleic acid and ribonucleic acid) were extracted from the 84 maize leaf samples collected from the farmers’ field in Kirinyaga, Narok and Bomet Counties using a modified cetyltrimetyl-amoniumbromide (CTAB) protocol where 0.4 g of maize leaf tissue was ground in 2 ml of extraction buffer using a mortar and pestle (Semagn Citation2014). The RNA pellet was suspended in 50 µL of deionized water.
A Nano-Drop spectrophotometer was used to measure the RNA concentration at maximum absorbance of 260 nm, and the purity was assessed by measuring the 260/280 and 260/230 absorbance ratios.
cDNA synthesis and RT-PCR
cDNA was prepared from 1 µg of RNA following the NEB #M0253 first Strand cDNA Synthesis Standard Protocol (New England BioLabs, Ipswich, USA) as per the instruction manual. Subsequently, the synthesized cDNA was used as a template for polymerase chain reaction (PCR) using MCMV and SCMV-specific primers. MCMV-specific primers used were MCMV F 5′- AACATTCACAGCAGACACC -3′ and MCMV R 5′- GATAGCCACAATGAATCGTCC-3′ whereas SCMV specific primers were SCMV F 5′-TCTACTGAGCGATACATGCC-3′ and SCMV R 5′-CGTGTGTTTGAACCACGAAC-3′ to produce an amplicon of 259 and 169 bp in length, respectively.
The PCR conditions for MCMV reaction were 94°C for 2 min, followed by 35 cycles of 94°C for 30 s, 50°C for 30 s, and 72°C for 1 min, with a final extension at 72°C for 7 min. The PCR conditions for SCMV reaction were similar to those of MCMV but with the annealing temperature being set at 60°C. The PCR product was analysed on 2% agarose gel in TBE buffer (70 V, 75 min) and visualized under UV light.
Sanger sequencing
Ten samples were selected for Sanger sequencing based on the geographical location and the quality of cDNA. The amplified PCR product was sent to Inqababiotec for sequencing. Both forward and reverse sequencing was performed on an automated DNA sequencer (ABI 3500XL Genetic Analyzer) using the specific MCMV and SCMV F and R primers. The sequences were viewed in FinchTV and ambiguous and contaminated sequences were removed. Both forward and reverse sequences were merged and aligned in Bioeditto get the consensus sequence for use in further analysis.
The partial coat protein gene sequences of 6 samples in this study were registered in GenBank and the accession numbers for MCMV (OL461943, OL461944, and OL461945); SCMV (OL461946, OL461947, and OL461948) were provided. The 6 isolates were analysed by BLASTN to determine the sequence identity and similarity. Phylogenetic trees were constructed for the coat protein genes isolates determined in this study and those retrieved from GeneBank. The phylogenetic tree was constructed in MegaX where the sequences were aligned in Clustal Omega and saved in a Fastaformat, the best model for phylogenetic tree construction was identified as Jukes-Cantor model for both MCMV and SCMV data. Bootsrap analysis with 1000 replicates was performed to evaluate the significance of the interior branches.
Results and discussion
Socio-demographic profile of the interviewed farmers
Of the 406 farmers who were interviewed in the present study, there was almost equal representation by both males (56.4%) and females (43.6%) (Table ). Statistically, there was a significant association between the gender of the farmer and the county where they were from, with Embu (23.6%) and Kirinyaga (36.7%) counties reporting a high number of females (Pearson Chi-Square = 49.869, p = 3.845e-10). This is in contrast to most survey studies done in Africa which report a higher representation of the males than the females citing the engagement of females in domestic activities which limit their mobility and crucial opportunities for interaction with other stakeholders(Mudde et al. Citation2017). Therefore, efforts to promote MLN disease management strategies should be structured in such a way that they are easily accessible to both males and females.
Table 1. Profile of sample farmers.
The average age was 44.1 years, with many farmers (44.4%) aged between 36 and 45 years. This age represents a young population of maize farmers, presenting great prospect of introducing new MLN management practices as young people easily adapt to new ways of doing things. The majority of the respondents (95%) had formal education, either primary school (grade 1–8), secondary school (grade 9–12) or university. Only 5% of the farmers were illiterate. Bometcounty had the highest number of university graduates (17.9%) while Narok had none. Hence it is easier for extension officers to relay relevant MLN control information to farmers. The average household size of the farmers was 5 individuals and 4 people as dependants (mostly school-going children). Therefore, less family labour to work in the farm is available unless the farmer hires workers. Hence MLN control measures devised should be less labour intensive for them to be easily adaptable to the farmer.
Maize production systems
The study indicated that maize production in the study area is small scale, and the average field size was 1.58 acres (0.64 hectares) (Table ). However, there was a statistically significant difference in the means of the land owned with the county F(4, 401) = 63.25, p = 2e-16. Respondents from Narok had on average 3.41 acres under maize cultivation while Embu county had the least area under maize cultivation, 0.42 acres on average. Age of the respondents had no statistically significant effect on the average land owned, however, gender had a significant effect F(1, 404) = 58.99, p = 1.21e-13. This is in contrast with a report by Kansiime et al. (Citation2019) where older farmers owned larger parcels of land. Male farmers owned on average 1.1 more acres of land compared to female farmers. A study done in Zambia also reported male farmers owning larger parcels of land compared to female farmers (Kansiime et al. Citation2019).
Table 2. Maize production practices for 2021.
In the present study, farmers reported maize production at 3.3 t/ha when the season is favourable (timely rains and no biotic stresses). However, the farmers noted that it is a while since these yields were obtained as the agricultural sector has been hit by many biotic and abiotic stresses. Estimated maize production for the 2020/2021 cropping season was 1.48 t/ha. Majority of farmers (77.3%) obtained an average yield of less than 2 t/ha. Respondents from Nakuru county (9.6%), reported an average yield of 4.41 t/ha with these farmers reporting MLN incidence rates of less than 10% in their farms. These farms were the ones located in Molo area at an altitude of 2411.02 m above sea level (GPS data). Previous studies have reported low MLN incidence at higher altitude areas due to unfavourable weather for insect survival (Guadie et al. Citation2019).
Farmers spent approximately Ksh. 6065 on organic fertilizer (47.8 Kgs of fertilizer per acre), for the whole season (Table ). Farmers cited low use of fertilizers to be due to lack of capital and inaccessibility to government subsidised fertilizers. Use of herbicides for weed control in combination with manual weeding was also common among farmers in Kirinyaga (41%). Use of biological and cultural methods for pest control was common in Nakuru (26%). Farmers in Nakuru(altitude of 2411.02 m above sea level, GPS data)reported having minimal pest damage saying when it rains, the pests are washed off. Other biological control methods reported included the use of ash, soil, tobacco, and ariel (detergent). In addition, some farmers reported to being against the use of pesticides especially when growing maize for their own use while others cited lack of money to buy the pesticides. However, some farmers were of the opinion that there has been a change in the pests affecting the maize crop, as the cultural control methods were very effective (eliminating the pests by 90–100%, according to farmers’ observation) 5–10 years ago, but they were noting these methods becoming less effective.
Maize production in the study area was mainly rainfed, with irrigation being practiced in Kirinyaga (60%) and Embu (34%). Most respondents (73.5%) reported to have noticed the impact of climate change on farming reporting less and delayed rains, changes in rainfall patterns making it hard for the farmers to predict planting times. Therefore, with the changes in weather patterns being noticeable, there should be emphasis to educate the farmers on the use of irrigation, and the use of drought tolerant varieties.
The majority respondents (87%) planted improved maize varieties (Table ) with maize varieties planted being different per county. This is supported by the results of the ANOVA test showing statistically significant differences between the maize variety planted and the county F(4,401) = 28.01, p = 2e-16. In Kirinyaga county, DUMA 43 (52%) was the most planted hybrid selected for its drought tolerant and fast maturing traits followed by Pioneer 3253 (31%) selected for its favourable markets quality traits as it is sold as green maize. Other hybrids planted in Kirinyaga county included DK777, Pannar, Sungura, babycorn, DK8031 and DK9089. In Nakuru county and Narok, H6213 is the most planted while in Bomet, DK777 and H614 hybrid varieties in Embu county. These hybrids are selected for their adaptability to the agro-ecological zones. However, only a selected few of the hybrids are planted per county and there is a need to introduce and diversify maize varieties planted per region.
Respondents in Narok reported that the OPV (Sirare) was more tolerant to MLN compared to the hybrid varieties. The average maize production of hybrids in Narok was 2.1 and 1.75 t/ha for the OPV. Therefore, though the OPVs were perceived to be more disease tolerant, their yield was still low. Hence, the OPVs have unexploited genetic diversity for novel traits that can be adopted into breeding programmes.
Though pests and diseases are considered to be a major constraint to maize production, in the present study, most farmers (91%) reported poor market prices as the main drawback to maize farming (Table ). Appropriate policies and regulations should be put in place to make maize farming economically attractive to the farmers. Weevils was reported to be a problem in Nakuru county. Resistance to pests and diseases is considered favourable to the farmers if the trait is combined with other traits such as high yielding, favourable traits to the market which are more favourable to the farmer. Breeders breeding for biotic and abiotic stresses should also take into account farmers’ preferences.
Farmers’ knowledge of MLN and if they have observed it in their farms
During the survey, farmers were shown photos of MLN infected maize plants. The majority of the farmers (80%) could identify MLN, though given different names in the different counties (Table ). The severity of MLN is noted in the present study as 93% of the farmers reported to having observed MLN in their farms. Statistically, there was no difference in the knowledge of MLN and the age or gender of the farmer. However, there was a statistically significant difference in the means of the farmers that observed MLN and the county, F (4,392) = 4.22, p = 0.00231. Fewer farmers in Kirinyaga reported having observed MLN compared to farmers in Bomet and Narok. Farmers (78%) also reported MLN as the most problematic maize disease they have observed. Other diseases observed included common rust, ear rots, leaf blight, smut. Common rust was reported as a major problem by 30% of farmers in Nakuru (data not shown). Farmers also indicated the unpredictability of MLN, with farmers in Bomet noting a reduction in incidence and severity of MLN in 2018 and then a resurgence in 2019.
Table 3. Percent of farmers knowing and having observed MLN in their farms.
Across all the study areas, more than 70% of the respondents reported that MLN is higher during the short rains (sunny season) and off-season plantings (Table ). Similarly, Regassa et al. (Citation2020) reported relatively high MLN incidence during the off-season plantings which support our findings. Environment plays a critical role in disease development and the dry and hot conditions during off season and short rains/sunny season would be a favourable environment for reproduction and movement of vectors to transmit MLN causing viruses. A few of the farmers (6%) reported MLN being high any season that maize is planted. This could be attributed to maybe late plantings in long rains which have been reported to contribute to high MLN incidence or delayed rains (which simulate sunny season in the long rains) (Jumbo et al. Citation2015).
Yellowing was reported by 60% of the farmers as the most common symptom associated with MLN. Few farmers could identify the early onset MLN symptoms (chlorotic mottle on leaves), making it difficult for the farmer to control the disease early before it spreads. Premature plant death and male sterility were not identified by farmers as MLN symptoms. Therefore, yellowing was the prominent MLN symptoms recognized by the farmers who had limited know-how of other symptoms associated with MLN.
About 46.3% of the farmers reported to observe MLN when the maize was at the pre-flowering stage and 36.5% at the vegetative stage. Other prominent stages were post-flowering stage (7.3%), 4–5 leaf stage (5.3%) and flowering stage (2.2%). About 2% of the farmers noted that MLN can affect the maize plant at any growth stage. The results of this study are concurrent with previous studies reporting that MLN can affect maize plants at all growth stages (Beyene et al. Citation2017).
Farmers’ perception on MLN causal pathogens and spreading mechanisms
Concerning the causal agent of MLN, 42.3% of the respondents did not answer this question. However, of those who responded (80.7%) did not know the causal agent of MLN, 8.5% of the farmers mentioned environmental factors and 2% poor seed. However, 13% of the farmers (majority being from Nakuru and Bomet) mentioned viruses as the causal agent of MLN.
Though most of the farmers (84.2%) did not know how MLN is spread from one area to another and from one farm to another, 15% of the respondents identified insects, wind, contaminated seed, infected debris/soil and contaminated farm tools as possible mechanisms of MLN distribution. Statistically, there was a significant association between the farmers who had some idea on how MLN is spread and their education level (Pearson Chi-Square = 190.85, p = < 0.001). In addition, there was a significant association between gender and the respondents who answered the question on MLN spread mechanisms (Pearson Chi-Square = 67.471, p = < 0.001) with more men (70%) responding to the question compared to 30% of the women.
MLN causing pathogens can be spread from one farm to another by insects and wind, therefore the practice of neighbouring farmers could have an effect on an otherwise MLN free farm. The lack of knowledge on MLN spreading mechanism limits how the farmers view the importance of concerted effort in MLN control.
Maize yield losses to MLN
About 31% of the respondents estimated the maize yield loss to MLN at 50–70% (Table ). Statistically, there was a significant association between farmers’ estimation of yield loss to MLN and their location/ county (Pearson Chi-Square = 384.11, p = 2.2e-16). Nakuru was least affected by MLN, with 43% of farmers reporting less than 10% maize yield losses to MLN (Table ). The results of this study show that farmers could appreciate the potential magnitude of yield loss due to MLN where experimental studies have shown yield reductions of up to 70% in highly susceptible hybrids (Uyemoto et al. Citation1980).
Table 4. Perceived yield loss due to MLN in different counties of Kenya.
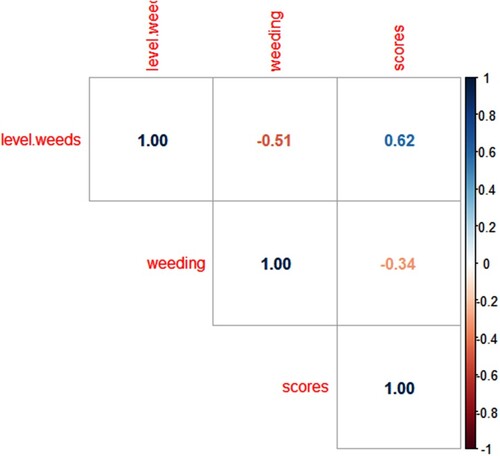
Further analysis of the data showed that there was a significant association between estimated maize yield losses and the cropping system practiced (monocropping, intercropping or both) (Pearson Chi-Square = 97.524, p = <0.01) and whether the farmer practised crop rotation (Pearson Chi-Square = 79.388, p = <0.01). A study by Regassa et al.(Citation2020) reported a significant association between cropping systems, crop rotation and MLN incidence. In addition, level of weeds had a positive correlation with MLN severity scores (Figure ). Findings of this study confirmed the reports of Gudero Mengesha et al. (Citation2019), that MLN severity was high in plots with high weed levels. Pearson correlation between frequency of weeding and MLN scores is −0.34, meaning that the two variables vary in opposite directions. This means that the more you weed, there will be low MLN severity scores, since a higher frequency of weeding reduces the number of weeds on the farm.
Figure 2. Correlation between level of weeds in the farm, number of times of weeding per season and mean MLN scores of randomly selected maize plants in the farm (MLN mean scores of 25 maize plants in each farm. Correlogram plotted in R studio: https://www.R-project.org/. (More intense colours for more extreme correlations)).
MLN control measures
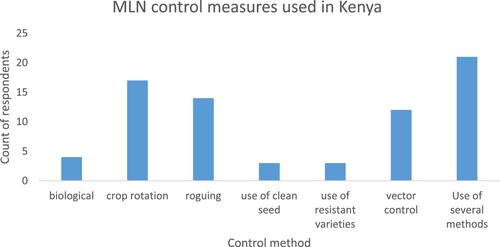
In the present study, farmers’ management of MLN was limited with 74.8% reporting to not controlling the disease. These farmers reported that MLN spreads very fast, making any control attempt not effective. Other reasons given for not controlling MLN included: expensive, not knowing how to control the disease, not knowing MLN is a disease that needed to be controlled, not being economically viable.
The control method that most farmers thought was not economically viable was roguing, the preference being to let the maize crop grow to cut the stalks for the animals or leave them as manure. In addition, some farmers also reported that roguing was not effective as MLN control method. Similarly, Mudde et al.(Citation2017) reported that 40% of the farmers practising roguing to control MLN said the practice was ineffective. When the data were extrapolated by county and gender, there was a statistically significant association between farmers’ management of MLN and their geographical location (Pearson Chi-Square = 384.11, p = 2.2e-16) and gender (Pearson Chi-Square = 11.585, p = 0.00305). A Tukey post-hoc test revealed that more men than women tried to control MLN (p = 0.0006962). Hence there should be more emphasis to reach and educate more women on MLN control methods.
Of the farmers who tried to control MLN, crop rotation and roguing were the most used methods. Generally, most farmers practised crop rotation for 3–6 months (one season interval) which is ineffective for disease management. A few of the farmers used a combination of methods which included: crop rotation, roguing, vector control and use of clean seeds (Figure ). In addition to the use of resistant varieties, decontamination of farm tools was also used though to a smaller scale.
Source of information on MLN management
The majority of the farmers mentioned having received information on MLN management. The main source of information (41.6%) was fellow farmers. Statistically, there was a significant association between the farmers who received information on MLN and the education level (Pearson Chi-Square = 512.12, p > 0.01). Furthermore, 56.4% of the farmers who got access to information had secondary education. Farmers acknowledged receiving information from multiple sources which included: extension workers, field visits, radio, television and agricultural offices. Few farmers (8%) relied on mobile phones as a source of information on MLN. This seems to indicate that though fellow farmers are the main source of information on MLN management, other platforms are also gaining traction.
Farmers’ use of mobile phones and community-based system
Most of the farmers (96.78%) in the study area owned a mobile phone, of whom 78.5% had a smartphone. These results are consistent with previous studies that have reported mobile phone ownership of up to 95.1% among the Kenyan adult population (Krell et al. Citation2021). Most of the farmers (59.2%) reported that using their mobile phones for communicating with family. In addition 24.9% of the farmers used their mobile phones to coordinate farm activities and marketing of the maize crop. Only 9.5% of the farmers used their mobile phones to get information on maize management while 15.9% accessed social media platforms (Facebook, WhatsApp, Twitter). Statistically, there was a significant association between education level and farmers use of mobile phones (Pearson Chi-Square = 278.07, p > 1.434e-14). More farmers (60%) whose education level was above secondary level used mobile phones for coordination of farm activities and getting information on maize management.
When farmers were asked about Mbeguchoice (www.mbeguchoice.com), an online platform developed to assist farmers in buying the best seeds for their agro-ecological zone, none of the farmers knew about it. A study by Wyche and Steinfield (Citation2016) also reported the lack of awareness by farmers on how they can use their mobile phones to gain access to agricultural information, market information.
All of the farmers also reported lack of a community-based system to discuss issues on maize production citing the lack of monetary gain in the sector. However, this is contradictory as maize is an important crop which can be used in several applications including use as for animal feed and in industry (fuel production). The lack of information leads to farmers in the study areas planting maize for their own use (50%) or selling through middlemen (23%) (Data not shown) leading to lack of gain from maize cultivation.
MLN disease severity, incidence and prevalence
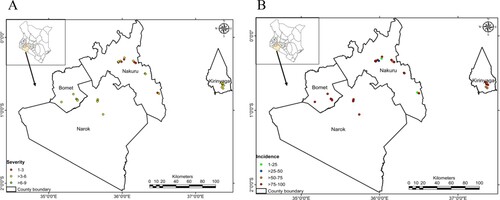
All the fields evaluated in Kirinyaga, Bomet, Nakuru and Narok had plants infected with MLN disease. Narok region had the highest MLN incidence, followed by Bomet, whereas the lowest incidence was noted in Nakuru (Figure ). BometCounty registered the highest disease symptom severity 4.928 while Nakuru registered the lowest 3.93 on the 1−9 MLN disease symptom severity scale.
The samples collected from the farmers field were at different growth stages including 4–5 leaf stage, vegetative, pre-flowering and flowering stage. Statistically, there was no significant difference in MLN mean scores and the maize growth stage (f (3) = 2.044, p = 0.154). In addition, maize crop existing at different growth stages in the field simultaneously due to farmers’ practise of planting maize at different times facilitates easy transmission of MLN from older to younger plants by the insect vectors and leads to continuous MLN disease infection (Table ).
Table 5. MLN severity analysis, incidence and prevalence. The difference in the mean level of severity among the different counties was statistically not significant at α = 0.05 (F-Value = 0.863, P-value = 0.4446).
Results of RT-PCR
MCMV and SCMV were detected by RT-PCR in 69 and 73 pooled leaf samples respectively out of the 84 maize leaf samples. Double infection of MCMV and SCMV was reported in 60 maize leaf samples. Interestingly in Kirinyaga, 3 asymptomatic maize leaf samples collected tested positive for MCMV with 1 of the samples testing positive for both MCMV and SCMV (Figure and Table ).
Figure 5. Results of MCMV and SCMV on a 2% agarose gel: total RNA was extracted from the samples collected from farmers’ field, cDNA was then prepared followed by RT-PCR for MCMV and SCMV using specific primers for these viruses.
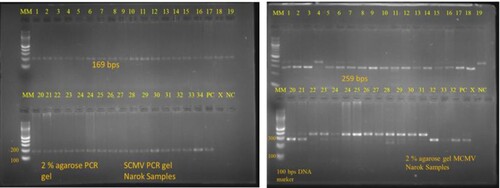
Table 6. MLN viruses testing results using RT-PCR for MCMV and SCMV.
Sequence comparisons of partial coat protein region of SCMV
Blast analysis of the aligned SCMV sample sequences (Kirinyaga, Bomet and Narok) identified the organism as SCMV. The SCMV isolates used in this study shared 99-100% nucleotide sequence identity among themselves. Analysis of the sample sequences in BLASTn, using the default parameters revealed nucleotide identity of 96.45% to 100% with SCMV sequences deposited in the GenBank. A high similarity was noted between East African isolates (Tanzania, Rwanda and Ethiopia) and previously deposited Kenyan isolates.
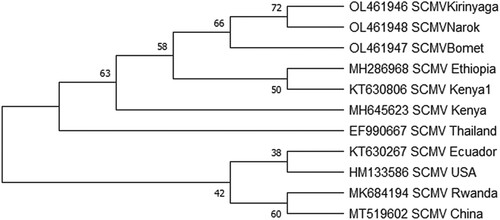
To further understand the genetic relationships among the global SCMV isolates, the 3 SCMV sequences from this study and genomes from GenBank representing different parts of the world were used on a phylogenetic analysis. Phylogenetic analysis of the partial gene sequences showed that they all belong to a single monophyletic clade of SCMV (Figure ). Previous studies have also reported that SCMV sequences are phylogenetically diverse and tend to cluster together by geographical origin (Mahuku et al. Citation2015).
Figure 6. Phylogenetic relationships among SCMV coat protein genes of isolates determined in this study (SCMVKirinyaga, SCMVBomet, SCMVNarok) and those retrieved from GenBank (accession number and geographic region is given). The evolutionary history was inferred by using the Maximum Likelihood method and Jukes-Cantor model. Evolutionary analyses were conducted in MEGA X.
Sequence comparisons of the partial coat protein region of MCMV
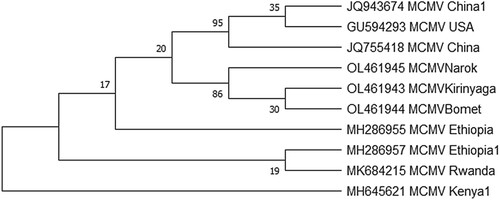
The 3 MCMV isolates used in the analysis shared 98.84% to 100% nucleotide identity with other sequences in the GenBank. The highest identity was observed with other East African isolates. The MCMV isolates used in the study were aligned in Clustal Omega among themselves using the default set parameters, the nucleotide identity identified by Sequence Manipulation Suite (Ident and Sim) was 100%. The results from the present study support results from previous studies which report low genetic variability of MCMVv(Guadie et al. Citation2019) (Figure ).
Figure 7. Phylogenetic tree reconstructed based on coat protein genes of isolates determined in these study (OL461945, OL461943, OL461944) and those retrieved from the GenBank: The evolutionary history was inferred using the Maximum Likelihood method based on the Jukes-Cantor model at 1000 bootstraps. GenBank accession numbers and country of origin are indicated.
Conclusions
The present study has shown that MLN, caused by the combination of MCMV and SCMV which were present in all the study regions, is widely distributed and still a major problem to maize production in Kenya. From the results of the study, farmers are still planting maize varieties which are susceptible to MLN. This is evident from the symptoms observed on the farms. In addition, the varieties being planted had been screened for their tolerance to MLN, and the results of the study showed that they were susceptible to MLN (Semagn et al. Citation2014). Since breeding for tolerant varieties is an economically and environmentally friendly way to control plant viral diseases, efforts should be made to release MLN tolerant varieties to the farmers.
The findings of the study showed that, though most farmers knew MLN, their understanding of MLN was limited in regard to its causal agents, spread mechanisms and visible symptoms on infected maize plants. Therefore, the lack of a better understanding of MLN, limited the farmers to adopt effective management practices. For example, the farmers uprooting maize plants with visible symptoms, threw them at the farm side or in ditches. Studies have shown that MCMV can be transmitted through MLN infected maize residues (Kinyungu et al. Citation2019). Therefore, improper disposal of MLN infected plants can lead to spreading of the disease instead of controlling it. In addition, the duration of crop rotation practised (3–6 months) is insufficient to lead to effective MLN management. Some farmers reported to practising crop rotation by changing the variety of maize planted. There should be sufficient efforts to introduce new plant varieties favourable to the farmer and the consumer to minimize overdependence on maize.
Most farmers owned a mobile phone, meaning that there is an opportunity to use the platform for information sharing on MLN disease, its management and also on other aspects of maize production such as access to tolerant seed varieties and marketing. However, before the use of the mobile-based platform is adopted, sufficient study should be conducted to find out the most acceptable, affordable and adaptable way for the farmers to adopt the new technologies.
From the results, MLN can attack maize crop at any stage of growth as the farmers reported to observing the disease from when the maize plant was at the 4–5 leaf stage. The presence of MLN (MCMV and SCMV) was also confirmed by the results of RT-PCR and sequencing analysis. This corroborates with findings from previous studies that MLN attack crops at any stage of growth (Frank et al. Citation2016).
Asymptomatic plants also tested positive for MCMV. Therefore, there should be deployment of field rapid test to be able to detect MCMV early on for implementation of proper control measures.
Therefore, the present study depicts the complexity of MLN and its significant effect on maize production, farmers’ income and food security. Maize production is mainly small scale and MLN mitigation measures should be adaptable to be incorporated in the farmers’ daily practices. Farmers should be sensitized on diversification of their cropping systems with research being done to identify alternative crops attractive to the farmers, the different agro-ecological zones and the market. In addition, the government should invest in establishing irrigation schemes as most of the maize farming was rainfed with farmers citing unpredictability of the weather as constraint to maize farming.
Author contributions statement
Faith Njeru was involved in concept conceptualization, study design, data acquisition, data analysis, and writing of the manuscript. Samuel Mwaura was involved in data acquisition, molecular work and design of the molecular experiments. Paul Kusolwa contributed to supervision of the project, review of the paper and change in its write up. Gerald Misinzo contributed to supervision and guidance for the project. All authors agree to the work done and for its publication.
Supplemental Material
Download PDF (141.7 KB)Acknowledgements
I, Faith Njeruacknowledge Kenya Agricultural and Livestock Research Organization (KALRO), Food Crops Research Centre, Njoro where the molecular work was conducted. I acknowledge the farmers who contributed to the study by giving their views on MLN and allowing us to collect samples from their fields.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available in the manuscript, and within the articles referenced and their supplementary materials. The raw data used for analysis is found at: njeru, faith (2021), ‘Maize production systems, farmers’ perception and current status of Maize Lethal Necrosis in selected counties in Kenya’, Mendeley Data, V2: https://doi.org/10.17632/n5cpfr4536.2. The raw data that has been deposited include the MLN survey data, the QGIS raw data, the sequencing raw data and the unprocessed RT-PCR gel images which are in the folder named new folder.
The partial coat protein gene sequences of 6 samples in this study were registered in GenBank(https://www.ncbi.nlm.nih.gov) and the accession numbers for MCMV (OL461943, OL461944, and OL461945); SCMV (OL461946, OL461947, and OL461948) were provided.
Ethics approval and consent to participate declaration
The project which is part of the Ph.D. project was approved by the Board College of Veterinary Medicine and Biomedical Sciences, Sokoine University of Agriculture under the reference number: SUA/PVM/D/2020/0005/03, and by the National Commission for Science, Technology & Innovation (NACOSTI) under the reference number: 742958.
Farmers were explained to the purpose of the study and the reason for the survey, and asked if they were willing to participate and they would respond verbally with Yes/No.
Supplementary data
The full version of the questionnaire is provided as a separate attachment.
Additional information
Funding
References
- Beyene Y, Gowda M, Stephen LMS, Olsen M, Oikeh SO, Juma C, … Prasanna BM. 2017. Genetic analysis of tropical maize inbred lines for resistance to maize lethal necrosis disease. Euphytica. 213(9):1–13. doi:10.1007/s10681-017-2012-3.
- Cabanas D, Watanabe S, Higashi CHV, Bressan A. 2013. Dissecting the mode of maize chlorotic mottle virus transmission (Tombusviridae: Machlomovirus) by Frankliniella williamsi (Thysanoptera: Thripidae). J Econ Entomol. 106(1):16–24. doi:10.1603/EC12056.
- FAO. 2019. FAOSTAT. Food and Agriculture Organization of the United Nations, Rome, Italy. https://www.fao.org/faostat/en/#data.
- Frank K, Robert G, Brian EI. 2016. Status of maize lethal necrosis in eastern Uganda. Afr J Agric Res. 11(8):652–660. doi:10.5897/ajar2015.10616.
- Guadie D, Knierim D, Winter S, Tesfaye K, Abraham A. 2019. Survey for the identification and geographical distribution of viruses and virus diseases of maize (Zea mays L.) in Ethiopia. Eur J Plant Pathol. 153(1):271–281. doi:10.1007/s10658-018-1568-7.
- Gudero Mengesha G, Kedir Mohammed B, Sultan Salo K. 2019. Management of maize lethal necrosis disease through hand weeding and insecticide applications at Arba Minch in Southern Ethiopia. Cogent Food Agric. 5(1):1705746. doi:10.1080/23311932.2019.1705746.
- Hutchens NJ. 1978. LD2668T41981H87.pdf. Kansas State University.
- Jensen SG, Wysong DS, Ball EM, Higley PM. 1991. Seed transmission of maize chlorotic mottle virus. Plant Dis. 75:497–498.
- Jumbo B, Makumbi D, Kimunye JN, Mahuku GS. 2015. Integration of maize Lethal Necrosis disease management in crop-livestock intensification to enhance productivity of smallholder agricultural production … , 2. https://cgspace.cgiar.org/bitstream/handle/10568/58478/esa_mln_poster_mar2015.pdf?sequence=1&isAllowed=y.
- Kansiime MK, Mugambi I, Rwomushana I, Lamontagne-godwin J, Rware H, Phiri NA, … Day R. 2019. Farmer perception of fall armyworm (Spodoptera frugiderda J. E. Smith) and farm-level management practices in Zambia. Pest Manage Sci. 75(May): 2840–2850. doi:10.1002/ps.5504.
- Kinyungu TN, Muthomi JW, Subramanian S, Miano DW, Olubayo FM, Maobe MA. 2019. Role of maize residues in transmission of maize chlorotic mottle virus and effect on yield. Int J Biosci. 6655:338–349.
- Krell NT, Giroux SA, Guido Z, Hannah C, Lopus SE, Caylor KK, Evans TP. 2021. Smallholder farmers’ use of mobile phone services in central Kenya. Clim Dev. 13(3):215–227. doi:10.1080/17565529.2020.1748847.
- Louie R. 1980. Sugarcane mosaic virus in Kenya. Plant Dis. 64(10):944. doi:10.1094/PD-64-944.
- Mahuku G, Lockhart BE, Wanjala B, Jones MW, Kimunye JN, Stewart LR, … Redinbaugh MG. 2015. Maize Lethal Necrosis (MLN), an emerging threat to maize-based food security in Sub-Saharan Africa. Phytopathology®. 105(7):956–965. doi:10.1094/PHYTO-12-14-0367-FI.
- Miano DW. 2014. A real threat to food security in the Eastern and Central Africa. University of Nairobi Digital Repository. 1973(Mcmv). http://hdl.handle.net/11295/79053.
- Mudde B, Olubayo F, Miano D, Kilalo D. 2017. Farmer knowledge, perceptions and management of maize lethal necrosis disease in selected agro-ecological zones of Uganda. Afr J Rural Dev. 2(September):247–261.
- Mwatuni FM, Nyende AB, Njuguna J, Zhonguo X, Machuka E, Stomeo F. 2020. Occurrence, genetic diversity, and recombination of maize lethal necrosis disease-causing viruses in Kenya. Virus Res. 286(January):198081. doi:10.1016/j.virusres.2020.198081.
- Nault LR, Styer WE, Coffey ME, Gordon DT, Negi LS, N CL. 1978. Transmission of maize chlorotic mottle virus by chrysomelid beetles. Phytopathology. 68(7):1071–1074. http://www.apsnet.org/publications/phytopathology/backissues/Documents/1978Articles/Phyto68n07_1071.pdf.
- Odendo M, De Groote H, Odongo OM. 2001. Assessment of farmers’ preferences and constraints to maize production in moist midaltitude zone of Western Kenya. Paper presented at the 5th International Conference of the African Crop Science Society, p. 21–26.
- Redinbaugh MG, Stewart LR. 2018. Maize lethal necrosis: an emerging, synergistic viral disease. Annu Rev Virol. 5(1):301–322. doi:10.1146/annurev-virology-092917-043413.
- Regassa B, Abraham A, Fininsa C, Wegary D, Wolde-Hawariat Y. 2020. Distribution of maize lethal necrosis epidemics and its association with cropping systems and cultural practices in Ethiopia. Crop Prot. 134(November 2019):105151. doi:10.1016/j.cropro.2020.105151.
- Semagn K. 2014. Leaf tissue sampling and DNA extraction protocols BT – molecular plant taxonomy: methods and protocols. In: P. Besse, editor. Totowa, NJ: Humana Press; p. 53–67. https://doi.org/10.1007/978-1-62703-767-9_3.
- Semagn K, Beyene Y, Babu R, Nair S, Gowda M, Das B, … Worku M. 2014. Molecular breeding for developing stress resilient maize for Sub-Saharan Africa. Crop Sci. 55(February 2015): 1449–1459. doi:10.2135/cropsci2014.09.0646.
- Uyemoto J, Bockelman D, Claflin L. 1980. Severe outbreak of Corn Lethal Necrosis Disease.PDF.
- Wamaitha MJ, Nigam D, Maina S, Stomeo F, Wangai A, Njuguna JN, … Garcia-Ruiz H. 2018. Metagenomic analysis of viruses associated with maize lethal necrosis in Kenya. Virol J. 15(1):90. doi:10.1186/s12985-018-0999-2.
- Wyche S, Steinfield C. 2016. Why don’t farmers use cell phones to access market prices? Technology affordances and barriers to market information services adoption in rural Kenya. Inf Technol Dev. 22(2):320–333. doi:10.1080/02681102.2015.1048184.