Abstract
Gastrointestinal tumor is a common malignancy that is dangerous to human health. Some of the patients exhibit familial hereditary syndromes; however, the molecular genetics of hereditary gastrointestinal tumors remain unclear. Here, a Chinese family including 21 people was investigated. Among them, three cases were respectively diagnosed with gastric cancer, colon cancer, and liver cancer; one case was diagnosed with cystic ovarian. Whole-exome sequencing (WES) and Sanger sequencing were applied to identify the pathogenic mutation of four patients. A novel frameshift mutation in exon 49 (c.7141_7151del) of ataxia telangiectasia mutated (ATM) gene was detected in three patients with gastric cancer, colon cancer, and cystic ovarian but absent in patient with liver cancer. This mutation was co-segregated with the disease phenotype and was predicted to be pathogenic. The deletion mutation in the ATM gene led to a frameshift mutation of the bases after ATM, ultimately causing the protein code to terminate at 2,401st amino acids (p.N2381fs). Our results detected a novel mutation of ATM in a family with hereditary gastrointestinal tumors and expanded the mutation spectrum of ATM gene. Taken together, these findings provide vital information about the possible detection of tumor occurrence and progression, contributing towards hereditary cancer prevention and screening.
Abbreviations: ATM: Ataxia telangiectasia mutated; InDels: Insertions and deletions; NCCN: National Comprehensive Cancer Network; SNVs: Single nucleotide variations; WES: Whole-exome sequencing
Introduction
Gastrointestinal tumors are one of the malignancies that threaten human health and are an important cause of cancer-related morbidity and mortality. According to the China National Cancer Center statistics in 2019, malignant tumors account for 23.91% of all deaths among domestic residents, and the incidence of gastric and colorectal cancers is ranked second and third, respectively, only after lung cancer (Xia et al. Citation2022). Genetics and pathology research provides better characterization of some well-defined inherited gastrointestinal cancer syndromes, including familial adenomatous polyposis, Lynch syndrome, Peutz-Jeghers syndrome, and juvenile polyp syndrome are common symptoms (Ma et al. Citation2018). It has been reported that approximately 5–10% of gastric adenocarcinomas arise in individuals with a family history of gastrointestinal tumors, and 3–5% are estimated to be associated with germline mutations concerning inherited cancer syndromes (Zhang et al. Citation2018). Family history is also one of the strongest predictors of risk of colorectal cancer (Kastrinos et al. Citation2020). Therefore, identifying these hereditary cancer cases is important for patients and at-risk relatives, and has clinical management significance for both affected and unaffected individuals.
In the past decades, great advances have been made in our understanding of the genomics of gastrointestinal neoplasms. It has been confirmed that the pathogenic mutations in several germline genes, such as CDH1, APC, and MLH1, are associated with higher gastrointestinal tumor susceptibility (Michailidi et al. Citation2015; Oue et al. Citation2019). A growing focus of research suggests a strong relationship between homologous DNA recombination, cell cycle checkpoints of genes, and tumorigenesis (Wahl Citation2020). To some extent, the identification of gene mutation in hereditary cancers is significantly important for patients and families, since it facilitates risk assessment, guiding clinical management, and treatment options (Pearlman et al. Citation2017; Zhou et al. Citation2020). Nevertheless, the molecular genetics of hereditary gastrointestinal cancers has not yet been adequately studied.
The National Comprehensive Cancer Network (NCCN) reports that germline mutations in genes involved in DNA repair, such as ataxia telangiectasia mutated (ATM), increase the risk of breast cancer, ovarian cancer, pancreatic cancer, and prostate cancers (Economopoulou et al. Citation2015; Grant et al. Citation2015). The ATM gene locates on chromosome 11q 22–23 and includes 66 exons with a 9168 base pair coding, which encodes a PI3K-related serine/threonine protein kinase that helps maintain genome integrity. The ATM gene encodes a vital cell cycle checkpoint kinase protein. Numerous studies have shown that ATM regulates DNA repair signals and is strongly associated with hereditary tumors (Frimer et al. Citation2016; Li et al. Citation2019; Lin et al. Citation2019). Numerous evidences have shown ATM gene mutations recognized as genetic predisposition factors for many cancers. For example, deleterious ATM mutations are observed in aggressive prostate cancers (Neeb et al. Citation2021); heterozygous mutation in ATM gene can increase the risk of breast cancer (Weigelt et al. Citation2018). However, the types of ATM mutations in patients with gastrointestinal cancers have not been studied.
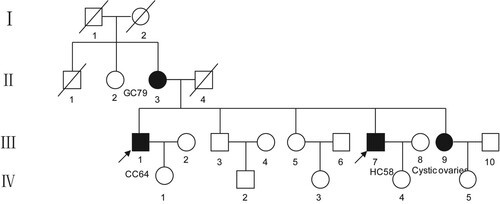
This study focuses on a 21-member family, in which the female subject II-3 was diagnosed with gastric cancer at the age of 67 years. Regarding the younger generation, her two sons were diagnosed with colon cancer (III-1) and liver cancer (III-7) at the age of 64 and 58 years, respectively. The remaining three are healthy. Utilizing whole-exome sequencing (WES), subjects II-3, III-1, and III-9 were found to share an ATM frameshift mutation, while III-7 had the wild-type genotype. It seems that frameshift mutations in ATM may lead to abnormalities in its protein function, which may have caused the disease of subjects II-3 and III-1. Sanger sequencing showed that the mutation was co-segregated with the disease phenotype, further suggesting that a mutation in the ATM gene might be related to the occurrence of gastrointestinal cancer.
Methods
Patients and samples
A family with hereditary gastrointestinal cancer was investigated in this study (Figure ). This family contained 21 persons, and the blood samples of four patients (II-3, III-1, III-7, and III-9) were collected at the Fourth Affiliated Hospital of China Medical University. This study protocol was approved by the Ethics Committee of China Medical University (ethical approval number: EC-2018-HY-007). These four subjects were informed of the study purpose and signed written informed consent, and this study followed the basic principles of the Declaration of Helsinki.
DNA extraction and whole exome sequencing
Genomic DNA was extracted from peripheral blood leukocytes using the QIAamp DNA Blood Midi Kit (Qiagen; Valencia, CA, USA) for whole-exome sequencing (WES). In brief, DNA libraries were prepared using the Roche NimbleGen v3.0 PLUS and then fragment analysis was performed using Agilent 4200 TapeStation. Finally, all data were sequenced using the NovaSeq 6000 System (PE150) with an average sequencing depth of 100×.
Bioinformatics analysis of variants
After data preprocessing, the clean reads were mapped to the reference human genome (hg19) using BWA (v0.7.11), followed by classification and deduplication. Base quality recalibration and indel realignment were performed using the GATK BaseRecalibrator (v4.1.0.0). Hereafter, HaplotypeCaller (v4.1.0.0) was applied to sample the BAM files for detection of germline single nucleotide variations (SNVs) and small insertions and deletions (InDels).
Furthermore, the screening criteria were as follows: (1) variant allele frequency (VAF) > 30%; (2) allele frequency < 0.001 in gnomAD database; (3) no benign or likely benign evidence of more than two stars in Clinvar data; (4) variants were in accordance with ACMG guidelines. In this study, all variants identified were manually inspected using Integrative Genomics Viewer (IGV 2.7.2).
Sanger sequencing
Next, Sanger sequencing was conducted to verify the identified candidate variants. PCR primers were designed by using the Primers5 tool and the sequences were: F, 5’-GGGTTGGACAAGTTTGCAATAGT-3’; R, 5’-CAGGAGAGCTTGCTTGTTTTCA-3’. The amplified products were sequenced on an ABI PRISM 3730 automated sequencer (Applied Biosystems, Foster city, CA, USA) and raw data were analyzed using Blast (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
Results
Family history and the process of diagnosis and treatment
This family included four deaths (I-1, I-2, II-1, and II-4), three cases with cancer (II-3, III-1, and III-7), one case with cystic ovarian (III-9), and the rest of subjects were healthy people (Figure ). Concretely, a 67-year-old woman (II-3) was first diagnosed with gastric cancer. Her two sons were diagnosed with colon cancer (III-1) and liver cancer (III-7) at the age of 64 and 58 years, respectively. Here, we listed the specific clinical manifestations and treatment process of each patient (Table ).
Table 1. The clinical characteristics of four patients in this family.
In 2011, subject II-3 underwent gastroscopy and pathological examination due to abdominal distension and stomach discomfort. She was diagnosed with gastric cancer. In the same year, she underwent a palliative gastrojejunostomy, followed by FOLFOX and capecitabine chemotherapy after the operation.
Six years later (2017), subject III-1 presented similar clinical manifestations, with right lower abdominal pain without obvious causes, paroxysmal pain, occasional diarrhea, no mucus pus and blood in the stool, no tenesmus, along with occasional nausea and vomiting; in addition, he had no fever with normal eating and sleeping. He lost 5 kg per month. In April 2017, he underwent a colonoscopy and results showed that his ascending colon was about 80 cm away from the anus with a raised and recessed annular cavity lesion, erosion ulcers on the surface, and a narrow intestinal cavity. Meanwhile, the pathological results indicated poorly differentiated adenocarcinoma of the right hemicolon. In May 2017, he underwent radical right hemicolectomy. After the operation, he received FOLFOX chemotherapy.
In 2017, subject III-7 had clinical manifestations of abdominal distension without obvious causes, recurrent attacks, gradual aggravation, abdominal pain, mucus pus or blood in the stool, nausea or vomiting, poor appetite, normal sleep, and no significant recent weight change. The enhanced CT results of the abdomen showed a round-shaped low-density shadow with a diameter of approximately 5.3 cm in the upper right posterior lobe of the liver. There were also several smaller ring-shaped enhanced low-density shadows in the right lobe of the liver. Multiple enlarged lymph nodes were observed in the hepatic portal area, retroperitoneum, and gastric cardia. The diameter of the largest one was approximately 6.6 cm, and some were fused. Taken together, he was diagnosed with liver cancer. In August 2017, subject III-7 underwent percutaneous hepatic artery chemoembolization with 20 mg of epirubicin. Prevention of infection, rehydration, nutrition, and other follow-up treatments were performed after surgery.
Identification and characterization of candidate mutation
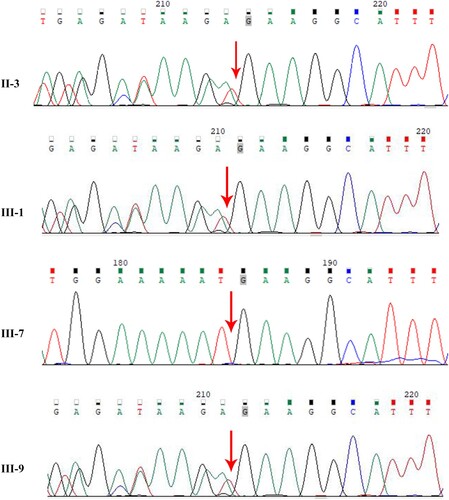
The exomes of four patients (II-3, III-1, III-7, and III-9) in the family were examined using WES analysis. An ATM deletion mutation was detected in three of them (subjects II-3, III-1, and III-9) (Figure ), which was a 10-base deletion in exon 49 of the ATM gene (c.7141_7151del p.N2381fs). The mutation was not found in the OMIM database (https://omim.org/), implying that it might be a novel mutation that could affect the function of the protein. Our analysis revealed that deletion mutations in the ATM gene lead to a frameshift mutation in ATM at latter bases, ultimately causing the protein code to terminate at 2401 amino acids (Figure ). Through the cBioPortal database (https://www.cbioportal.org/) and Clinvar database (https://www.ncbi.nlm.nih.gov/clinvar/), no significant hotspot mutations in ATM gene were found (Figure B, Supplementary Figure 1).
Figure 2. Identification of this truncation mutation c.7141_7151del in ATM. Visualization of ATM sequencing reads containing c.7141_7151del mutation using the Integrative Genomics Viewer (IGV). The big red box represents the 11 missing nucleotides. The small red box represents the stop codon.
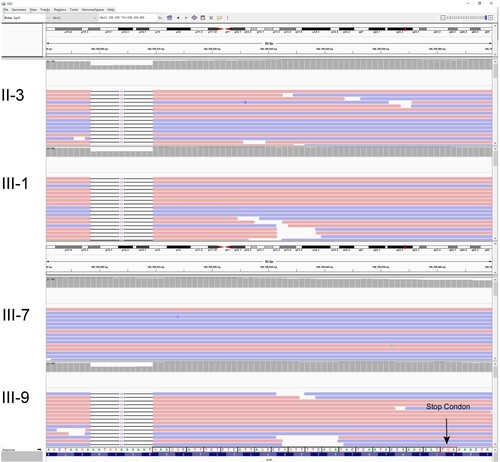
Figure 3. Mutations in ATM. (A) Protein structure of ATM dimer. On the left is the normal ATM protein structure, which serves as a control. The pink part represents the spiral region, the green part is the pincer region, the purple is the FAT structure region, and the blue is the kinase domain. On the right, the gray part is the unexpressed part after ATM mutation. The enlarged red part represents the unshifted region of a normal ATM protein. The enlarged yellow portion represents the amino acid sequence encoded by ATM after a code-shift due to deletion mutations. (B) The circle represents the mutation site, and the height represents the mutation frequency. ATM proteins are showing the spiral region, pincer region, FAT region, and kinase domain. Mutation p.N2381fs was found in the kinase domain of ATM.
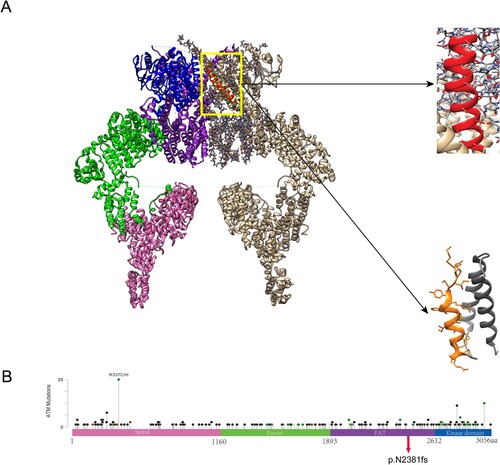
As shown in Figure , ATM functions in a dimeric form. On the left was the normal ATM protein structure, which served as a control. The mutation caused the amino acid to terminate prematurely at p.N2381fs, and the gray portion on the upper right was the part that was not expressed after the ATM frameshift mutation. This part includes the kinase domain and a portion of the FAT domain (Baretić et al. Citation2017).
Moreover, this mutation was not presented in the 1000 genome database, ExAC database, and gnomAD database. According to the guidelines of the College of Medical Genetics and Genomics, the mutation was highly pathogenic.
Family separation
To validate the true positive result of the mutation, Sanger sequencing was further performed. The mutation was confirmed to segregate well in the phenotype of liver cancer patients (III-7) and other cancer patients (II-3, III-1). The frameshift mutation caused a deletion of nucleotides from No. 7141–7151 in the coding region, resulting in a change of 2381 amino acid by asparagine (Figure ). Furthermore, it was not found in unaffected family members or in normal controls.
Discussion
Previous studies revealed that aggregation within families occurred in a sizable fraction (about 10%) of colorectal cancer and gastric cancer patients. However, the mutational spectrum and clinical features of hereditary CRC and GC in China are not well understood. Early screening and management can significantly reduce the risk of cancer in these carriers of pathogenic mutations. Recently, many scholars are dedicated to the research of hereditary tumors to better understand hereditary cancer syndromes and promote an accurate diagnosis and early treatment.
In the present pedigree, a novel frameshift mutation in ATM was detected in patients with gastric cancer, colon cancer, and cystic ovarian. This heterozygous mutation in ATM was named c 7141_7151del, and it resulted in an abnormal ATM protein that terminated at 2401st amino acids. This mutation was a loss-of-function mutation that might be pathogenic due to haploinsufficiency. Moreover, this mutation was not presented in the 1000 genome database, ExAC database, or gnomAD database. According to the guidelines of the College of Medical Genetics and Genomics, the mutation had a very strong pathogenicity (PVS) (Richards et al. Citation2015) (see Supplementary Table 1).
Evidence has indicated that hereditary and sporadic ATM mutations span the entire functional domain of ATM gene and these mutations occur at the C-terminus which interacts with the PI3 kinase domain (Armstrong et al. Citation2019). DNA damage response and repair (DDR) is impaired when the ATM protein is dysfunctional, over time, the loss of this DDR mechanism designed for DNA double-stand breaks may result in the accumulation of mutations, which theoretically can start the process of tumorigenesis (Carter et al. Citation2019). In this family, subject II-3 was diagnosed with gastric cancer at age 67 years, and her son (III-1) was diagnosed with colon cancer at age 64 years. Both had been found to have an ATM pathogenic mutation. Although subject III-7 developed liver cancer at age 58 years, he did not carry the causative mutation, while the patient had been drinking alcohol for about 30 years, at an average of 100 mL/day. Long-term alcohol abuse may be a cause of liver cancer in the patient. Subject III-9 carries a pathogenic mutation associated with a high risk of tumors, and regular screening is recommended. This time, the heterozygous c.7141_715 del mutation in exon 49 of the ATM gene was first reported in cases with gastrointestinal cancer, and this study helps to understand the types of genetic variants of hereditary gastrointestinal cancer.
ATM gene is involved in repairing DNA lesions and maintaining genome stability, which is associated with tumor invasion and metastasis. As reported, Helicobacter pylori infection could initiate DNA damage to active ATM, and downregulation of ATM expression was related to an unfavorable prognosis of gastric cancer (Han et al. Citation2017; Santos et al. Citation2018). Previous study (Tao et al. Citation2020) reported ATM rs189037 G > A polymorphism was related to increase susceptibility and poorer prognosis in gastric cancer in the Chinese population. Moreover, previous study revealed that the frequency of deleterious ATM mutations in patients with gastric cancer was significantly higher than that in general population (Huang et al. Citation2015). These findings confirmed the potential role of ATM mutation in the pathogenesis of gastric cancer. Furthermore, ATM deficiency was also significantly inhibited the proliferation, migration, and invasion of colon cancer cell lines, suggesting that ATM might serve as an attractive target for improving cancer treatment (Liu et al. Citation2017).
A systematic review and meta-analysis showed that heterozygous carriers of pathogenic ATM mutation had a decreased life expectancy and an increased risk of cancer, especially breast cancer and possible gastrointestinal cancer (van Os et al. Citation2016). In this study, a heterozygous c 7141_7151del mutation in the ATM gene was identified for the first time, and the clinical phenotype of cases was mainly gastrointestinal cancer. Previous studies also confirmed that carriers of ATM pathogenic variants had four- to fivefolds increased risk of gastric cancer (Helgason et al. Citation2015). Thus the high prevalence of ATM in the general population supports the importance of precise and comprehensive estimates of cancer risk associated with ATM pathogenic variants (Swift et al. Citation1986; Swift et al. Citation1987), especially those with evidence for familial predisposition. With the popularity and increase of genetic testing, the identification of germline ATM pathogenic mutations may help reduce the incidence of cancer in many disease sites. In addition, tumors with DNA repair-deficient mutations, including ATM, are expected to enhance chemosensitivity to drugs, such as platinum salts or topoisomerase inhibitors (Sato et al. Citation2017). A clinical trial confirmed that a patient with locally advanced colon cancer carrying a germline ATM mutation exhibited a complete response to neoadjuvant FOLFOXIRI plus panitumumab (McGillivray et al. Citation2021). Taken together, genetic testing can often improve understanding and clarification of risk for cancer development, and then knowing whether patients carry germline ATM mutations may help guide treatment decisions (Mahon Citation2016). Furthermore, previous evidence indicated that no differences in age at diagnosis were observed for gastric cancer patients with or without ATM pathogenic mutations (Hall et al. Citation2021). However, the relationship has not been validated. Thus it is necessary to further explore how ATM mutation affects screening guidelines for patients with gastrointestinal tumors.
However, our study still had limitation. First, we did not collect the blood from the healthy member due to the lack of informed consent from other participants. Second, this mutation was only detected in this one family with hereditary gastrointestinal tumors. Third, ‘two-hit’ hypothesis proposes that germline variation and somatic variation are independent mutational events, which ultimately lead to the loss of function of tumor suppressor genes. Nevertheless, whether these patients had the somatic ‘second hits’ event of ATM mutation were not investigated. Thus the source and somatic second hit of ATM mutation need to be further analyzed in other families with hereditary gastrointestinal tumors.
Conclusion
In conclusion, we detected a heterozygous mutation designated as c. 7141_7151del p.N2381fs in the exon 49 of ATM gene in a Chinese family with gastrointestinal tumors. This mutation caused an abnormal ATM protein that terminated at 2,401st amino acids. Hence, this finding expands the variants spectrum of ATM gene, which may contribute to the genetic counseling of gastrointestinal tumors. Meanwhile, WES is a useful method for diagnosing congenital diseases, which has great benefit in disease screening, genetic diagnosis, and counseling. Further studies should be investigated to unravel the factors which account for phenotypic variability present in patients with ATM gene mutations.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Author contributions
Xiaoxia Li came up with the study concept and design and obtained funds. Gongping Sun and Guanyu Fu wrote the paper. Junjie Yi and Guanyu Fu drew a figure of genetic reports and a pedigree chart. Xiaobo Lu performed the bioinformatics analysis. Gongping Sun, Yuanxin Tang, Rongjun Su, and Wei Liu were involved in the therapy, patient management, and family disease information collection.
Consent for publication
Written informed consent for publication was obtained from all participants.
Data availability statement
The data supporting the findings of this study are available in the figshare repository [https://figshare.com/] at doi:10.6084/m9.figshare.19709965 [https://figshare.com/s/3e18591f29c0c9c8c397].
Ethics approval and consent to participate
All patients were informed of the study purpose and signed consent forms, and the Ethics Committee of China Medical University approved the study protocol (ethical approval number: EC-2018-HY-007). This study followed the basic principles of the Declaration of Helsinki.
Additional information
Funding
References
- Armstrong SA, Schultz CW, Azimi-Sadjadi A, Brody JR, Pishvaian MJ. 2019. ATM dysfunction in pancreatic adenocarcinoma and associated therapeutic implications. Mol Cancer Ther. 18:1899–1908.
- Baretić D, Pollard H, Fisher D, Johnson C, Williams R. 2017. Structures of closed and open conformations of dimeric human ATM. Sci Adv. 3:e1700933.
- Carter HB, Helfand B, Mamawala M, Wu Y, Landis P, Yu H, Wiley K, Na R, Shi Z, Petkewicz J, et al. 2019. Germline mutations in ATM and BRCA1/2 Are associated with grade reclassification in Men on active surveillance for prostate cancer. Eur Urol. 75:743–749.
- Economopoulou P, Dimitriadis G, Psyrri A. 2015. Beyond BRCA: new hereditary breast cancer susceptibility genes. Cancer Treat Rev. 41:1–8.
- Frimer M, Levano K, Rodriguez-Gabin A. 2016. Germline mutations of the DNA repair pathways in uterine serous carcinoma. Gynecol Oncol. 141:101–107.
- Grant R, Selander I, Connor A. 2015. Prevalence of germline mutations in cancer predisposition genes in patients with pancreatic cancer. Gastroenterology. 148:556–564.
- Hall MJ, Bernhisel R, Hughes E, Larson K, Rosenthal ET, Singh NA, Lancaster JM, Kurian AW. 2021. Germline pathogenic variants in the ataxia telangiectasia mutated (ATM) gene are associated with high and moderate risks for multiple cancers. Cancer Prev Res (Phila). 14:433–440.
- Han M, Ma L, Qu Y, Tang Y. 2017. Decreased expression of the ATM gene linked to poor prognosis for gastric cancer of different nationalities in Xinjiang. Pathol Res Pract. 213:908–914.
- Helgason H, Rafnar T, Olafsdottir HS, Jonasson JG, Sigurdsson A, Stacey SN, Jonasdottir A, Tryggvadottir L, Alexiusdottir K, Haraldsson A, et al. 2015. Loss-of-function variants in ATM confer risk of gastric cancer. Nat Genet. 47:906–910.
- Huang D-S, Tao H-Q, He X-J, Long M, Yu S, Xia Y-J, Wei Z, Xiong Z, Jones S, He Y, et al. 2015. Prevalence of deleterious ATM germline mutations in gastric cancer patients. Oncotarget. 6:40953–40958.
- Kastrinos F, Samadder NJ, Burt RW. 2020. Use of family history and genetic testing to determine risk of colorectal cancer. Gastroenterology. 158:389–403.
- Li J, Jing R, Wei H. 2019. Germline mutations in 40 cancer susceptibility genes among Chinese patients with high hereditary risk breast cancer. Int J Cancer. 144:281–289.
- Lin P, Yeh Y, Wu P, Hsu K, Chang J, Shen M. 2019. Germline susceptibility variants impact clinical outcome and therapeutic strategies for stage III colorectal cancer. Sci Rep. 9:1–10.
- Liu R, Tang J, Ding C, Liang W, Zhang L, Chen T, Xiong Y, Dai X, Li W, Xu Y, et al. 2017. The depletion of ATM inhibits colon cancer proliferation and migration via B56γ2-mediated Chk1/p53/CD44 cascades. Cancer Lett. 390:48–57.
- Ma H, Brosens LAA, Offerhaus GJA, Giardiello FM, de Leng WWJ, Montgomery EA. 2018. Pathology and genetics of hereditary colorectal cancer. Pathology. 50:49–59.
- Mahon SM. 2016. Management of individuals with a mutation in the ataxia telangiectasia mutated gene. Oncol Nurs Forum. 43:114–117.
- McGillivray E, Farma J, Savage M, Hall MJ, Luo B, Jain R. 2021. Pathologic complete response in patient With ATM mutation after neoadjuvant FOLFOXIRI plus panitumumab therapy for locally advanced colon cancer: A case report. Clin Colorectal Cancer. 20:e96–e99.
- Michailidi C, Theocharis S, Tsourouflis G, Pletsa V, Kouraklis G, Patsouris E, Papavassiliou AG, Troungos C. 2015. Expression and promoter methylation status of hMLH1, MGMT, APC, and CDH1 genes in patients with colon adenocarcinoma. Exp Biol Med (Maywood). 240:1599–1605.
- Neeb A, Herranz N, Arce-Gallego S, Miranda S, Buroni L, Yuan W, Athie A, Casals T, Carmichael J, Rodrigues DN, et al. 2021. Advanced prostate cancer with ATM loss: PARP and ATR inhibitors. Eur Urol. 79:200–211.
- Oue N, Sentani K, Sakamoto N, Uraoka N, Yasui W. 2019. Molecular carcinogenesis of gastric cancer: Lauren classification, mucin phenotype expression, and cancer stem cells. Int J Clin Oncol. 24:771–778.
- Pearlman R, Frankel W, Swanson B, Zhao W, Yilmaz A, Miller K, Bacher J, Bigley C, Nelsen L, Goodfellow P. 2017. Prevalence and spectrum of germline cancer susceptibility gene mutations among patients with early-onset colorectal cancer. JAMA Oncol. 3:464–471.
- Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody W, Hegde M, Lyon E, Spector E. 2015. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American college of medical genetics and genomics and the association for molecular pathology. Genet Med. 17:405–423.
- Santos JC, Gambeloni RZ, Roque AT, Oeck S, Ribeiro ML. 2018. Epigenetic mechanisms of ATM activation after Helicobacter pylori infection. Am J Pathol. 188:329–335.
- Sato Y, Hirakawa M, Ohnuma H, Takahashi M, Okamoto T, Okamoto K, Miyamoto H, Muguruma N, Furuhata T, Takemasa I, et al. 2017. A triplet combination with capecitabine/oxaliplatin/irinotecan (XELOXIRI) plus cetuximab as first-line therapy for patients with metastatic colorectal cancer: a dose escalation study. Cancer Chemother Pharmacol. 80:1133–1139.
- Swift M, Morrell D, Cromartie E, Chamberlin AR, Skolnick MH, Bishop DT. 1986. The incidence and gene frequency of ataxia-telangiectasia in the United States. Am J Hum Genet. 39:573–583.
- Swift M, Reitnauer PJ, Morrell D, Chase CL. 1987. Breast and other cancers in families with ataxia-telangiectasia. N Engl J Med. 316:1289–1294.
- Tao Y, Mei Y, Ying R, Chen S, Wei Z. 2020. The ATM rs189037 G > A polymorphism is associated with the risk and prognosis of gastric cancer in Chinese individuals: A case-control study. Gene. 741:144578.
- van Os NJH, Roeleveld N, Weemaes CMR, Jongmans MCJ, Janssens GO, Taylor AMR, Hoogerbrugge N, Willemsen MAAP. 2016. Health risks for ataxia-telangiectasia mutated heterozygotes: a systematic review, meta-analysis and evidence-based guideline. Clin Genet. 90:105–117.
- Wahl R. 2020. The interaction of genomics, molecular imaging, and therapy in gastrointestinal tumors. Semin Nucl Med. 50:471–483.
- Weigelt B, Bi R, Kumar R, Blecua P, Mandelker D, Geyer F, Pareja F, James P, Investigators k, Couch F. 2018. The landscape of somatic genetic alterations in breast cancers from ATM germline mutation carriers. JNCI: J National Cancer Institute. 110:1030–1034.
- Xia C, Dong X, Li H, Cao M, Sun D, He S, Yang F, Yan X, Zhang S, Li N, et al. 2022. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl). 135:584–590.
- Zhang H, Feng M, Feng Y, Bu Z, Li Z, Jia S, Ji J. 2018. Germline mutations in hereditary diffuse gastric cancer. Chin J Cancer Res. 30:122–130.
- Zhou J, Zhao Z, Zhang Y, Bao C, Cui L, Cai S, Bai Y, Shen L XZ. 2020. Pathogenic germline mutations in Chinese patients with gastric cancer identified by next-generation sequencing 2020. Oncology. 98:583–588.