Abstract
A 2-year research was conducted to evaluate the effects of bean cultivar, herbicide and planting date on a plot scale in Zanjan Province, Iran. Weeds across plots were identified as 26 species. Weed density was often lower for the fourth planting date (25–30 June) than the first date (10–15 May) of Imazethapyr, Trifluralin, hand-weeding and control at seedling, flowering and maturity stages of bean growth. At seedling and flowering, there was a greater Rhizoctonia root rot incidence on the first planting date than the fourth date. Postponing cultivation to the fourth planting date decreased mean disease incidence up to 93% at flowering in 2014 and 75% at maturity in 2015. There were greater mean fly infestation ratings at the third (2014) and fourth (2015) planting dates of treatments. Fly infestation was lacking in beans planted at the second date of cultivar and herbicide treatments examined in 2015. Postponing planting from the first date to the fourth one increased mean plant dry matter up to 162% at flowering in 2015. The highest number of pods per plant and seeds per pod were recorded for the third (2014) and first-second (2015) planting dates. This study recommends a postponed planting to minimize disease, herbicide use and weed threats, and thus produce beans in sustainable agriculture.
Introduction
The common or dry bean (Phaseolus vulgaris) is an important edible crop in America, Africa and Asia (Naseri Citation2019). In Iran, the size of irrigated land under local cultivars of bean cultivation is 105,300 ha with an average production of ∼2.3 t/ha (Anonymous Citation2020). It is known that bean fly, root rot and weed are important factors affecting bean production worldwide (Talekar Citation1992; Ojwang et al. Citation2010; Naseri and Veisi Citation2019). Hence, field assessment of influential agronomic practices for sustainable disease-pest-weed management and bean cultivation deserves further consideration. A range of fly-rot-weed management methods such as biological and chemical control (Kalantari et al. Citation2018), cultural practices (Ampofo and Massomo Citation1998; Medvecky et al. Citation2007; Naseri and Hemmati Citation2017) and host plant resistance (Ojwang et al. Citation2010) have been suggested for bean cropping systems. However, the effect of planting date on this triple combination of threats to bean production is little understood.
Bean fly (Ophiomyia sp.) is considered the most predominant and destructive field insect pest of common bean in main Iranian bean growing areas (Naseri Citation2013a). Ophiomyia phaseoli Tyron and Ophiomyia spencerella Greathead are the two economically important species attacking eastern African beans (Greathead Citation1968). Yield reductions observed under field conditions resulting from bean fly are up to 100% (Talekar Citation1992). The distribution and infestation of bean fly vary with location and season (Ojwang et al. Citation2010; Naseri Citation2013a). In the lower semi-arid areas of East Africa where bean crops are more prone to drought stress, O. phaseoli is the most prevalent species. Ojwang et al. (Citation2010) studied the susceptibility reaction of 64 bean lines and cultivars to bean fly under diverse environmental conditions in Kenya. They reported that seed yield loss due to bean fly infestation ranged from 3% to 69%. Van der Goot (Citation1930) reported that sowing delayed by 3 weeks increased plant mortality due to bean fly infestation. Sariah and Makundi (Citation2007) also reported a greater fly infestation in later sown beans in northern Tanzania.
Rhizoctonia root rot caused by Rhizoctonia solani has been known as a devastating disease in beans grown worldwide (Dehghani et al. Citation2018; Naseri Citation2019; Gupta and Singh Citation2021). Exposure of bean crops to manure application, sprinkler irrigation, growing beans following potato and tomato, the lack of urea application, appropriate planting density, shallow seeding, avoiding furrow irrigation, manual cultivation, growing Red beans, sufficient soil organic matter, irrigating at 6–9 days intervals, improving rhizobial nodulation, and planting beans in soils with 15–30% silt content corresponded with low development of Rhizoctonia root rot in commercial bean fields (Naseri and Moradi Citation2015). Although Medvecky et al. (Citation2007) reported the efficiency of residue management practice for reducing bean-fly and root-rot damage, the identification of other influential agronomic practices to minimize losses to these pests needs further consideration.
Weed control in commercial bean fields in Iran is mainly accomplished through herbicide application. Herbicides usually suppress weeds that indirectly influence boost the yield. However, the widespread application of herbicides is considered faulty due to herbicide expenses, resistance of weed to herbicide, environmental pollution and health hazards (Oveisi et al. Citation2021). Therefore, it is interesting to minimize herbicide usage from sustainable bean production viewpoint. Increased bean competitiveness via cultural methods such as mix cropping besides herbicide use provided a solution to increase the efficiency of herbicide applied at lower doses (Oveisi et al. Citation2021). Despite much research on chemical weed and pest management in bean fields, environmental-friendly methods for effective and sustainable fly-rot-weed control are less understood. Naseri (Citation2013b, Citation2013c) advised that later sowing of bean crops restricted Rhizoctonia root rot according to plot- and regional-scale findings reported from Iran. This encouraged us to study whether adjusting the planting date is effective on the bean fly, root rot and weed interaction in the bean farming system, and how this cultural method interacts with bean cultivar and herbicide application. Thus attempts were made to explore how effectively planting date decreases herbicide use, fly-rot-weed infestation and improves productivity in two bean cultivars at a pilot scale.
Materials and methods
Experimental data collection
During the two growing seasons (2014–2015), the infestation of bean fly, Rhizoctonia root rot, weed density and productivity in common bean was studied in an experimental field under natural infestations of soil. Preliminary tests indicated that field soil (clay-loamy, mixed, mesic, Typic Haploxerepts) was infested with R. solani propagules. Infestations of previously grown beans showed that field soil was highly infested with bean fly and weed seeds. The experiments were conducted at Kheirabad Research Station (latitude 36°31′ north, longitude 48°47′ east; 1770 m a.s.l; 284.5 mm annual rainfall, 142 annual frost days). The experimental design was a split–split plot with four replicates for each of four sowing dates (main plot treatment), four herbicide applications and two bean cultivars (subplot treatments). The treatments comprised of different sowing dates (10–15 May, 26–31 May, 10–15 June and 25–30 June), herbicide applications (Imazethapyr, Trifluralin, hand-weeding and control) and two bean cultivars (Talash and COS16). Herbicides treatments included the pre-emergence application of Imazethapyr (PursuitTM 10% SL) at 1 l/ha and pre-planting Trifluralin (TreflanTM 48% EC) at 2.5 l/ha. Each experimental plot involved six 5-m rows at 0.30 m row spacing and 0.075 m plant spacing that provided a planting density of 40 plant/m2. To avoid mixing of herbicide treatments, experimental plots were spaced 5 m apart with no beans planted.
Weed density was recorded for each plot as the number of weed plants per quadrat (0.6 × 0.6 m; three quadrat per plot) at seedling (with three leaflets formed), flowering and maturity stages of bean growth. To measure Rhizoctonia root rot incidence, five plants per quadrat were dug up carefully to check root tissues for red-brown cankers (Naseri Citation2013a). The percentage of bean plants showing Rhizoctonia cankers on roots of five randomly selected plants per quadrat was considered as disease incidence. The incidence of bean Rhizoctonia root rot was recorded at seedling (with three leaflets formed), flowering and maturity stages. In the next step, 10 bean plants which were assessed for the disease incidence were used to measure bean plant dry matter per plot at first-leaf-formed seedling, third-leaf-formed seedling, flowering, podding and maturity stages. To rate fly bean infestation, five bean plants per plot were sampled arbitrarily at weekly intervals from the emergence of seedling to harvest and then transferred to the lab for further examination. The percentage of bean plants with damage or presence of pupae was determined for each experimental plot. The number of pods per plant and seeds per pod was recorded for 10 randomly selected plants per plot at the maturity stage.
Statistical methods
The development of bean Rhizoctonia root rot, fly infestation, weed density, dry matter, pod/plant and seed/pod was studied across 256 experimental plots over the two growing seasons. Statistical software SAS (SAS Institute Inc., Cary, NC, USA) was used to perform all statistical tests. To determine the effects of herbicide application, sowing-date and bean-cultivars factors on Rhizoctonia root rot, fly infestation, weed density, dry matter, pod number per plant, and seed number per pod, the factor levels were compared using the least significant differences (LSD) provided by the analysis of variance (ANOVA) procedure. These mixed models involved fixed (herbicide application, sowing-date and bean-cultivars) and random (assessment times and replications) terms.
Results
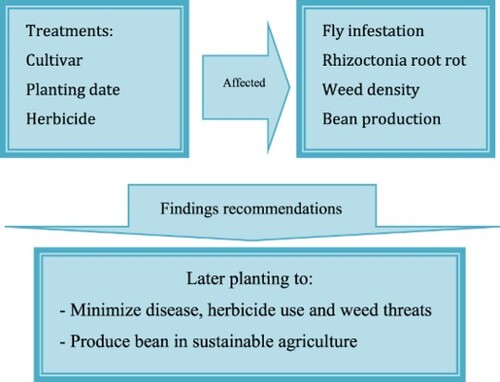
The density of weed, fly infestation, Rhizoctonia root rot incidence, bean dry matter, and number of pod/plant and seed/pod for different planting dates, herbicide applications and bean cultivars were determined during the two growing seasons at plot scale in Zanjan province. Over the two years of this study, 26 weed species were identified (). Planting date, herbicide application and cultivar affected the density of weed plants examined at seedling, flowering and maturity stages of bean growth (). In 2014, there were often significantly greater mean values for weed density of the control at each cultivar, planting date and growth stage in comparison with the Imazethapyr and Trifluralin treatments (). In this growing season, significantly lower mean weed densities were often recorded for the fourth planting date than the first date of the control and Trifluralin treatment at the three growth stages tested. For instance, postponing the date of planting control plots from 10–15 May to 25–30 June decreased mean weed density by 49% at seedling, 64% at flowering, and 82% at maturity in cv. COS16. In 2015, the highest mean weed density at the seedling stage with three leaflets formed was detected in the second planting date (26–31 May) of control-COS16, followed by the first planting date (10–15 May) of the Imazethapyr-COS16 and the second planting date of control-Talash treatments. The lowest mean weed density at this seedling stage was determined on the fourth planting date (25–30 June) of Imazethapyr-Talash treatment, followed by Trifluralin and Imazethapyr treatments of cv. COS16. At the flowering stage of bean growth, the highest mean weed density was obtained for the second planting date of control-COS16, the forth planting date of Imazethapyr-Talash and the third planting date (10–15 June) of control-Talash. The lowest weed density at flowering was recorded for the second planting date of Imazethapyr-Talash, the third and fourth planting dates of Imazethapyr-COS16 treatment. At maturity in 2015, the highest mean weed densities were detected on the first planting date Imazethapyr-COS16 treatment and the fourth planting date of control-COS16. There were significantly lower weed densities in the third planting date of Imazethapyr treatment in cvs. COS16 and Talash, and the second planting date of Trifluralin-Talash at maturity stage ().
Figure 1. Mean weed density in bean cultivars (Talash and COS16) planted at different planting dates under different herbicide applications at seedling (LSD = 51.1/2014 & 154.4/2015), flowering (LSD = 29.2/2014 & 130.2/2015) and maturity (LSD = 22.6/2014 & 47.9/2015) stages; D1–D4 refer to planting dates: 10–15 May, 26–31 May, 10–15 June, 25–30 June; Im, Tr and Co refer to Imazethapyr, Trifluralin, Control, respectively.
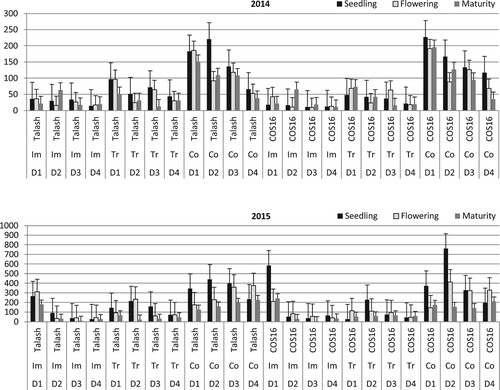
Table 1. Weed species were identified in 2-year study of weed density, fly infestation, Rhizoctonia root rot and productivity in bean cultivars planted at different planting dates under different herbicide applications.
Table 2. Analysis of variance for weed density affected by bean cultivar, herbicide application and planting date factors.
The ANOVA results demonstrated that the planting date affected the incidence of Rhizoctonia root rot at all growth stages and years studied (). The highest mean Rhizoctonia root rot incidence at the seedling stage in 2014 was detected on the first planting date of the Imazethapyr-Talash treatment, followed by the first and fourth planting dates of the Trifluralin-Talash treatment (). At the flowering stage in 2014, there were significantly greater mean disease incidence values for the first planting date of all the four herbicides and two bean cultivars tested when compared with the fourth planting date studied. Furthermore, the disease levels at this stage were often significantly greater on the second and third planting dates than the fourth date. Postponing the date of planting from the first date to the fourth one decreased the mean disease incidence up to 93% at flowering in 2014. At the podding stage in 2014, the highest mean disease incidence was recorded for the third planting date of hand-weeding-COS16, followed by the fourth and third planting dates of control-Talash and Trifluralin-COS16 treatments, respectively. In 2015 at the seedling stage, the highest disease incidence was detected on the first planting date of Imazethapyr-Talash treatment, followed by the third planting date of hand-weeding-Talash and Trifluralin-COS16 treatments. The lack of disease at seedling (2015) was observed in the fourth planting dates of hand-weeding-Talash, control-Talash, Imazethapyr-COS16 and control-COS16 treatments. At the flowering stage in 2015, the disease incidence was often decreased in all the treatments studied as the planting date was postponed. At podding in 2015, there were often significantly lower mean disease incidence values in the third–fourth planting dates than the first–second dates examined. At maturity in 2015, a significantly greater mean disease incidence was often observed in the third planting date than on the other dates of planting studied. Postponing the date of planting from the third date to the fourth one decreased the mean disease incidence by 52% and 75% in the control and hand-weeding treatments of cv. Talash was examined at the maturity stage in 2015 ().
Figure 2. Mean Rhizoctonia root rot incidence in bean cultivars (Talash and COS16) planted at different planting dates under different herbicide applications at seedling (LSD = 11.6/2014 & 12.3/2015), flowering (LSD = 24.8/2014 & 12.9/2015), podding (LSD = 28.2/2014 & 16.3/2015) and maturity (LSD = 20.2/2015) stages; D1–D4 refer to planting dates: 10–15 May, 26–31 May, 10–15 June, 25–30 June; Im, Tr and Co refer to Imazethapyr, Trifluralin, Control, respectively.
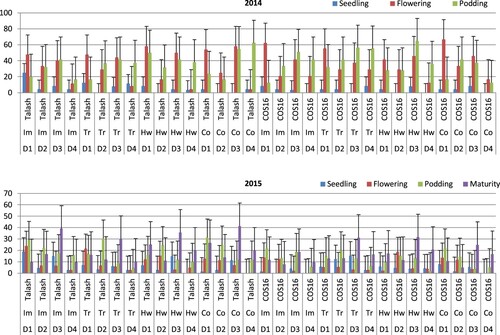
Table 3. Analysis of variance for Rhizoctonia root rot incidence affected by bean cultivar, herbicide application and planting date factors.
According to the ANOVA results, bean cultivar, herbicide application and planting date affected the rating of fly-infested plants (). In 2014 at the seedling stage, the highest mean fly infestation rating was determined for the third planting date of the Trifluralin-Talash treatment, followed by the hand-weeding-Talash treatment (). At the flowering stage in 2014, there were significantly greater mean fly infestation ratings at the third planting date of the Imazethapyr-Talash and Trifluralin-Talash treatments compared to those treatments planted at the first and second dates studied. At the podding stage in 2014, there were significantly greater mean fly infestation ratings at the third planting date of the Imazethapyr-COS16, hand-weeding-COS16, control-Talash and Trifluralin-Talash treatments in comparison with the treatments planted at the other dates tested. The fly infestation was lacking in beans planted at the second date of cultivar and herbicide treatments examined in 2014. In 2015 at the seedling stage, the highest mean fly infestation rating was detected at the fourth planting date of the hand-weeding-COS16 treatment, followed by the first planting date of Trifluralin-COS16 and the fourth date of control-Talash, hand-weeding-Talash, Imazethapyr-Talash, Trifluralin-COS16 and Imazethapyr-COS16 treatments. In 2015 at the flowering stage, mean fly infestation ratings were significantly greater at the fourth planting date of the hand-weeding-COS16, control-Talash, Imazethapyr-Talash, hand-weeding-Talash, Trifluralin-COS16 and Imazethapyr-COS16 treatments. At podding in 2015, the highest mean fly infestation was obtained for the second date of Imazethapyr-Talash, followed by the third planting date of control-COS16. At maturity (2015), mean values for fly infestation rating were significantly greater at the second planting date of hand-weeding-COS16 and the fourth planting date of Trifluralin-Talash treatment ().
Figure 3. Mean fly-infested plants in bean cultivars (Talash and COS16) planted at different planting dates under different herbicide applications at seedling (LSD = 13.3/2014 & 8.3/2015), flowering (LSD = 8.8/2014 & 6.4/2015), podding (LSD = 7.4/2014 & 7.2/2015) and maturity (LSD = 3.5/2015) stages; D1–D4 refer to planting dates: 10–15 May, 26–31 May, 10–15 June, 25–30 June; Im, Tr and Co refer to Imazethapyr, Trifluralin, Control, respectively.
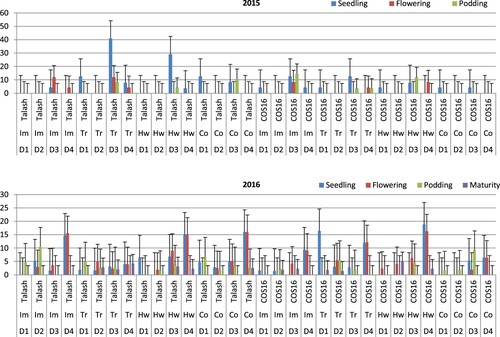
Table 4. Analysis of variance for fly-infested plants affected by bean cultivar, herbicide application and planting date factors.
According to ANOVA results, the tree-way interaction of bean cultivar, herbicide application and planting date affected the plant dry matter over the two growing seasons (). In 2014, the highest mean dry matter at the flowering stage was determined for the fourth planting date of the Trifluralin-COS16 treatment, followed by the hand-weeding-COS16 and Imazethapyr-COS16 planted at the fourth date tested (). Whereas the lowest mean dry matter values at the flowering stage in 2014 were obtained for the fourth planting dates of Imazethapyr, Trifluralin, hand-weeding and control of cv. Talash. At the maturity stage of bean growth in 2014, there were significantly greater mean dry matter values for the first planting dates of hand-weeding, Imazethapyr and Trifluralin treatments in cv. Talash. The lowest mean dry matter values at the maturity stage in 2014 were obtained for the first and second planting dates of the Trifluralin treatment and control in cv. COS16, respectively. In 2015 at both of the one- and three-leaflet seedling stages, there were significantly greater mean dry matter values for the fourth planting dates than the other three dates studied for all the four herbicide applications and two cultivars. In 2015 at the flowering stage, the highest mean dry matter was determined at the fourth planting date for the Imazethapyr-Talash treatment, followed by the Trifluralin-Talash and hand-weeding-COS16 treatments. Postponing the date of planting from the first date to the fourth one increased the mean plant dry matter for the Imazethapyr-Talash treatment by 162% at the flowering stage in 2015 ().
Figure 4 Mean plant dry matter in bean cultivars (Talash and COS16) planted at different planting dates under different herbicide applications at the first leaflet (LSD = 3.8/2014 & 7.6/2015), third leaflet (LSD = 10.7/2014 & 26.6/2015), flowering (LSD = 38.8/2014 & 35.8/2015) and maturity (LSD = 152.8/2014) stages; D1-D4 refer to planting dates: 10–15 May, 26–31 May, 10–15 June, 25–30 June; Im, Tr and Co refer to Imazethapyr, Trifluralin, Control, respectively.
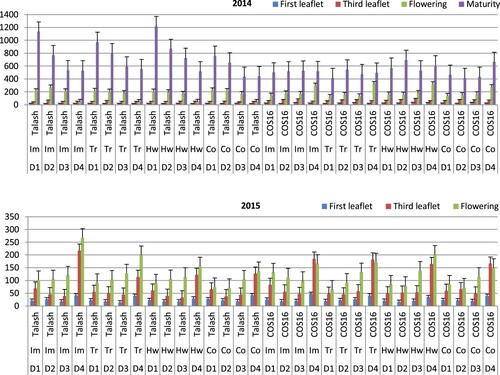
Table 5. Analysis of variance for plant dry matter (g/m2) affected by bean cultivar, herbicide application and planting date factors.
The number of pods per plant and seeds per pod was affected by the cultivar, herbicide application and planting date factors studied during the two growing seasons (). In 2014, the highest and lowest mean numbers of pods per plant were recorded for the third planting date of Trifluralin-COS16 and the first date of control-COS16 treatments, respectively (). Excepting the controls, the mean number of pods per plant at the fourth planting date was significantly lower than either the first, second or third dates of herbicide and cultivar treatments studied in 2014. The highest mean seed number per pod in 2014 was detected for the third planting date of Imazethapyr-Talash treatment, followed by the third-date plantings of Trifluralin and hand-weeding and the first-date planting of Imazethapyr for cv. Talash. In 2015, the highest pod number per plant was observed at the first planting date of hand-weeding-Talash, followed by the first and fourth planting dates of Imazethapyr-Talash. The lowest pod number per plant in 2015 was detected for cv. COS16 at the third planting date of control and Imazethapyr treatment. In 2015, the highest and lowest mean seed numbers per pod were recorded for the second-date-hand-weeding and first-date-control of cv. COS16 ().
Figure 5. Mean pod (LSD = 1.5/2014 & 2.9/2015) and seed (LSD = 0.9/2014 & 0.7/2015) numbers per plant and pod, respectively, in bean cultivars (Talash and COS16) planted at different planting dates under different herbicide applications; D1–D4 refer to planting dates: 10–15 May, 26–31 May, 10–15 June, 25–30 June; Im, Tr and Co refer to Imazethapyr, Trifluralin, Control, respectively.
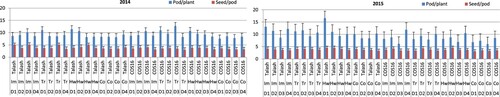
Table 6. Analysis of variance for pod and seed numbers per plant and pod, respectively, affected by bean cultivar, herbicide application and planting date factors.
Discussion
Sustainability in bean production appears to be dependent on developing integrated environmentally friendly crop management programs (Naseri Citation2019). Therefore, attempts were made to evaluate the effectiveness of planting date on chemical weed control, bean fly infestation, Rhizoctonia root rot and productivity at an experimental-plot scale. The present research demonstrated that weed density is reduced by delaying the plantation of bean cultivars in control plots over the two growing seasons. Furthermore, there were similar or lower weed populations in the later planted control (neither herbicide application nor hand-weeding) plots compared to the earlier planted herbicide treatments. Improved bean competitiveness via later planting not only enhanced the efficiency of herbicides but also reduced weed density nearly as effective as herbicides applied to early plantings in the current study. Despite much research on chemical weed management in bean cropping systems (Oveisi et al. Citation2021), the comparison of planting date and herbicides for weed control efficiency is little understood. In Italy, weed infestation reduced bean pods up to 60% (in 2004), 65% (in 2005) and 59% (in 2006) of the weed-free plots, suggesting that the French bean crop in such Mediterranean areas must be kept weed-free between 11 and 29 days after emergence for yield losses lower than 5% (Stagnari and Pisante Citation2011). Although the date of planting corresponds to the critical period for weed competition in bean, there are several controversial reports on the association of planting date with weed populations in bean fields (Esmaeilzadeh and Aminpanah Citation2015; Byiringiro et al. Citation2017). To the best of our knowledge, this field-scale finding is the first report of either similar or better efficiency of later plantings to herbicides in weed control and bean productivity under environmental conditions encountered in this study. This finding is highly valuable for bean experts and producers to replace the application of herbicides with a properly-timed planting in sustainable bean cropping systems.
According to the present findings, Rhizoctonia root rot incidence at seedling and flowering stages of bean growth was lower in the late-planted plots than in early-planted ones. This is in agreement with previous documents from Iran (Naseri Citation2013b, Citation2013c) reporting that later sowing of bean crops restricted Rhizoctonia root rot according to experimental plot and regional-scale findings. This observation was attributed to a faster emergence of late-planted bean seedlings in warmer soils (Abawi Citation1989) and thus, lower root rots and crop damage compared with earlier plantings (Naseri Citation2013b, Citation2013c). However, a reverse trend of disease levels in interaction with the planting date was observed at podding and maturity stages, indicating greater disease levels at podding-maturity in later plantings than earlier ones. The mid-summer hot and dry soil stresses might be partially responsible for such root rot increases, as warmth and drought stresses have been linked to Rhizoctonia root rot intensity (Allen and Lenné Citation1997; Haddoudi et al. Citation2021).
It was highly desired by bean experts and producers to know the effectiveness of later planting on bean fly infestation and weed population for sustainable bean production purposes. Besides the above-mentioned promising outcomes of late-planted beans on root rot and weed control, the current study suggested similar or higher dry matter, pod/plant and seed/pod production in the late-planted control (neither herbicide application nor hand-weeding) plots than in the earlier herbicide-treated plantings. From a bean-fly-infestation viewpoint, this pest-infested bean plants more severely at the seedling and flowering stages and declined as the growth season progressed in both study years. Furthermore, there were greater infestations of bean roots with this pest in later planted plots, with or without herbicide application. Sariah and Makundi (Citation2007) also reported a higher fly infestation in late-planted beans in northern Tanzania, suggesting a peak-fly-population escape of the most vulnerable growth stage of early sown beans. These Iranian and Tanzanian findings are in agreement with the previous report from Van der Goot (Citation1930) on greater mortality of fly-infested beans sown lately in Java. In Tanzania, chemical seed dressing reduced pest infestation during the early stages of bean growth, while mulch and fertilizer improved plant survival (Ampofo and Massomo Citation1998). Ojwang et al. (Citation2010) reported higher resistance of 64 bean genotypes to natural bean fly infestation under long rainfalls and subsequent better performance of beans in Kenya, 2008–2009. Considering late-planted beans with lower levels of Rhizoctonia root rot (Naseri Citation2013b, Citation2013c) and weed populations based on the current findings, a later planting appears to benefit the sustainable management of bean farming systems. Similar or greater productivity in late-planted plots studied over the two growing seasons demonstrated an improved performance of later sown beans in warmer soils presumably due to reduced weed and early-season root rot, regardless of increased fly infestation and late-season root rot. This confirms the previous large-scale finding on the lack of significant association between bean fly, Rhizoctonia root rot and yield at large scale (Naseri Citation2013a). Whereas Rhizoctonia root rot incidence and weed density significantly affected bean production in Iranian commercial bean fields (Naseri and Veisi Citation2019).
In conclusion, it is wise to recommend late planting of bean for getting high production because of having less Rhizoctonia root rot infection and weed infestation. This agronomic practice appears a potential bean management tool to minimizing herbicide and fungicide applications by bean growers. Although this work ignored increases in bean fly infestation in later plantings, the effectiveness of late planting on fly-root-rot-weed warrants further examination in different geographical areas.
Ethical statement
The research presented in this manuscript did not involve any animal or human participants.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Authors’ contributions
Nazer Kakhki SH: Designing and performing the work, Taghaddosi MV: data collecting, Moini MR: data collecting, Veisi M: assistance with revising the manuscript, Naseri B: interpreting the data and writing the paper.
Data availability
The data that support the findings of this study are openly available in figshare at http://doi.org/10.6084/m9.figshare.19728145.
Additional information
Funding
References
- Abawi GS. 1989. Root rot. In: Schwartz HF, Pastor Corrales MA, editor. Bean production problems: disease, insect, soil and climatic constraints of Phaseolus vulgaris. Cali: Centro Internacional de Agricultura Tropical; p. 105–157.
- Allen DJ, Lenné JM. 1997. The pathology of food and pasture legumes. Cambridge: CAB International.
- Ampofo JKO, Massomo SM. 1998. Some cultural strategies for management of bean stem maggot (Diptera: Agromyzidae) on beans in Tanzania. African Crop Science Journal. 6:351–356.
- Anonymous. 2020. Agricultural production report. Tehran, Iran: The Iranian Ministry of Agriculture.
- Byiringiro B, Birungi S, Musoni A, Mashingaidze AB. 2017. The effect of planting date on weed density, biomass and seed yield in common bean (Phaseolus vulgaris L.) in the semi-arid region of Nyagatare, Rwanda. Tropical Agriculture (Trinidad). 94:335–345.
- Dehghani A, Panjehkeh N, Asadi Rahmani H, Salari M, Darvishnia M. 2018. Effectiveness of simultaneous application of indigenous rhizobium and arbuscular mycorrhiza on root rot disease and yield of red bean (Phaseolus vulgaris L.) in Lorestan Province. Biocontrol in Plant Protection. 6:43–58.
- Esmaeilzadeh S, Aminpanah H. 2015. Effects of planting date and spatial arrangement on common bean (Phaseolus vulgaris) yield under weed-free and weedy conditions. Planta Daninha. 33:425–432.
- Greathead DJ. 1968. A study in East Africa of the bean flies (Diptera: Agromyzidae) affecting Phaseolus vulgaris and their natural enemies, with the description of a new species of Melana gromyza Hend. Bulletin Entomological Research. 59:541–561.
- Gupta SK, Singh G. 2021. Major fungal French bean diseases: epidemiology and management. In: Singh KP, Jahagirdar S, Sarma BK, editor. Emerging trends in plant pathology, 141–174. Singapore: Springer; doi:10.1007/978-981-15-6275-4_7.
- Haddoudi I, Mhadhbi H, Gargouri M, Barhoumi F, Romdhane SB, Mrabet M. 2021. Occurrence of fungal diseases in faba bean (Vicia faba L.) under salt and drought stress. Eur J Plant Pathol. 159:385–398.
- Kalantari S, Marefat AR, Naseri B, Hemmati R. 2018. Improvement of bean yield and Fusarium root rot biocontrol using mixtures of Bacillus, Pseudomonas and Rhizobium. Tropical Plant Pathology. 43:499–505.
- Medvecky BA, Ketterings QM, Nelson EB. 2007. Relationships among soilborne bean seedling diseases, Lablab purpureus L. and maize stover residue management, bean insect pests, and soil characteristics in trans Nzoia district, Kenya. Appl Soil Ecol. 35:107–119.
- Naseri B. 2013a. Epidemics of Rhizoctonia root rot in association with biological and physicochemical properties of field soil in bean crops. Journal of Phytopathology. 161:397–404.
- Naseri B. 2013b. Interpretation of variety × sowing date × sowing depth interaction for bean–fusarium–rhizoctonia pathosystem. Archives of Phytopathology and Plant Protection. 46:2244–2252.
- Naseri B. 2013c. Linkages of farmers’ operations with Rhizoctonia root rot spread in bean crops on a regional basis. Journal of Phytopathology. 161:814–822.
- Naseri B. 2019. The potential of agro-ecological properties in fulfilling the promise of organic farming: a case study of bean root rots and yields in Iran. In: Unni MR, Sarathchandran Veloormadom C, Sabu Thomas, editor. Organic farming, 361–389. Singapore: Singapore: Woodhead Publishing.
- Naseri B, Hemmati R. 2017. Bean root rot management: recommendations based on an integrated approach for plant disease control. Rhizosphere. 4:48–53.
- Naseri B, Moradi P. 2015. Farm management strategies and the prevalence of Rhizoctonia root rot in bean. Journal of Plant Diseases and Protection. 5:238–243.
- Naseri B, Veisi M. 2019. How variable characteristics of bean cropping systems affect Fusarium and Rhizoctonia root rot epidemics? Archives of Phytopathology & Plant Protection. 52:30–44.
- Ojwang PPO, Melis R, Songa JM, Githiri M. 2010. Genotypic response of common bean to natural field populations of bean fly (Ophiomyia phaseoli) under diverse environmental conditions. Field Crops Res. 117:139–145.
- Oveisi M, Pourmorad Kaleibar B, Rahimian Mashhadi H, Müller-Schärer H, Bagheri A, Amani M, Elahinejad M, Masoumi D. 2021. Bean cultivar mixture allows reduced herbicide dose while maintaining high yield: A step towards more eco-friendly weed management. Eur J Agron. 122:126173.
- Sariah JB, Makundi RH. 2007. Effect of sowing time on infestation of beans (Phaseolus vulgaris L.) by two species of the bean stem maggot, Ophiomyia spencerella and Ophiomyia phaseoli (Diptera: Agromyzidae). Archives of Phytopathology and Plant Protection. 40:45–51.
- Stagnari F, Pisante M. 2011. The critical period for weed competition in French bean (Phaseolus vulgaris L.) in Mediterranean areas. Crop Prot. 30:179–184.
- Talekar NS. 1992. The bean fly pest complex of snap beans in the tropics. In: Henry G, Janseen W, editor. Cartillas para CIAL Los comités de investigación Agrícola local cartilo. Cali: CIAT; p. 2.
- Van der Goot P. 1930. Agromyzid flies of some native legume crops in Java. Shanhua, Taiwan: Asian Vegetable Research and Development Center (AVRDC); p. 98.