Abstract
A novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged in the sprawling capital of Central China’s Hubei province Wuhan in 2019. Since late 2019, SARS-CoV-2 has been responsible for a global pandemic that has resulted in 405,961,201 confirmed cases of infection and 5,789,567 deaths as of February 11, 2022, according to the World Health Organization (WHO).
Here we report the development of an inactivated SARS-CoV-2 vaccine candidate and show that its efficacy and safety in preclinical studies warrant further clinical evaluation. Efficiency and possible side effects of vaccine candidate on different doses were evaluated using histopathology, K18 hACE2 transgenic mice challenge, T-cell response, and serum neutralization test in Balb/c mice. All pilot production and propagation experiments of SARS-CoV-2 were performed in the Level 3 Negative Pressure Biosafety Facility (BSL3/ABSL-3) according to COVID-19 interim guidance of WHO.
All analytical studies have shown that the quality control parameters of the vaccine candidate meet the requirements for inactivated viral vaccines according to European Pharmacopeia. The vaccine candidate (KOCAK-19) has been authorized by the Turkish Medicines and Medical Devices Agency and has now entered phase 2 clinical development (NCT04838080).
Abbreviations: SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2.; MERS-CoV: Middle East Respiratory Syndrome Coronavirus.; qRT-PCR: Quantitative Real-Time Polymerase Chain Reaction.; TEM: Transmissible Electron Microscopy.; SEC: Size Exclusion.; AEX: Anion exchange.; BPL: β-Propiolactone.; CPE: Cytopathic Effect.; TCID50: 50% Tissue Culture Infectious Dose.; hACE-2: Human Angiotensin-Converting Enzyme 2.; mRNA: Messenger RNA.; RBD: Receptor binding domain.; Nab: Neutralizing antibody.; MOI: Multiply of Infection.
HIGHLIGHTS
A new inactivated vaccine candidate to protect against COVID −19
SARS-CoV-2 inactive vaccine development in disposable wave bioreactor systems with microcarrier technologies and analytical analysis
Preclinical toxicity and immunity trials for COVID-19 inactive vaccine candidate in Balb/c mice, K18 transgenic mice and, ferrets.
Introduction
The new coronavirus, SARS-CoV-2, the causative agent of 2019 coronavirus disease (COVID-19), belongs to the genus Beta coronaviruses. This genus also includes SARS-CoV and MERS-CoV (Gao et al. Citation2020). SARS-CoV-2 is closely related to severe acute respiratory syndrome coronavirus (SARS-CoV) and several bat coronaviruses. SARS-CoV-2 has a linear single-stranded positive-sense RNA genome of 30 kb in length encoding four structural proteins (spike (S), envelope (E), membrane (M), and nucleocapsid (N)), sixteen non-structural proteins, and several accessory proteins. Spike (S) is a binding domain and structural protein that elicits highly potent neutralising antibodies (Nabs). SARS-CoV-2 binds to ACE2 receptors via the spike protein's RBD (Receptor Binding Domain) to initiate membrane fusion and enter human cells. Some critical amino acid residues (AA) in the RBD were different between SARS-CoV-2 and SARS-CoV (Gu et al. (Citation2020 Sep 25); Tang et al. (Citation2020); Lu et al. (Citation2020); Andersen et al. (Citation2020)).
Neutralizing antibodies (Nabs) are key components of the protective immune response to viral infections, as they can bind to and block viral particles and help to serve APC (antigen-presenting cells) (Spruth et al. (Citation2006); Gao et al. (Citation2020 Jul 3); Wang et al. (Citation2020)).
Since the beginning of the epidemic, many variant strains of the SARS-Cov-2 virus have been found around the world. Most of these variant strains have observed mutations caused by a change in the surface protein (Spike). Variants with spike mutations have been found in many countries, especially in America, England, and South Africa. Variants B.1.1.7, B.1.351, B.1.617.2 and P.1 have been reported worldwide as being of particular concern. Variants B.1.1.7 and B.1.351 were first reported in the UK and South Africa. Although the B.1.351 variant contains several spike protein mutations, these mutations did not have a serious impact on the spread and severity of the disease. The Delta variant (B.1.617.2) was spread around the world by mid-2021, becoming the main variant. As of December 2021, the Omicron variant has been identified in at least 27 countries worldwide. Omicron is the most recently detected variant strain, and at least 50 mutations were detected in the spike protein compared to the reference strain. Of these mutations, 26 were found to be completely unique and were observed in addition to mutations in Delta and Beta. It has been observed that its transmissibility is high, but its mortality is low so far at this time. (Kumar et al. (Citation2021); Lou et al. (Citation2021)).
More than 300 vaccines of different technologies are currently in development, many of which have been approved for emergency use in countries. The first vaccines approved by the Food and Drug Administration (FDA) for use in the mass vaccination program against COVID-19 disease are Pfizer/BioNTech, Moderna COVID-19, and Janssen Ad26.COV2 S vaccines. The European Commission has granted widespread use permission for Novavax's vaccine. CoronaVac has received conditional approval for emergency use in China and overseas, such as in Brazil, Chile, Colombia, and Uruguay. BBIBP-CorV is already licenced for usage in China, the United Arab Emirates, and Bahrain, and is allowed for emergency use in a number of other countries. India has also approved Covaxin for emergency use only (Kumar et al. (Citation2021); Mohamed et al. (Citation2021)),(COVID-Citation19 Vaccines Authorized for Emergency Use,(Citation2019); Amanat et al. (Citation2021)).
Material method
Development and preparation of KOCAK-19 vaccine candidate
A SARS-CoV-2 strain (Vial no.31242/12.05.2020) isolated from bronchoalveolar lavage specimens or throat swabs from a hospitalized patient in the recent COVID −19 outbreaks by the Turkish Ministry of Health was used to develop a candidate vaccine. This strain was isolated from Vero CCL-81 cells, which are approved by WHO as a cell line to produce inactivated vaccines for human use. This primary seed was further amplified to create a master seed virus bank, a working virus bank, and a production virus bank in the Class III isolator cabinet in the BSL-3 plant.
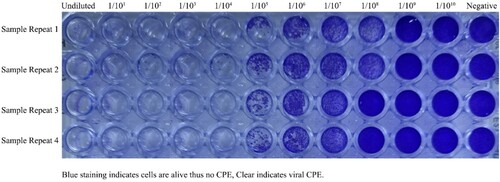
Highly efficient, propagation and high genetic stability are key characteristics of the development of an inactivated vaccine. It was found that the strain showed optimal replication and generated high virus yields on Green Monkey Kidney Cells (Vero CCL-81, ATCC) were cultured in Eagle’s modified Essential medium (EMEM) (Sigma, Germany) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Sigma, Germany) and L-glutamine. In the present study, the strain showed optimal replication and produced high virus yields. Here, we set out to determine the most appropriate times post-infection to harvest. We determined that the titers depend on the cytopathic effect (CPE) caused by SARS-CoV-2 in Vero cells is most apparent at 48 h post-infection. The highest titer is at 62 h post-infection by crystal violet (0.1% w/v) (Sigma-Aldrich) staining (Figure ).
Figure 1. Viral CPE (Vero cells seeded at 5 × 104 cells/well with SARS-CoV-2 5th passage log10 dilutions). Each of the samples was performed in the well-plate at four repeats. After 4 days in culture, 96 well plates were fixed and stained with formaldehyde and crystal violet, respectively. Plates were washed in water, dried, and scanned. The end-point titers (TCID50) were calculated according to the Reed & Muench method based on four replicates for titration
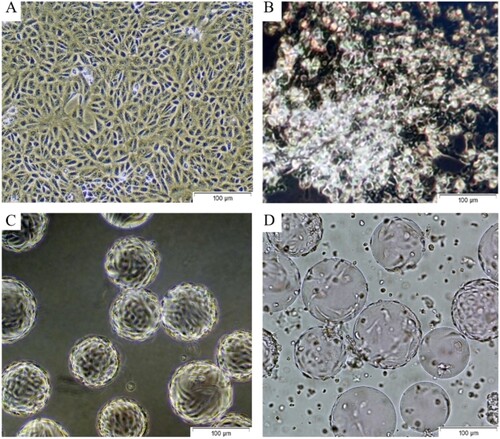
Initially, Vero cells CCL-81 were seeded in T-flasks (NEST, USA) at 2.105 cm2 and incubated in a humidified incubator at 37 °C with 5% CO2. Four days after seeding, confluent Vero cells (Figure A) were trypsinized at a ratio of 0.25% trypsin-EDTA (Sigma, USA) and transferred to the 3.165 cm2 BioFactory Chamber (NEST, USA). In addition, two T-175 flasks were infected with SARS-CoV-2 virus seed to produce a bioreactor inoculum. Infected Vero cells show typical CPE under the inverted light microscope at a magnification of 20 x (Nikon Eclipse Ti2) (Figure B). After the 4th day of incubation, the titer was determined by microtitration assay. Four days after the cells were seeded in the BioFactory chamber, they were passaged onto a microcarrier system (3–5 mg/L Cytodex-1, Cytiva, USA) in a 20-liter single-use bag bioreactor (Biostat RM20, Sartorius, Germany) (Figure C). Vero cells were cultured in a 20-liter bioreactor at a temperature of 37 ± 1°C. The pH of 7.2 was regulated by CO2 and the addition of NaHCO3 at 88 g/L, and dissolved oxygen was adjusted to 35-98% air saturation by continuous surface aeration. To produce the SARS-CoV-2 virus, the virus seeds were inoculated at a ratio of 0.05 MOI after washing the cells with DPBS (Sigma Aldrich, USA) solution, the pH was 94 maintained at 6.8 - 7.1, the pO2 at 35–98% air saturation, and the temperature at 37°C. The rotation speed was 12 - 18 rpm and the angle was 6°−7° depending on the different cell growing and viral incubation stages. Growth kinetic analysis of the 5th passage (P5) of the virus (Working Seed Lot) in Vero cells showed that the strain virus could replicate efficiently and reached a peak titer of 3.6 × 106 PFU after 56 - 72 h at infection multiplicities (MOI) of 0.05 (Gao et al. (Citation2020 Jul 3)). The virus mass was harvested at 62 h (Figure D) after the inoculation and then clarified using 50 µm PES (Polyethersulphone) and 0.45 µm PES filter. Clarified harvest was inactivated with β-propiolactone (Sigma, USA) at a 1:1500 ratio at 2 - 6°C for 16 - 20 h, followed by benzonase-endonuclease (Merck, USA) treatment at room temperature, concentration by 100 - 300 kDa molecular weight ultrafiltration cassettes, and multimodal chromatographic (SEC and AEX) purification. During the inactivation process, the pH was maintained at 6.8-7.1. The final mass was prepared by adding 0.05% (v/v) Alhydrogel (Croda, Denmark) as adjuvant and dilution buffer containing Phosphate Buffer Saline (1x pH 7.2 PBS, Sigma Aldrich) with stirring at 20 - 30 rpm at 25 °C for 2 h (Jureka and Silvas (Citation2020)).
Figure 2. Propagation of SARS-CoV-2 in VERO CCL-81 cell culture. A. Healthy and confluent Vero cells in T-175 Flasks. B. 62. h after the inoculation of SARS-CoV-2 in T-175 Flasks. C. Healthy and confluent Vero cells on Cytodex-1 microcarriers in Biostat RM 20L Bioreactor. D. 62. h after the inoculation of SARS-CoV-2 on Cytodex-1 microcarriers in Biostat RM 20L Bioreactor.
Since safety and efficiency are critical for vaccine development, all sterility, extraneous agents tests, Mycoplasma sp., and endotoxin analyses were performed according to European Pharmacopeia and acceptable levels were found. However, the Vero cell bank and virus seed lots were found to be free of Mycoplasma by culture and NAT (Nucleic Acid Amplification Technique) according to European Pharmacopoeia. Mycoplasma NAT test is based on a nucleic acid amplification technique using a very sensitive touch-down PCR profile. The presence of Mycoplasmas’ nucleic acids in the test sample is determined by approximately 450 bp of the target sequence was amplified and this amplicon is finally detected by polyacrylamide gel electrophoresis. No mycoplasma species’ DNA was detected using specific primers. Bacterial endotoxin levels were measured by the gel-clot technique using amoebocyte lysate (LAL) from the horseshoe crab. Our endotoxin levels always are detected below 12.5 IU/dose compared to standards in the final product.
In the present study, a purified inactivated SARS-CoV-2 virus vaccine candidate was prepared at a pilot scale to induce specific neutralizing antibodies in 6–8 week old Balb/c mice. Five immunization doses (1, 4, 6, 10 µg per dose, and placebo) mixed with 0.05% (v/v) Alhydrogel adjuvant was administered to five groups of mice (n = 10). Partial and complete protection against challenge by observing an increase in serum neutralizing antibody levels pneumonia symptoms, and histopathological examination of the lungs. In transgenic mice, we observed protection against challenge and viral loads in the tissues (Spruth et al. (Citation2006)), (Gao et al. (Citation2020 Jul)).
Virus titration by plaque assay and microtitration assay
SARS-CoV-2 viral titers were determined using a plaque assay. Serial 10-fold dilutions of virus-containing samples were inoculated into 12-well culture plates seeded with confluent Vero cells. 1 h later, the inoculums were removed and then covered with 0.4% agarose MEM. After 3 days of culture in 5% CO2 incubators at 37 °C, the cells were fixed with 10% neutralized formalin for at least 1 h at room temperature. After the fixation with neutralized formalin, the agarose layer was removed with pipette tips and stained with crystal violet solution in methanol (0.4% v/v). After the washing process with pure water, the wells were observed for plaque formation and the titer was calculated as Plaque Forming Unit (PFU/ml) (Jureka and Silvas (Citation2020)).
Virus titers were also determined using microtitration assay and samples were serially diluted (from 1 log to 10 logs) in 96-well, clear bottom plates containing confluent monolayers of Vero cells. For each dilution, a total of four replicate wells were infected. The infected plates were incubated at 37°C and 5% CO2 in a humidified incubator (Inforce HT, Switzerland) for 4 days, and then individual wells were visually inspected for the presence of virus-induced cytopathic effects (CPE) with crystal violet (0.1% w/v) (Sigma-Aldrich) staining macroscopic observation at each dilution as compared a negative control. The end-point titers(TCID50) were calculated according to the Reed & Muench method based on four replicates for titration Kärber (Citation1931; Manenti et al. Citation2020).
Validation of the inactivation
β-Propiolactone (BPL) modifies the structure of nucleic acids of the viruses after reaction with purine residues to prevent the replication of the virus. BPL is widely used for inactivation viruses to produce inactivated viral vaccines (Morgeaux (Citation1995)). SARS-CoV-2 with BPL at a ratio of 1:1500 for 16–20 h at 6 °C followed by degradation of BPL 2 h at 37 °C was found effective inactivation of the SARS-CoV-2 which was validated in a sample from each batch of the COVID-19 vaccine candidate. 5 ml of inactivated SARS-CoV-2 was used to inoculate confluent Vero CCL-81 cell monolayers in 25 cm2 flasks, and negative control cells were prepared; the cells were then cultured at 37°C and 5% CO2 in an 80% humidified incubator for 4 days. Then, 5 ml supernatant of cells in the flask was inoculated onto two more Vero cell monolayers in 25 cm2 flasks (5 ml each) at the same incubation conditions. To pass the inactivation test, CPE must not be seen after 3 consecutive passages for the inactive bulk (Jureka and Silvas (Citation2020)).
Transmission electron microscopy (TEM) sample preparation
Inactivated purified bulk samples and SARS-CoV-2 infected and gluteraldehit fixed Vero cells were mounted on pyeloform-coated nickel grids, stained with 2% uranyl acetate, washed with sterile pure water, and then visualized in a transmission electron microscope (Jeol, Tokyo, Japan) (Kumar et al. (Citation2021)). A specialized histologist and a virologist made the observations.
Nucleic acid extraction and first-strand cDNA synthesis
Viral supernatants from viral seed lots were clarified by centrifugation and 0.45 µm filtration. All nucleic acids were extracted from clarified viral supernatants using the Mini Pathogen Nucleic Acid Kit (QIAGEN) according to the manufacturer's instructions. The viral RNA was reverse transcribed using the Moloney murine leukemia virus reverse transcriptase (Thermo Scientific, USA) and random hexamers according to the manufacturer’s recommendations. The reaction mixtures were incubated for 60 min at 42˚C, heated at 95˚C for 5 min and then taken on the ice.
Sequencing
For whole-genome sequencing of SARS-CoV-2 samples, Quant-it RNA HS Assay kit (Invitrogen, USA) and Qubit fluorometer were used for measurement of isolated RNA samples. Library preparation was performed using the CleanPlex® SARS-CoV-2 Panel (Paragon Genomics) with isolated RNA samples following the kit's recommended instructions. Libraries were sequenced on the Illumina NextSeq (Illumina, USA) platform with a 2x loop kit with an average of 500,000 reads. The quality of raw data was examined with FastQC v.0.11.5 and low-quality bases and primers were trimmed using Trimmomatic v.0.32. Reads were aligned to the known SARS-CoV-2 genome (GenBank Access: MN908947.3) using the Burrows–Wheeler aligner v.0.7.1. Variants were detected using the Genome Analysis Toolkit -HaplotypeCaller (GATK) v.3.8.0 and analyzed on GenomeBrowse v2.1.2 (GoldenHelix). The genome sequences of the first viral sample were obtained and the genome sequence of the last sample was aligned using BLAST+ (Pavel et al. (Citation2020)).
Quantitative real-time pcr
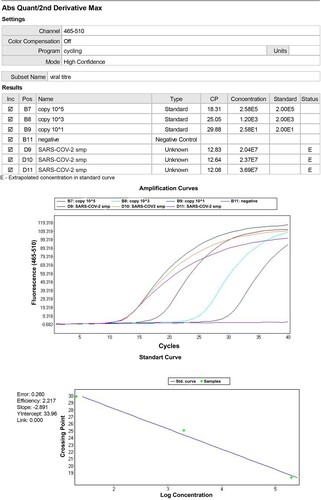
A commercial one-step RT–PCR Master Mix (Primer design, USA) was used for the polymerase chain reaction. The Primer design 2019-nCoV genesig® Advanced Kit is a Vitro diagnostic real-time reverse transcriptase PCR (RT–PCR) assay developed for the quantitative detection of nucleic acid from SARS-CoV-2. The assay utilizes real-time technology targeting the RNA-dependent RNA polymerase (RdRp). Each 20-µL reaction contained 10 µL 2X Master Mix (Genesig/primer design, USA), 2 µL 5 µmol/L probes, 20 µmol/L forward and reverse primer mix, 5 µL nuclease-free water, and 5 µL nucleic acid extract. We performed amplification in 96-well non-transparent plates on a LightCycler 480 Real-Time PCR instrument (ROCHE, Germany). Real-time PCR conditions RT consisted of 10 min at 55°C for reverse transcription (RT) reaction, 2 min at 95°C for Taq polymerase enzyme activation, and 40 cycles of 3 s at 95°C and 30 s at 55°C in the polymerase chain reaction. Viral load (copy number/µl) is calculated depending on the standard curve (Dhama et al. (Citation2020)).
SDS-PAGE and WESTERN BLOTTING
25 µl β-Propiolactone inactivated and purified SARS-CoV-2 final vaccine bulk was separated using the 10% polyacrylamide gels described by Laemmli and visualized using the Commosie Blue staining kit (BIO-RAD). Another SDS -PAGE (Bio-Rad TGX Mini, Bio-Rad, Hercules, CA, USA) gel was transferred to a 0.2 µM PVDF membrane according to the manufacturer's protocols (Bio-Rad Transblot Turbo; Bio-Rad, Hercules, CA, USA). PVDF membrane was blocked with 5% non-fat dry milk in TBST (10 mM Tris, 150 mM NaCl, 0.5% Tween-20, pH8) for 1 hr and then the membrane was incubated overnight at 4 °C with SARS-CoV N (Sino Biological, Wayne, PA, USA; 40588-T62) and SARS-CoV S (Sino Biological, Wayne, PA, USA; 40589-T62) antibodies. After the incubation, the membrane was washed in TBST and incubated with an HRP-conjugated rabbit secondary antibody (Cell Signaling, Danvers, MA, USA; 7074) for 1 hr at 21 ±1 °C. PVDF membrane was then washed, developed with ECL, and imaged on a Chemidoc imaging system (Bio-Rad, USA). The molecular weights of the full-length S and N proteins are approximately 190 and 48, respectively.
SARS-CoV-2 spike protein quantity detection by sandwich-ELISA method
SARS-CoV-2 Spike Protein was quantified by sandwich ELISA using the SARS-CoV-2 (2019-nCoV) Spike Protein ELISA kit (Creative Biolabs, Life Technology, NY, USA) according to the manufacturer's instructions. The SARS-CoV-2 (2019-nCoV) Spike Protein ELISA kit is based on a solid-phase sandwich enzyme immunoassay. The wells of the plate strips are coated with a monoclonal antibody specific for SARS-CoV-2 spike protein and nucleoprotein. Since the 10000 pg/ml standard serves as a high standard, we diluted the samples hundreds to thousands of times. We determined the optical density of each well at 450 nm. The results were analyzed using EPOCH Gen 5 software. We calculate the spike protein levels in a dose with a standard curve.
Humoral immunogenicity analysis and T-cell response analyses of the vaccine candidate by neutralization assay and ELISPOT
An ideal serological assay should measure the binding of neutralizing antibodies to the SARS-CoV-2 spike protein. For this purpose, Balb/c mice were randomly divided into five groups (13 animals in each group) and injected with the experimental vaccine at five different doses (0, 1, 4, 6, and 10 µg per dose, mixed with alum adjuvant) on the day 0th and 21st (Wang et al. (Citation2020)), blood was collected from the mice at 0th, 7th, 14th, 21st and 35th days after the first immunization. Each animal was injected with 0.2 ml of the test sample.
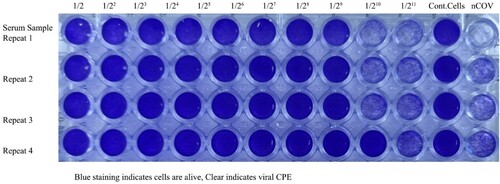
Serum from each group of mice to be tested was diluted 1:2 in advance and inactivated in a 56°C water bath for 30 min. The serum was diluted 1:2 by a 2-fold dilution series to the required concentration, and an equal volume of a challenge virus solution containing 100 TCID50/50 µl viruses was added. After neutralization in a 37°C incubator for 1 h, a 1 × 106/ml cell suspension was added to the wells (0.1 ml/well) and cultured in a CO2 incubator at 37°C for 4 days. The CPE observation was used to calculate the neutralization endpoint (conversion of serum dilution to logarithm) according to the Karber method (Kärber (Citation1931; Manenti et al. Citation2020)), i.e. the highest serum dilution that can protect 50% of the cells from infection by 100 TCID50 viruses is the antibody potency of the serum. Each of the samples was performed in the well-plate at four repeats. A neutralization antibody potency of 1:8 is negative, while one of >1:8 is positive (Manenti et al. (Citation2020); Muruato et al. (Citation2020)).
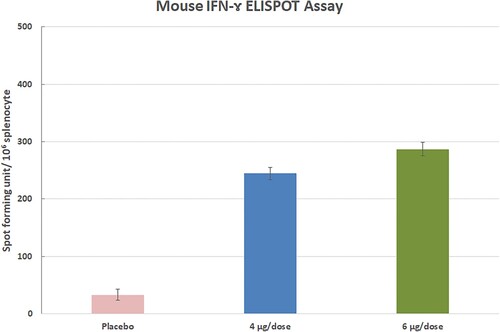
Three animals for the 4 and 6 µg group were euthanized on day 35th and splenocytes were collected to evaluate T-cell response by IFN-ɤ ELISPOT assay.
IFN-ɤ elispot Assay
The ELISPOT assay constitutes an ideal tool in the investigation of Th1 / Th2 responses. ELISPOT assay was performed with Murine IFNγ ELISPOT Kit (Abcam, USA) according to the manufacturer's instructions. A total of 2.5 × 105 splenocytes per 100 µl were stimulated at 37 °C for 48 h with MOI 0.05 of the SARS-CoV-2 and controls (splenocytes from PBS-inoculated Balb/c mice); culture media alone (background); concalvine A (conA) 0.5 µg/mL (cell viability). IFN-ɤ-secreting cells were revealed by adding streptavidin-alkaline phosphatase and ready to use BCIP/NBT substrate. Incubate the plate for 5-15 min monitoring spot formation visually throughout the incubation period to assess sufficient colour development. Spots were counted under a stereomicroscope. Spot-forming units (SFUs) per million cells were represented after background subtraction from unstimulated cells.
Repeated dose toxicity studies in ferrets
Some studies have demonstrated that ferrets are a suitable mammalian model for SARS CoV-2, which efficiently replicates in its upper respiratory tracts. To examine whether the KOCAK-19 can cause toxicity or local reactions in the ferrets. Ferrets (n = 6) received 8 µg of KOCAK-19 th on the 0th, 8th, and 15th days via the intramuscular route (IM). Four ferrets received physiologic saline solution (0,9% NaCl) on the 0th, 8th, and 15th days via the same route (IM). Clinical evaluations included local reactions to injection locations, temperature, weight loss, fever, dyspnoea, lethargy, anorexia, and grooming. Monitoring of ferrets was performed twice a day for two months. Also, biochemical and hematological examinations were carried out.
Challenge studies in transgenic K18 hACE-2 mice
K18 hACE-2 Transgenic male mice (Jackson Lab., USA) were randomly divided into three groups (13 animals in each group) and intraperitoneally (IP) injected with the experimental vaccine at three different doses (0, 4, and 6 µg per dose, mixed with alum adjuvant) on the 0th and 21st (Wang et al. (Citation2020)). All groups of mice were challenged by intranasally 104 TCID50/50µl live viruses SARS- CoV-2, which was administered into both nasal passages using a micropipette in an isolator (220 Pa negative pressure) on day 35th. Symptoms (weight loss, fever, dyspnoea, lethargy, anorexia, and grooming) and death were monitored to day 45 after the first immunization.
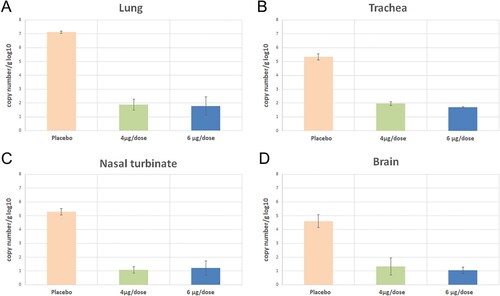
Three animals for each group were euthanized three days following the challenge. Lungs, nasal turbinate, trachea, and brains were collected for virus isolation and virus load detection by real-time RT–PCR kit (Primer design, USA).
Experimental method in Balb/c mice
To evaluate the efficacy and safety of the prepared vaccine, 50–60 days old, 20 female Balb/c mice were randomly divided into 6 groups. Animals were kept in IVC cages (22°C, 55-65% relative humidity), all groups were separated, they were fed with ad libitum and animals had access to food and water whenever they wanted. Group N1 animals received virus only (n = 2), group N2 received no application (n = 2), group G1 received virus and 1 µg vaccine (n = 4), group G2 received virus and 6 µg vaccine (n = 4), group G3 received virus and 10 µl vaccine (n = 5), group G4 received 4 µl vaccine only (n = 2). The vaccine was administered on days 0 and 21. On day 35, N1, G1, G2, and G3 received intranasally 104 TCID50 live viruses SARS-CoV-2, which was administered into both nasal passages using a micropipette in an isolator cabinet (220 Pa negative pressures). All animals were sacrificed on day 45 for histopathological examination.
Animals were subjected to routine necropsy, organ tissue samples were collected in 10% formalin solution and subjected to routine tissue examination and embedded in paraffin blocks. Sections 3–4 µm thick were collected from each block using a rotary microtome (Leica RM2255, Germany), stained with hematoxylin & eosin (H&E), and analyzed under a light microscope (Olympus BX50, Japan). All histopathological examinations were double-blinded and scored by each pathologist and their mathematical mean was accepted as the true value.
Results
Vaccine preparation
Microcarrier-based production in disposable wave bioreactors (SARTORIUS Biostat RM20, Germany) was very efficient for vaccine production. Disposable bags are easily maintained, and aseptic connections of bags are very useful for dangerous pathogens. Cells are growing very fast and healthy in appropriate conditions (rocking, pH, angle, gases control). All manipulations are carried out aseptically and safely using with bio-welder and bio-sealer (SARTORIUS, Germany). However, 0.01 - 0.05 MOI was found optimal multiply of infection ratio to infect Vero CCL-81 cells. Bio-welder and bio-sealer (SARTORIUS, Germany) were used to connect pipes and transferring of inoculums, collect samples, and harvest.
SARS-CoV-2 Spike ELISA
We found the SARS-COV-2 Spike protein quantity is equal to 7.53 ng/ml in 1:75 dilution of the main bulk of the vaccine candidate. Each dose of the vaccine candidate contains about 562 ng viral spike protein in a 6 µg vaccine (Table ). Also, we determine the Alum-antigen binding capacity before and after the adjuvant binding process by quantitative SARS-COV-2 Spike protein ELISA and found about 95–99% in different trials. Alum-antigen binding is important for immunogenicity and long-time stability of viral proteins at +2 to +8 °C.
Table 1. SARS-COV-2 Spike protein quantities
Virus titration by plaque assay and microtitration
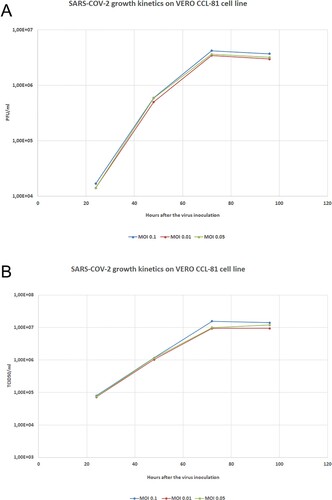
Virus titration of 3rd passage of the virus (master seed lot) was reaching a peak titer over 3.4 x106 PFU/ml or 9.9 × 106 TCID50/ml by 56 - 72 h post-infection (hpi) at multiplicities of infection (MOI) of 0.01. Growth kinetic analysis of the 5th passage of virus stock (working seed lot) in Vero cells showed that the stock virus could replicate efficiently and reached a peak titer 3.6 × 106 PFU/ml or 1 × 107 TCID50/ml by 56 –72 h post-infection (hpi) at multiplicities of infection (MOI) 0.05 (Figure ). Viral kinetics of the KOCAK-19 strain at different multiplicities of infection (MOI) was given in Figure (A and 4B).
Figure 3. Quantification of SARS-CoV-2 in CCL-81 cell culture. Plaque assays were performed on the harvested virus at 62 h post-infection, fixed, and stained with crystal violet to visualize. A, B, C, D, E wells are ten-fold dilutions of virus (10−3–10−7) and F well is the negative control.
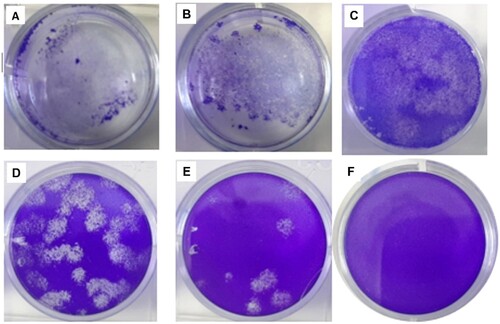
Figure 4. Viral kinetics of the KOCAK-19 strain at different MOIs. A. Growth kinetics titers as a PFU/ml B. Growth kinetics titers as a TCID50/ml
Quantitative RT- PCR Genesig/Primerdesign qRT-PCR (RdRp gene) kit results are correlated with viral titer results at 56–72 h. qRT- PCR is a fast way to the determination of harvesting time (Figure ). However, the preparation of viral stocks’ viral titers should be determined before and after the storage in liquid nitrogen and inoculation.
Inactivation validation
No CPE was observed for three sub passages of inactivated virus bulk. Inactivation was validated by passaging the treated samples 3 generations (n = 3) without the appearance of CPE. β-propiolacton (BPL) inactivation provides a rapid and safe method for the inactivation of viral particles. If there are any live residual viruses and infective RNA in the bulk, Vero cells must be infected and cytopathic effect (CPE) must be observed under the inverted light microscope.
Sequencing
Sequence distance matrix of first, third, and, ninth passages of SARS-COV-2 in Vero cell culture. All these passages correlate to SARS-COV-2 NC 045512 isolate at a ratio of 97.891–99.117% in the NCBI database. It was determined that the 1st passage (ON653597), 4th (ON653599) passage, and 9th passage (ON653598) of SARS-CoV-2 genome sequences matched 100%, and all the mutations in the first sample were also observed completely in the other samples. Some negligible deletions were observed in the sequences, not in the affected structure of the virus. The variations were found to correspond to the genomic positions 313, 5554, 8782, and 17259 (ORF1ab gene), 22468 (S gene c.906G > T), 28878 (N gene), 28144 (ORF 8 gene), 26873 (M gene), and 29742 (3’ UTR). No change was observed in the spike RBD subunit, which was reported to be very antigenic in previous studies (Harvey et al. (Citation2021)).
SDS-PAGE and WESTERN BLOTTING
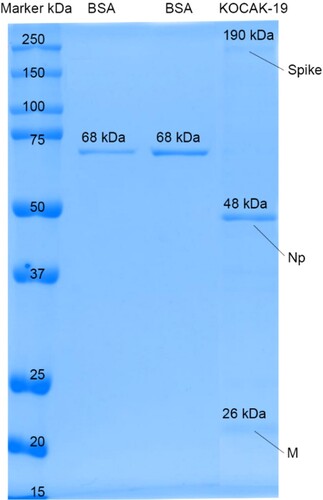
It is observed that Spike, Nucleoprotein, and Membrane proteins are approximately 190, 48, 26 kDa, respectively after the purification step by SDS page in 25 µl loading volume of final bulk (Figure ).
Figure 6. 25 µl loading volume of KOCAK-19 SARS-CoV-2 final bulk SDS gel electrophoresis against known BSA (150 and, 250 ng/25µl) standards
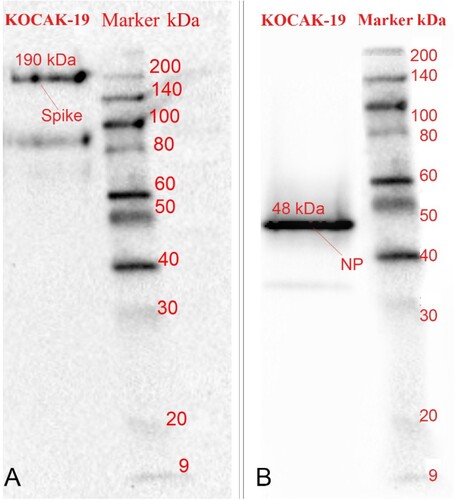
SARS-CoV-2 N and SARS-CoV-2 S proteins are determined by incubation with anti-SARS-COV-2 N, S antibodies and, HRP conjugated rabbit antibodies in Western Blot. It is observed that Spike and Nucleoprotein bands are approximately 190 and 48 kDa in Western Blot (Figure A and 7B).
Figure 7. A. SARS-COV-2 Spike bands, approx.190 kDa B. Nucleoprotein bands, approx. 48 kDa in Western Blotting
Transmission electron microscopy (TEM)
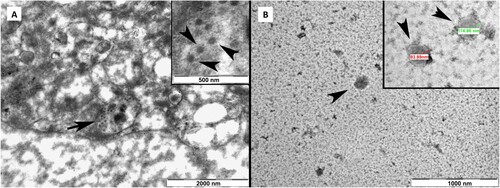
TEM analysis verified the viral replication in Vero cells and showed intact, oval-shaped particles with diameters of 90–110 nm of the virus after the final purification step (Figure ).
Vaccine candidate immunogenicity analysis and microneutralization assay (MNT)
The preliminary results in mice showed that the Nab titers reached the peak post-priming and boosting in 21 days. The geometric mean titers were respectively <1/4, 1/39, 1/1/ 595, 1 /791, and 1/1048 in placebo, 1, 4, 6, and 10 µg groups (Figure ). Statistical significance was tested with the ANOVA corrected for multiple comparisons in vaccinated and placebo groups and then P values are shown for comparisons that resulted in statistical significance (P < 0.05). No statistical significance was observed in the 4, 6, and 10 µg vaccinated groups.
Figure 9. Mouse neutralization antibody (NAb) levels with different doses by two-dose immunization. Mice were injected intraperitoneally (IP) route by using two-time immunization (D0/D21), and the NAb levels 35 days after the first immunization were tested by the microneutralization method (n = 10).
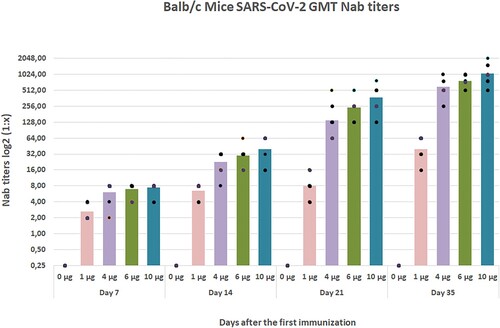
Figure 10. Microneutralization analysis of Balb/c mice Nab(s) was carried out in 96 well plates coated by Vero CCL-81 cells. The assay was performed on 0,7,21, and 35 days after the first immunization, neutralized by 100 TCID50 SARS-CoV-2 viruses, incubated for 4 days, fixed, and stained with crystal violet to visualize. The first 10 well columns belong to two-fold dilutions of Nab, the 11th well columns are the negative control, and the 12th well columns are positive virus controls. Each of the samples was performed in the well-plate at four repeat
SARS-CoV-2-specific cellular responses were assessed in 4 and 6 µg vaccinated animals by IFN-ɤ ELISPOT. T-cell immune responses in the 4 µg (n = 3) and 6 µg dose group (n = 3) and control group (n = 3) were analyzed on day 35 following the first immunization by an IFN-γ-based ELISPOT assay (Figures –). The statistical significance was assessed using an ANOVA test p values less than 0.01 were considered to be statistically significant. Error bars represent mean ± standard deviation.
K18 hACE-2 transgenic mice challenge, findings of viral loads of tissues
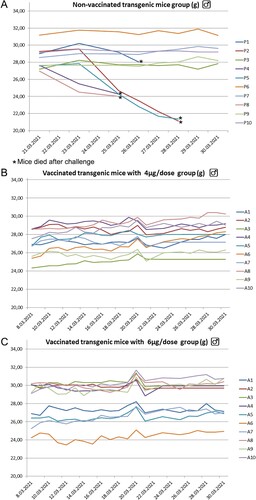
6–8 weeks old, non-vaccinated K18 hace-2 transgenic mice exhibited a significant decrease in body weight at 3–5 dpi and 5 of 10 mice (P1, P2, P4, P5, and P8) died until 7 dpi compared with vaccinated K18 hACE-2 transgenic mice. More than 50% of non-vaccinated K18 hACE-2 transgenic mice died at 5–7 dpi. All vaccinated mice survived during the study (Figure A, 12B, and 12C).
Figure 12. K-18 hACE 2 transgenic mice placebo, 4µg, and 6 µg groups (each of 10 animals) mean weight changing graphs. Five animals in the placebo group died at 5–7 dpi. No any symptoms in both of the vaccinated groups
More viral RNA copy number was detected in all unvaccinated transgenic mice than in vaccinated groups’ tissues. Especially lung and trachea have more viral RNA load than the brain and nasal turbinate in unvaccinated controls (Figure A, 13B, 13C, and 13D). Viral loads are significantly less in all vaccinated animals than in control animals at 3 days post-infection. Error bars represent mean ± standard deviation.
Repeated dose toxicity studies in ferrets
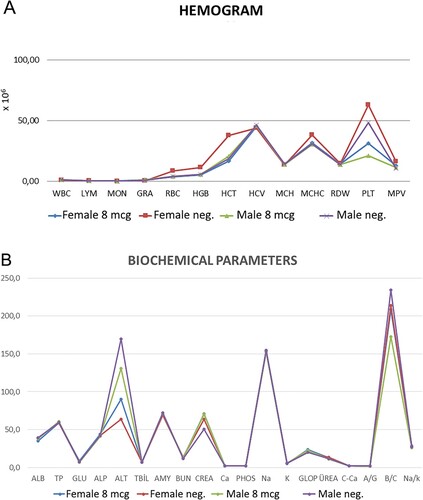
The most common adverse reaction was injection site swelling, which was mild and limited. Fever, inappetence, and weight loss were not observed in Balb/c mice and transgenic ferrets. When the inactivated vaccine candidate (n = 6) and placebo (n = 4) was applied in 8 µg doses three times, It was well tolerated in all dose groups with no vaccine-related serious adverse effects. Two months after the first application, biochemical and hematological parameters were found in the normal range compared to the placebo group (Figure A, 14 B).
Figure 14. A. Hematological parameters in control and 8 µg doses vaccinated ferrets B. Biochemical parameters in control and 8 µg doses vaccinated ferrets after the end of the 2 months of the first injection
Macroscopic findings
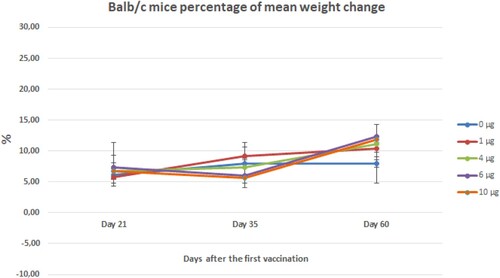
The weight gain percentage of Balb/c mice inoculated with the lowest and highest doses of the vaccine candidate was significantly more than control groups after the challenge (Figure ). Although there were no significant differences between the groups in the macroscopic examination, depending on the euthanasia method applied (decapitation), bleeding areas were observed in the head, neck, and back regions, and in some animals, blood accumulation/collections in the lungs due to blood aspiration were observed.
Histopathologic findings
Histopathologic changes observed in animals are given in Table . In the histopathologic evaluation, the main differences between groups were observed especially in the lung, heart, abdominal lipid tissue, and spleen.
Table 2. Histopathologic changes observed in animals
In the lungs, the most significant findings were the perivascular and peribronchial mononuclear cell infiltrates, especially in animals that received vaccination and they were more prominent in the 6 and 10 µL vaccine groups (Figure A, 16B). And only in G3 bronchial epithelial hyperplasia/hypertropia is observed. Hemorrhages were observed in all groups in which we assumed blood aspiration due to the decapitation process. Interstitial pneumonia is only observed in one animal (N2) which only received a virus. Thrombosis in small vessels and inside alveoli and hyaline accumulation inside alveoli were observed especially in G4 in animals (Figure C, 16D) who only received 4 µL vaccination.
Figure 16. A) Changes in the lungs. Hematoxylin-eosin, G1-1, Perivascular and peribronchial lymphocytic infiltrations (arrow). B) Changes in the lungs. Hematoxylin-eosin, G1-4, Fibrinoid necrosis in the small vessels (arrow). C) Changes in the lungs. Hematoxylin-eosin, G2-3, Hyperemia, and perivascular lymphocytic infiltrations (arrow). D) Changes in the lungs. Hematoxylin-eosin, G4-2, Thrombosis in small vessels (arrow). E) Changes in the central nervous system. Hematoxylin-eosin, G2-4, Per diapedesis bleeding in the meninges (arrowhead). F) Changes in the central nervous system. Hematoxylin-eosin, G2-3, Perivascular lymphoid infiltrations, and micro thrombosis (arrow). G) Changes in the heart. Hematoxylin-eosin, G2-2, Widespread Zenker degenerations (arrow) H) Changes in the heart. Hematoxylin-eosin, G2-1, Widespread Zenker degenerations (arrow). I) Changes in the abdominal fat tissue. Hematoxylin-eosin, G4-4 Granulomatous changes; lymphocytic infiltrations, macrophages, and small necrosis (arrow). Wide calcified necrosis in a granuloma (star). J) Changes in the spleen. Hematoxylin-eosin, G2-2, Lymphocytic hyperplasia throughout the spleen (yellow arrow) and increased megakaryocytes (black arrow).
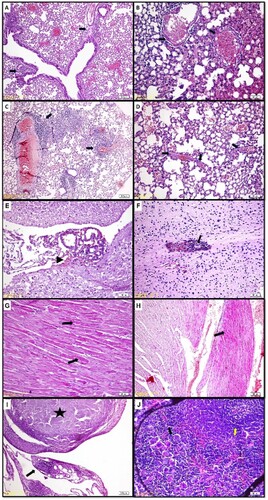
In the central nervous system, some meningeal lymphoid infiltrations and per diapedesis bleeding were observed in 5 animals; N1-1, G2-1, G2-4, G3-5, and G4-1 (Figure E), and hemorrhage, micro thrombosis, and perivascular lymphoid infiltrations are observed only in G2-3 (Figure F).
Varying degrees of hemorrhages, myofiber degenerative changes (Zenker degenerations), and necrosis, mononuclear cell infiltrations were detected in the heart (Figure G). Only the animals in the group that did not receive any treatment or virus application (N2) heart tissues were healthy. The degenerative changes were distinct, especially in G2 (Figure H) and they are generally located on the left side of the heart.
Widespread parenchymatous degeneration and varying degrees of fatty degeneration were detected in the livers of all animal groups. Only hepatitis and hemorrhages were observed in one animal (N1).
In all groups which received intraperitoneal vaccination varying degrees of changes were observed in the abdominal lipid tissue. G3 animals which received 10 µL vaccination demonstrated the severest cases. Lymphocytic infiltrations, macrophages, necrosis, granulomatous changes, and granulomas are observed (Figure I). In one animal (G2-4) where these changes were close to the pancreatic tissue, degenerative changes and lymphocytic infiltrations were observed in the pancreatic tissue.
In the splenic tissue of animals, lymphocytic hyperplasia was significant in all groups with vaccinations (Figure J). Increased megakaryocytes in the spleen are observed in all animals.
In kidneys, hyperemia and interstitial nephritis were only observed in groups G3 and G4, and small degrees of lymphocytic infiltration especially in the renal pelvis were determined.
Discussion
In the development of the vaccine candidate, the candidate strain generated the highest virus yields in Vero cells CCL-81 and had no amino acid variations within 9 passages, suggesting its good genetic stability. We observed only some deletions in the full genome sequence but not affected the amino acid structure of the basic structure of the virus. Pavel et. al. (Dhama et al. (Citation2020)) found variations that genomic positions 1397 and 11083 (ORF1ab gene), 23876 (S gene), 26688 (N gene), 29563 (ORF 10 gene), and 29742 (3’ UTR). Also, Gao et al. (Gao et al. (Citation2020 Jul 3)) reported that some genetic alterations in the passaging levels of the virus, but not affecting to immunity. In this study, we found variations that genomic positions 313, 5554, 8782 and 17259 (ORF1ab gene), 22468 (S gene c. 906G > T), 28878 (N gene), 28144 (ORF 8 gene), 26873 (M gene), and 29742 (3’ UTR). Harvey et. al. (Harvey et al. (Citation2021)) reported that residues at positions 614 and 222 have a relatively low effect on the serum neutralizing antibody response. In the same report, Harvey et. al. (Harvey et al. (Citation2021)) reported amino acid changes in the RBD subunit have been reported to be of primary importance in immunity, as they induce neutralization titers. No change was observed in the spike RBD subunit of three different passages (1st, 4th, and 9th passage) of SARS-CoV-2, which was reported to be very antigenic in previous reports (Harvey et al. (Citation2021)). In this study, one amino acid variation was observed at position 906 in the S2 subunit of the spike protein. These differences may be affected immunity to wild-type virus strains slightly or never, but it will become clear after the cohort studies are carried out on large scale.
Due to high viral yield, microcarrier size and concentration, Vero cell seeding quantity, multiply of infection ratio, time and duration of viral infection, maintenance of pO2, pH, bioreactor angle and shaking, and time of harvesting are critical factors in the upstream process. The pO2 saturation must not be decreased below 35% and pH should be maintained at around 7 to avoid the aggregation and lyses of viral proteins and to support cell growth and viral production. 56–72 h was found optimal harvesting time in these production conditions. Our data agrees with a study by Manenti et al. (Manenti et al. (Citation2020)) in which SARS-CoV-2 replicated rapidly in Vero CCL-81 cells after an initial eclipse phase and increased gradually, peaking at 56 h post-infection.
However, the latest report shows in the preparation of vaccines, BPL inactivation provides a rapid and safe method for the inactivation of viral particles (Xia et al. (Citation2020)). Also, intact viral particles are apparent by transmissible electron microscopy (TEM). In TEM analysis showed intact, oval-shaped particles with diameters of 90–110 nm of the virus after the final purification step. Our data agrees with that of Jureka et. al. (Jureka and Silvas (Citation2020)), in which TEM analysis showed 90 −110 nm virus in purified bulk.
Quantitative SARS-CoV-2 Spike antigen ELISA test verified SDS gel antigen quantities with working BSA standards in SDS gel electrophoresis. Also, qRT-PCR results from the Genesig Advanced COVID-19 RT–PCR kit gave information about the viral titers and viral protein quantity with invert microscopy examination to decide the harvesting time of viral bulk.
We demonstrated that both nucleoprotein and spike (full-length and cleaved) of SARS-CoV-2 in all downstream process steps are detectable by western blot in these samples.
The two-dose immunizations with median doses (4 and 6 µg) at days 0/21, were found efficient. SARS COV-2 vaccine candidate induces high levels of Nab titers, and T-cell response and efficiently protects them in Balb/c mice. Also, these two-dose immunizations with 4 and 6 µg doses at days 0/21, were found efficient in K18 hACE-2 transgenic mice challenge studies compared to the placebo group. SARS-CoV-2 vaccine candidate efficiently protects them in suitable animal models against challenge. Viral loads in vaccinated animals’ tissues (Lung, trachea, nasal turbinate, and brain) are much less than in the unvaccinated group.
We used the ANOVA method to compare the log-transformed antibody titer depending on the normality test results that showed normal distribution. When the comparison among all five groups showed a significant difference between vaccinated and non-vaccinated (Placebo) groups, we then did pair-wise comparisons. Median doses (4 and 6 µg) showed a good immune response and not any gross and pathological findings. SARS CoV-2 vaccine candidate is efficiently produced, genetically stable, and safe in Balb/c mice, K18 hACE-2 transgenic mice, and ferrets. Clinical evaluations included temperature, weight loss, fever, dyspnea, lethargy, anorexia, and grooming. The most common adverse reaction was injection site swelling, which was mild and limited. Fever, inappetence, and weight loss were not observed in Balb/c mice and transgenic ferrets (Gu et al. (Citation2020 Sep 25); Zeiss et al. (Citation2021)).
During the study, no differences in body weight, body temperature, or clinical pathology were observed or detected in Balb/c and K18 hACE-2 transgenic mice inoculated with the lowest and highest doses of the vaccine candidate, compared with control groups (Gu et al. (Citation2020 Sep 25); Gao et al. (Citation2020)).
No obvious systemic toxicity was observed in any animal species during the dosing period and at the end of the 2-week recovery period. Local skin irritation reactions related to alum adjuvants were noted on injection sites, but they disappeared within a week (Gao et al. (Citation2020 Jul)).
The results showed that the vaccine candidate stimulated immoral immune responses and presented no toxicity in animals tested. In conclusion, a study on the safety and immunogenicity of the inactivated, whole virus vaccine showed the vaccine stimulated a strong humoral immune response. In this study, the preliminary results in mice showed that the Nab titers reached the peak post-priming and boosting in 21 days. Titers in 1, 4, 6, and 10 µg groups found about 1/39, 1/ 595, 1/791, and 1/1048, respectively.
Even the placebo and vaccination of Balb/c mice were challenged with 105 TCID50 /50 µL doses of SARS-CoV-2 by the intranasal route, lethality and clinical pneumonia were not reported. SARS-CoV–2 showed good binding for human ACE2 but limited binding to mice ACE2. The SARS-CoV-2 virus showed slight pneumonia without apparent clinical signs and replication was remained in limit in other epithelial tissues (Gao et al. (Citation2020)).
Based on the results presented here, a Phase I clinical trial of candidate COVID-19 vaccine is currently in six months of evaluation progress (NCT04838080), and a Phase II of clinical trial has recently been initiated. These clinical trials have been designed using the same aluminium adjuvant formulation described here, with two different groups of 4, and 6 µg dose groups to evaluate the appropriate dose for further clinical application.
About the histopathologic examination of the organs on Balb/c mice, there were not any reports we found that performs systemic examinations. The published report generally investigates changes in lung tissue and olfactoric bulbs in Balb/c mice (Gu et al. (Citation2020 Sep 25); Dinnon et al. (Citation2020)).
In all groups that received intraperitoneal vaccination, varying degrees of changes were observed in the abdominal lipid tissue. These findings were similar to the findings of Portuondo et. al. (Portuondo et al. (Citation2017)) where they observed palpable nodules at the injection site in all animals injected with aluminium hydroxide adjuvants. Meningeal lymphoid infiltrations observed in animals were similar in cases in humans with focal leptomeningeal inflammation described by Lee et. al. (Lee et al. (Citation2021)). However, we could not detect any reports regarding the changes observed in Balb/c mice. The most significant finding was varying degrees of hemorrhages and Zenker degenerations in the heart of animals that receive treatment and/or virus application. These changes were similar to the findings reported in Syrian hamsters (Zeiss et al. (Citation2021); Chan et al. (Citation2020)) and humans (Diaz et al. (Citation2021)). The findings of mild focal myocardial degeneration in Syrian hamsters after virus application were similar to our findings and observed more intensely in non-vaccinated animals than vaccinated animals. Widespread parenchymatous degeneration and varying degrees of fatty degeneration were detected in the livers of all animal groups. Detecting these changes in all animals gives rise to the thought of changes due to ad libitum feeding, type of feed, or inactivity inside the cages. The changes observed in the kidneys were limited to 2 animals from different groups, and due to small populations, we thought they could be negligible and no literature data in Balb/c to compare was determined.
In the literature about Balb/c mice generally, lung tissue changes were observed in aged mice (Gu et al. (Citation2020); Dinnon et al. (Citation2020); Zhang et al. (Citation2021)). When the necropsies were performed our mouse population was aged between 90–100 days. According to Hongjing et. al. (Gu et al. (Citation2020)), modified virus applications similar to our modification can cause some changes in the young Balb/c mouse at early ages, and this finding was like our findings in the lung tissues. The increased lymphocytic infiltration especially in the vaccinated group was thought to be due to the immune response gained by the vaccine. Small doses of vaccination were observed to cause vascular changes but reported that much larger populations, where statistical analyzes can be performed should be used. Like in the lung tissue increased lymphocytic hyperplasia in the splenic tissue of vaccinated animals was thought to be due to the immune response gained by the vaccine. Increased megakaryocytes in the spleen are observed in all animals which we think is a result of repetitive blood collections for titrations.
Conclusion
In the presented study the preclinical development of an inactivated COVID-19 vaccine candidate is described. Initial results describing the immunogenicity of the COVID-19 vaccine candidate, are promising, and it is particularly encouraging to measure neutralization antibodies, T-cell response, and weight losses in Balb/c mice. Also, protection against challenge was demonstrated in transgenic mice compared to the control group. P values are shown for comparisons that resulted in statistical significance (P < 0.05) between vaccinated groups and placebo. Median doses (4 and 6 µg) showed a good humoral and T-cell immune response and not any gross and pathological findings. We provide evidence for the safety of inactivated COVID-19 vaccine candidates in Balb/c mice and ferrets did not observe infection enhancement and immunopathological changes in two doses of vaccinated Balb/c mice and three doses of vaccinated ferrets. SARS CoV-2 vaccine candidate is efficiently produced, genetically stable, and safe in Balb/c mice, K18 hACE-2 transgenic mice, and ferrets. The vaccine candidate (KOCAK-19) has been authorized by the Turkish Medicines and Medical Devices Agency and has now entered phase 2 clinical development (NCT04838080).
Declarations
Ethics declarations
The Animal Local Ethics Committee of Kocak Pharmaceuticals approved all animal experiments in this study (No. 2020-5).
Availability of data and materials
The key information and data generated and/or analyzed during this study were included in this article.
Human tissue, human participants, and human data
Not applicable.
Consent for publication
Not applicable.
Competing interests
Engin Alp ONEN is working in the Kocak Pharmaceuticals Vaccine R&D department as a viral vaccine project manager and senior scientist. The other authors declare no conflict of interest.
Author Contribution Statements
E.A.O. directed the project. E.A.O and K.G. designed the project. E.A.O. developed and formulized the vaccine candidate for Preclinical and Phase I trials. K.G., F.Y. and A.G. performed the histopathological experiments. E.K.D. performed electron microscopy. E.A.O K.G., F.Y. and E.K.D performed the experiments, and the analytic calculations and analysed the data. E.A.O. and K.G. wrote the paper with input from all authors. All authors have read and agreed to the published version of the manuscript.
Preprint Statement
The preprint of this study is available on https://doi.org/10.21203/rs.3.rs-1073686/v1 in Researchsquare.
Data Availability Statement
The raw data that support the findings of this study are available on http://doi.org/10.11922/sciencedb.o00025.00001 in Science Data Bank.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
REFERENCES
- Amanat F, Strohmeier S, Meade P, Dambrauskas N, Mühlemann B, Smith DJ, et al. 2021. Vaccination with SARS-CoV-2 variants of concern protects mice from challenge with wild-type virus. PLoS Biol. 19(12):1–14. doi:10.1371/journal.pbio.3001384
- Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. 2020. The proximal origin of SARS-CoV-2. Nat Med. 26:450–452. doi:10.1038/s41591-020-0820-9
- Chan JF-W, Zhang AJ, Yuan S, Poon VK-M, Chan CC-S, Lee AC, et al. 2020. Simulation of the clinical and pathological manifestations of coronavirus disease 2019 (COVID-19) in a golden Syrian hamster model: implications for disease pathogenesis and transmissibility. Clin Infect Dis. 71(9):2428–2446. doi:10.1093/cid/ciaa325
- COVID-19 Vaccines Authorized for Emergency Use. FDA 21.01.22 https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines#authorized-vaccines.
- Dhama K, Khan S, Tiwari R, Sircar S, Bhat S, Malik YS, Singh KP, Chaicumpa W, Bonilla-Aldana DK, Rodriguez-Morales AJ. 2020. Coronavirus disease 2019–COVID-19. Clin Microbiol Rev. 33:e00028–20. doi:10.1128/CMR.00028-20.
- Diaz GA, Parsons GT, Gering SK, Meier AR, Hutchinson I V, Robicsek A. 2021. Myocarditis and pericarditis after vaccination for COVID-19. JAMA. 326(12):1210–1212. doi:10.1001/jama.2021.13443
- Dinnon KH, Leist SR, Schäfer A, Edwards CE, Martinez DR, Montgomery SA, et al. 2020. A mouse-adapted model of SARS-CoV-2 to test COVID-19 countermeasures. Nature. 586:560–566. doi:10.1038/s41586-020-2708-8.
- Gao Q, Bao L, Mao H, Wang L, Xu K, Yang M, Li Y, Zhu L, Wang N, Lv Z, et al. 2020 Jul 3. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 369(6499):77–81. DOI: 10.1126/science.abc1932. . Epub 2020 May 6. PMID: 32376603; PMCID: PMC7202686.
- Gu H, Chen Q, Yang G, He L, Fan H, Deng YQ, Wang Y, Teng Y, Zhao Z, Cui Y, et al. 2020 Sep 25. Adaptation of SARS-CoV-2 in BALB/c mice for testing vaccine efficacy. Science. 369(6511):1603–1607. DOI: 10.1126/science.abc4730. . Epub 2020 Jul 30. PMID: 32732280; PMCID: PMC7574913.
- Harvey WT, Carabelli AM, Jackson B, et al. 2021. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. 19:409–424. doi:10.1038/s41579-021-00573-0.
- Jureka A, Silvas J. 2020. Basler C. propagation, inactivation, propagation, inactivation, and safety testing of SARS-CoV-2. Viruses. 12(6):622. doi:10.3390/v12060622
- Kärber G. 1931. Beitrag zur kollektiven behandlung pharmakologischer reihenversuche. Archiv f. experiment. Pathol. u. Pharmakol. 162:480–483. doi:10.1007/BF01863914
- Kumar A, Dowling WE, Román RG, et al. 2021. Status report on COVID-19 vaccines development. Curr Infect Dis Rep. 23(6):9. doi:10.1007/s11908-021-00752-3
- Lee MH, Perl DP, Nair G, Li W, Maric D, Murray H, Dodd SJ, Koretsky AP, Watts JA, Cheung V, et al. 2021. Microvascular injury in the brains of patients with COVID-19. N Engl J Med. 384(5):481–483. doi:10.1056/NEJMc2033369. Epub 2020 Dec 30. PMID: 33378608; PMCID: PMC7787217.
- Lou F, Li M, Pang Z, Jiang L, Guan L, Tian L, Hu J, Fan J, Fan H. 2021. Understanding the secret of SARS-CoV-2 variants of concern/interest and immune escape. Front Immunol. 12:744242. doi:10.3389/fimmu.2021.744242.
- Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. 2020. Genomic characterization and epidemiology of 2020 genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The Lancet. 395:565–574. doi:10.1016/S0140-6736(20)30251-8
- Manenti A, Maggetti M, Casa E, Martinuzzi D, Torelli A, Trombetta CM, et al. 2020. Evaluation of SARS-CoV-2 neutralizing antibodies using a CPE-based colorimetric live virus micro-neutralization assay in human serum samples. J Med Virol. 92(10):2096–2104. doi:10.1002/jmv.25986
- Mohamed NA, Solehan HM, Mohd Rani MD, Ithnin M, Che Isahak CI. 2021. Knowledge, acceptance and perception on COVID-19 vaccine among Malaysians: A web-based survey. PLoS ONE. 16(8):e0256110. doi:10.1371/journal.pone.0256110.
- Morgeaux SP. 1995. Inactivation of DNA by β-propiolactone. Biologicals. 23(3):207–211. doi:10.1006/biol.1995.0034. PMID: 8527119.
- Muruato AE, Fontes-Garfias CR, Ren P, Garcia-Blanco MA, Menachery VD, Xie X, et al. 2020. A high-throughput neutralizing antibody assay for COVID-19 diagnosis and vaccine evaluation. Nat Commun. 11:4059. doi:10.1038/s41467-020-17892-0
- Pavel STI, Yetiskin H, Aydin G, Holyavkin C, Uygut MA, Dursun ZB, et al. 2020. Isolation and characterization of severe acute respiratory syndrome coronavirus 2 in Turkey. PLoS One. 15:1–17. doi:10.1371/journal.pone.0238614
- Portuondo DL, Batista-Duharte A, Ferreira LS, de Andrade CR, Quinello C, Téllez-Martínez D, et al. 2017. Comparative efficacy and toxicity of two vaccine candidates against sporothrix schenckii using either montanide™ Pet Gel A or aluminum hydroxide adjuvants in mice. Vaccine. 35(34):4430–4436. doi:10.1016/j.vaccine.2017.05.046
- Spruth M, Kistner O, Savidis-Dacho H, Hitter E, Crowe B, Gerencer M, et al. 2006. A double-inactivated whole virus candidate SARS coronavirus vaccine stimulates neutralising and protective antibody responses. Vaccine. 24(5):652–661. doi:10.1016/j.vaccine.2005.08.055
- Tang X, Wu C, Li X, Song Y, Yao X, Wu X, et al. 2020. On the origin and continuing evolution of SARS-CoV-2. Natl Sci Rev. 7(6):1012–1023. doi:10.1093/nsr/nwaa036
- Wang H, Zhang Y, Huang B, Deng W, Quan Y, Wang W, et al. 2020. Development of an inactivated vaccine candidate, BBIBP-CorV, with potent protection against SARS-CoV-2, cell-182. Cell. 182(3):713–721. doi:10.1016/j.cell.2020.06.008
- Xia S, Duan K, Zhang Y, Zhao D, Zhang H, Xie Z, et al. 2020. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes. JAMA. 324(10):951–960. doi:10.1001/jama.2020.15543.
- Zeiss CJ, Compton S, Veenhuis RT. 2021. Animal models of COVID-19. I. comparative virology and disease pathogenesis. ILAR J. 62(1-2):35–47. doi:10.1093/ilar/ilab007.
- Zhang Y, Huang K, Wang T, Deng F, Gong W, Hui X, et al. 2021. SARS-CoV-2 rapidly adapts in aged BALB/c mice and induces typical pneumonia. J Virol. 95(11):e02477-20. doi:10.1128/jvi.02477-20.