Abstract
This prospective study investigated the relationship between the lipoprotein-associated phospholipase A2 (Lp-PLA2) level and cognitive impairment (CI) in type II diabetes mellitus (TIIDM) patients with white matter hyperintensity (WMH). A total of 87 TIIDM patients diagnosed with WMH were included in this study. They were grouped into CI group and noncognitive impairment (NCI) group based on the MoCA scales. The serum Lp⁃PLA2 levels and MoCA scores of the patients with WMH were compared with those of control (Ctl). Logistic regression was used to analyze the risk factors affecting CI and diagnostic value of serum Lp⁃PLA2 for CI. The WMH group had significantly higher serum level of Lp⁃PLA2 and significantly lower MoCA score than Ctl group. There were significant differences in serum levels of homocysteine, high-density lipoprotein cholesterol (HDL-C) and Lp⁃PLA2 (P < 0.05) between the groups. Logistic regression showed that HDL-C, homocysteine and Lp⁃PLA2 were the risk factors for CI in the patients (P < 0.05); receiver operating characteristic curve analysis showed that HDL-C and Lp⁃PLA2 had significant diagnostic value for CI in WMH patients. Therefore, Lp⁃PLA2 can be assessed in the elderly as a screening to identify putative CI patents for preventive treatment and management.
Introduction
White matter hyperintensity (WMH) is a subtype of cerebral small vessel disease (CSVD), also known as leukoencephalopathy (Graff-Radford et al. Citation2019; Wardlaw et al. Citation2015). Diagnostically, it is shown up as areas of increased brightness when visualized by T2-weighted magnetic resonance imaging (MRI) (Merino Citation2019; Stewart et al. Citation2021). The main pathological features of WMH are abnormal white matter areas with demyelination, apoptosis and vacuolation of the oligodendrocyte (Barkovich and Deon Citation2016). Clinical manifestations of WMH include cognitive impairment (CI, defined as a condition in which individuals demonstrate cognitive impairment with minimal impairment of instrumental activities of daily living (Petersen Citation2004; Winblad et al. Citation2004)), dementia, abnormal gait and urinary incontinence (Vipin et al. Citation2018). Severe WMH increases the risk of myocardial infarction, symptomatic stroke and even all-cause death (Ederle et al. Citation2013; Nolze-Charron et al. Citation2015). WMH may progress to cause reduced cognitive ability, anxiety and depression, or even dysuria, gait instability, and loss of ability to control life (Hainsworth et al. Citation2017; Wardlaw et al. Citation2015) and is a core feature of Alzheimer's disease (AD) (Lee et al. Citation2016). Early diagnosis and treatment are considered to be the most important measures for CSVD management (Smith and Beaudin Citation2018). Clinically, it is useful to use blood markers to assist WMH diagnosis and evaluation in addition to costly MRI studies.
Lipoprotein-associated phospholipase A2 (Lp-PLA2), a member of the phospholipase superfamily, is an inflammation-related factor mainly synthesized and secreted by macrophage (McConnell and Hoefner Citation2006). It mediates vascular inflammation through the regulation of lipid metabolism in blood (Huang et al. Citation2020). The substrates of Lp-PLA2 are mainly oxidized phospholipids, in particular, those having a polar fatty acid moiety that are generated during the oxidation of LDL and apoptosis (Wilensky and Macphee Citation2009), generating proinflammatory lysophosphatidylcholine to impose inflammatory stress on brain microvascular endothelial cells (Lum et al. Citation2003) Clinically, Lp-PLA2 has become an emerging inflammatory marker that is used to assess the risk for cardiovascular disease (CVD) and associated events such as ischemic stroke (Carlquist et al. Citation2007; Han et al. Citation2017; Lin et al. Citation2015). It has been shown to play an important role in the pathophysiological process of atherosclerosis and high Lp-PLA2 level is associated with ischemic stroke and cognitive change (Pokharel et al. Citation2019; Swardfager et al. Citation2017; Tsimikas et al. Citation2007). This bioactive phospholipid can impact platelet activation and coagulation level (Kim et al. Citation2020; Tsironis et al. Citation2004). A number of studies demonstrate that inhibition of Lp-PLA2 can reduce peripheral measures of inflammation in nonclinical and clinical studies (Berger et al. Citation2011; Zhang et al. Citation2013). Recently, Lp-PLA2 inhibitors have been found to slow the progression of AD (Maher-Edwards et al. Citation2015).
Due to the disorder of glucose and lipid metabolism, diabetes often causes microangiopathy and complications in the central nervous system (CNS), such as CSVD and cerebral large vessel disease (CLVD) (Benjamin et al. Citation2014; Brundel et al. Citation2014; Roberts et al. Citation2014). Bleeding, occlusion, fibrinoidosis and amyloidosis are the main manifestations of CSVD, which has occult onset with no obvious clinical manifestations (Venkatesan et al. Citation2014) and can be deteriorated due to diabetes (de Havenon et al. Citation2019).
Although WMH has been associated with congestive encephalopathy (van Dijk and Willinsky Citation2003) and CI (Hu et al. Citation2021; van den Berg et al. Citation2018), the relationship between Lp⁃PLA2 level and cognitive decline in diabetes patients with WMH has not been elucidated. This study was conducted to investigate the relationship and diagnostic value of Lp⁃PLA2 for CI in type II diabetes mellitus (DMII) patients with WMH. The findings would be useful to provide convenient tool for CI diagnosis in DMII patients with WMH.
Materials and methods
Participants
Patients diagnosed WMH between December 2018 and January 2020 at our hospital were eligible for the study. Patients were prospectively included if he/she had WMH based on cranial T1-weighted MRI (T1WI), T2-weighted imaging (T2WI), and fluid-attenuated inversion recovery (FLAIR) sequence MR imaging and diagnosed with DMII based World Health Organization guidelines (American Diabetes Citation2019), was ≥ 40 years old with complete clinical records. Patients were excluded if he/she was complicated with stroke, cerebral microbleeds and subarachnoid hemorrhage, CNS infection, multiple sclerosis patients, serious deficiency of folic acid and vitamin B or complicated with severe mental disorder and AD. Patients were also excluded if they had type 1 diabetes, or acute complications of diabetes mellitus. Age- and sex-matched healthy people were recruited as control. He/she was recruited if aged ≥ 40 years without complaint of CI and had the MOCA score of ≥ 26, with no obvious WMH found in MRI, no lacunar infarction, cerebral microbleeds nor rheumatism in the past six months. The participants were excluded if he/she had severe liver and kidney diseases, mental illness and incomplete clinical data. This study was approved by the Ethic Committee of Qiqihar Medical University (reference number CS131), and written informed consent was obtained from every participant.
Data collection
Demographic and clinical data, including laboratory findings, were retrieved from the hospital databases, such as gender, age, education level, the history of hypertension, DM, smoking (average 6 cigarettes per day and more than three months in a row), drinking (more than 28 units of alcohol per week for three months, 1 unit alcohol = 285 ml beer or 25 ml spirits or 1 glass of wine). Laboratory tests were carried out within 24 h of admission and the findings were collected, including fasting blood glucose, the serum levels of homocysteine, blood fibrinogen (FIB), uric acid, triglyceride, total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C).
Lp⁃PLA2 measurement
Serum Lp⁃PLA2 levels in fasting venous blood collected in the morning from included participants were determined using a Beckman AU 5800 analyzer as described previously (Wang et al. Citation2019). Lp-PLA2 in the serum hydrolyzes the substrate, 1-myristoyl-2-(4-nitrophenyl succinyl) phosphatidylcholine to generate a colored reaction product, 4-nitrophenol. The formation rate of 4-nitrophenol is measured spectrophotometrically to quantify Lp-PLA2. The assay was carried out using the reagent Lp-PLA2 FS from DiaSys, Germany, according to the manufacturer’s instructions. The assay had a precision of <3% coefficient variation with a detection limit of 10 ng/mL and was performed as part of normal care procedure.
Assessment of cognitive function
Assessment of cognitive function was conducted independently by two neurologists who were blinded to the patient information using the Montreal cognitive assessment (MoCA) as described previously (Nasreddine et al. Citation2005). The total score was 30 and patients with total score of <23 was classified as having cognitive impairment. The assessment was conducted within five days of Lp-PLA2 assay for all patients.
Statistical analysis
SPSS 23.0 (IBM, USA) was used to analyze the data. The measurement data were tested for normality, and normal variables were expressed as means ± standard derivation (SD). Difference between means was compared with Student’s t-test. Counting data were expressed as % and compared using χ2-test. Influencing factors were analyzed using multivariate logistic regression. These analyses were adjusted for demographic characteristics (age, sex, education) and covariates related to higher levels of inflammation and health risk (smoking, drinking, hypertension, diabetes). The diagnostic value was analyzed using the receiver operating characteristic curve (ROC). P < 0.05 was considered statistically significant.
Results
Study population characteristics
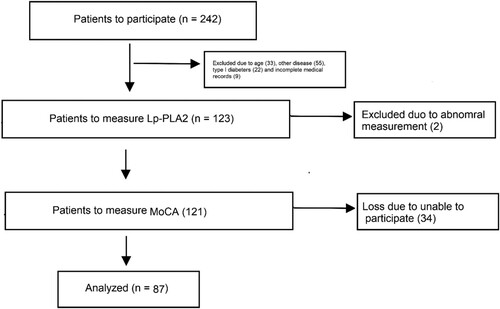
A total of 242 patients were identified and 87 of WMH patients were included in this study (Figure ), including 52 males and 35 females with an average age of 58.1 ± 4.7 years. Eighty-two healthy participants were recruited as control, including 48 males and 34 females with an average age of 57.3 ± 4.8 years. The demographic and clinical data of the participants are listed in Table . Analysis showed that there was no difference in age, gender, educational level, the histories of hypertension and smoking, the serum levels of uric acid, triglyceride, TC, LDL-C and FIB between WMH and healthy control. However, the levels of fast blood glucose, homocysteine, HDL-C and Lp-PLA2 were significantly different between the two groups. In addition, the MoCA scores were significantly lower in WMH group than in control (22.2 ± 3.7 vs. 28.2 ± 2.7, P < 0.01).
Table 1. Demographic and clinical data of patients with WMH and healthy participants.
Factors associated with cognitive ability
In this study, MoCA was used to assess CI. Therefore, factors that are associated with CI are also associated with MoCA. Among the WMH patients, 35 had a MoCA score of less than 23, and were classified into CI group, while the remaining 52 were grouped into NCI group. Comparison of these two groups showed that CI patients had significantly higher levels of homocysteine, HDL-C and Lp⁃PLA2 as compared with NCI patients (Table ), while other clinical parameters such as age, gender, educational level, the histories of hypertension and smoking, the serum levels of uric acid, triglyceride, TC, LDL-C and FIB were not significantly different between the two groups (Table ).
Table 2. Demographic and clinical data of WMH patients with CI and NCI.
Risk factors affecting cognitive ability
Logistic regression analysis was performed using CI as the dependent variable and four significant variables in univariate analysis as independent variables. The results showed that HDL-C, homocysteine and Lp⁃PLA2 were significant risk factors for CI (Table ) with OR varying from 1.497 (homocysteine) to 3.384 (Lp-PLA2).
Table 3. Significant risk factors of cognitive impairment identified by multivariate logistic regression analysis in DMII patients with WMH.
Diagnostic value of HDL-C and lp⁃PLA2 for CI
Since above analysis showed that HDL-C and Lp⁃PLA2 appeared to have greater OR for CI, the ROC curves were plotted for HDL-C and Lp⁃PLA2 to calculate if they could be diagnostic indicators for CI using CI and NCI as positive and negative outcomes, respectively. The analysis results showed that the AUCs were 0.718 (95%CI: 0.529–0.882) and 0.818 (95%CI: 0.629–0.932) for HDL-C and Lp⁃PLA2, respectively (Figure , Table ). In addition, if the two indicators were combined, the AUC were 0.858 (95%CI: 0.629–0.972), which is significantly higher than that of single indicator (P < 0.05).
Figure 2. Receiver operating characteristic (ROC) curves of the HDL-C and Lp⁃PLA2 within 24 h after admission for congestive impairment. Abbreviation: AUC, area under the curve.
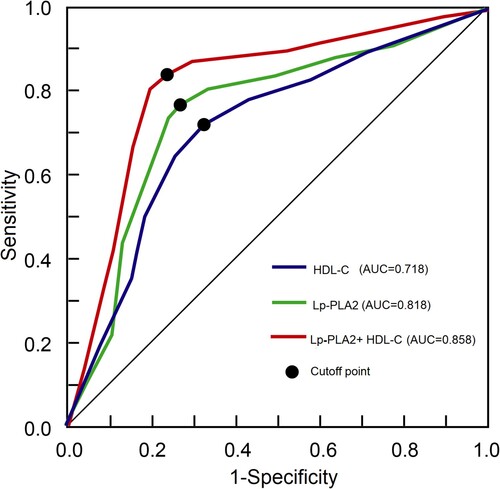
Table 4. Diagnostic value of high-density lipoprotein cholesterol and Lp⁃PLA2 for cognitive impairment in WMH patients.
Discussion
In this study, WMH patients with type II DM were analyzed for CI and the results showed that the MoCA scores were lower and the Lp⁃PLA2 level was significantly elevated in the WMH patients as compared with healthy individuals and NCI patients, suggesting that Lp⁃PLA2 may be involved in the pathogenesis of WMH, leading to CI.
Several factors have been identified as risk factors for WMH, such as age and hypertension. Aging may lead to gradual hardening of cerebral arterioles, affecting the blood supply to the brain, and eventually cerebral infarction, and decreased myelin sheath level and increased demyelination (Goldberg et al. Citation1997). In addition to age, studies have confirmed that hypertension is also a risk factor for WMH (Guevarra et al. Citation2020). However, hypertension is not associated with WMH in this study, which may be attributed to sample variation. Diabetic patients often have lipid metabolism disorder, which increases blood viscosity, leading to hypercoagulability and hyperviscosity, increasing the risk of vascular disease (Baktiroglu et al. Citation2016; Strain and Paldanius Citation2018). In type 2 diabetes, serum lipoprotein(a) concentration is independently associated with diabetic retinopathy (Tu et al. Citation2017). Diabetes mellitus could also be associated with WMH progression, leading to cognitive decline and functional disability (Tamura and Araki Citation2015). Therefore, diabetic patients with WMH were selected as study subjects in our study.
WMH is known to cause dementia and reduced cognitive ability (Lampe et al. Citation2019; van den Berg et al. Citation2018). This is consistent with our findings. Recent studies show that WMH is associated with CSVD-induced CI (Lampe et al. Citation2019; Tozer et al. Citation2018), and inflammation plays an important role in the pathogenesis of CSVD and dementia (King et al. Citation2018). However, it is unclear if WMH-associated CI is related to the serum level of Lp-PLA2. By comparing the demographic and clinical data between WMH and healthy groups, significant differences were found in diabetes, fasting blood glucose, homocysteine, HDL-C and Lp⁃PLA2 between CI and NCI groups; when the two groups were compared, they were also significantly different in homocysteine, HDL-C and Lp⁃PLA2. Therefore, it is likely that these are among many factors that may contribute to WMH and CI.
Logistic regression analysis showed that HDL-C, Lp-PLA2 and homocysteine are significant risk factors for CI. The relationship between homocysteine and CI has been demonstrated previously (Kim et al. Citation2018; Smith et al. Citation2018) and levels of plasma homocysteine is association with CI in healthy elderly community dwellers (Ravaglia et al. Citation2003) and in elderly Latinos participating in the Sacramento Area Latino Study on Aging (Miller et al. Citation2003), although in other studies, no association is found between serum homocysteine level and cognitive skills (Kalmijn et al. Citation1999; Ravaglia et al. Citation2000), suggesting that such association may be population-specific. This study suggests that high level of HDL-C and Lp-PLA2 may be involved in the occurrence and development of CI in patients with WMH. However, this is different from a previous cross-sectional study involving 78 cases of AD and 59 cases of amnestic mild CI, in which Lp-PLA2 was not found associated with cognitive function (Davidson et al. Citation2012). In our analysis, several demographic characteristics and covariates were adjusted as confounding variables such as age, sex, education level, smoking, drinking, hypertension and diabetes, because they may impact CI directly or indirectly. For example, heavy alcohol consumption and is negatively associate with cognitive function (Clare et al. Citation2017), while the number of years of formal education completed by individuals is positively correlated with their cognitive function and predicts lower risk of dementia late in life (Lovden et al. Citation2020). Since Lp-PLA2 is likely to exert effect on cognitive function through vascular inflammation, it is more likely to affect CI due to vascular inflammation. For example, Zhang et al. found that serum Lp-PLA2 level is negatively associated with the MoCA scores in patients with vascular dementia and is a risk factor for ischemic stroke complicated with vascular dementia (Zhang et al. Citation2018).
There are several mechanisms related to Lp-PLA2-induced inflammation. Lp-PLA2 may form instable plaques in blood to bind with LDL and deposited to the necrotic nucleus of macrophages and the fibrous cap of the plaque to hydrolyze oxidized phospholipids, leading to production of proinflammatory factors and plaque formation (Lin et al. Citation2015). Although previous studies have shown that in patients with low LDL-C levels, Lp-PLA2 has the strongest correlation with vascular events (Lin et al. Citation2015), in this study we found that the level of circulating LDC-L is not associated with CI. Therefore, Lp-PLA2 may play a role in the pathogenesis of WHM CI through regulating vessels and inflammation rather than LDC-L metabolism.
As a new type of biomarker, Lp-PLA2 appears to be a highly sensitive predictor for cardiovascular and cerebrovascular events with high sensitivity and specificity as compared with traditional inflammatory indicators (Zhang et al. Citation2021), which is confirmed in this study. Our ROC analysis showed that it has significant diagnostic value for CI with an AUC of 0.818 (95%CI: 0.629–0.932). In addition, we found that HDL-C also has diagnostic value for CI as well. HDL-C has been known to influx into the brain and be associated inversely with the risk of AD, although the association between serum HDL-C and AD risk are inconsistent in different studies (Gatz et al. Citation2010; Mielke et al. Citation2012). Since Lp-PLA2 as an endothelial lipase, it may play a role HDL-C metabolism such hydrolysis of HDL, leading to reduced HDL-C level (Ahmed et al. Citation2006; Cohen Citation2003). However, it is unclear whether this is a link between Lp-PLA2 and HDL-C in the patients, although combined use of them could have better diagnostic accuracy for CI.
Previously, interleukin-6 (IL-6) but not C-reactive protein (CRP) measured in midlife was found to predict cognitive decline after the analysis was adjusted for baseline age, sex, education, ethnicity, smoking, obesity and other clinical condition, suggesting that circulating levels of inflammatory markers are raised before clinical onset of circulating levels of inflammatory markers are raised before clinical onset of dementia and CI (Singh-Manoux et al. Citation2014). In other studies, high CRP but not IL-6 is associated with impaired cognition in healthy aging population (Teunissen et al. Citation2003). It is likely that these consistencies may result from difference in patient populations. Our finding that Lp-PLA2 and HDL-C are predictive for CI is derived from a cohort of type II diabetes patients with WMH. It would be interesting to know whether similar relationship exists in general populations or in subjects without WMH. Since CI is likely the consequences of AD and dementia, our finding may be used as a tool to assist CI diagnosis to help address public health issues, particularly in elderly populations.
There are limitations with this study. As a single center study, the sample size is relatively small. The prognostic value of Lp-PLA2 and HDL-C needs to be validated with independent populations. Furthermore, the relationship between Lp⁃PLA2 level and cognitive decline in diabetes patients without WMH was not investigated in this study and should be studied side by side to further elucidate the role of WMH in CI.
Conclusions
Our study indicates that there are association between CI and Lp-PLA2 or HDL-C in type II DM patients with WMH; Lp-PLA2 and HDL-C significantly diagnostic value for CI, particularly when used together. Since both Lp-PLA2 and HDL-C are serum-based markers, patient populations can be screened in appropriate clinical settings or community centers to collect blood samples and assessed for Lp-PLA2 and HDL-C levels. Patients whose Lp-PLA2 and HDL-C levels are over the diagnostic cutoff values may be referred to have further medical tests or consultation for potential CI. Implantation of this approach would assist early and differential diagnosis of CI for better management of the disease.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data are not available.
References
- Ahmed W, Orasanu G, Nehra V, Asatryan L, Rader DJ, Ziouzenkova O, et al. 2006. High-density lipoprotein hydrolysis by endothelial lipase activates PPARalpha: a candidate mechanism for high-density lipoprotein-mediated repression of leukocyte adhesion. Circ Res 98:490–498.
- American Diabetes A. 2019. 2. Classification and diagnosis of diabetes: standards of Medical Care in diabetes-2019. Diabetes Care. 42:S13–S28.
- Baktiroglu S, Yanar F, Ozata IH, Oner G, Ercan D. 2016. Arterial disease and vascular access in diabetic patients. J Vasc Access 17(Suppl 1):S69–S71.
- Barkovich AJ, Deon S. 2016. Hypomyelinating disorders: An MRI approach. Neurobiol Dis 87:50–58.
- Benjamin P, Lawrence AJ, Lambert C, Patel B, Chung AW, MacKinnon AD, et al. 2014. Strategic lacunes and their relationship to cognitive impairment in cerebral small vessel disease. NeuroImage Clin. 4:828–837.
- Berger JS, Ballantyne CM, Davidson MH, Johnson JL, Tarka EA, Lawrence D, et al. 2011. Peripheral artery disease, biomarkers, and darapladib. Am Heart J 161:972–978.
- Brundel M, Kappelle LJ, Biessels GJ. 2014. Brain imaging in type 2 diabetes. Eur Neuropsychopharmacol 24:1967–1981.
- Carlquist JF, Muhlestein JB, Anderson JL. 2007. Lipoprotein-associated phospholipase A2: a new biomarker for cardiovascular risk assessment and potential therapeutic target. Expert Rev Mol Diagn 7:511–517.
- Clare L, Wu YT, Teale JC, MacLeod C, Matthews F, Brayne C, et al. 2017. Potentially modifiable lifestyle factors, cognitive reserve, and cognitive function in later life: A cross-sectional study. PLoS Med 14:1–14.
- Cohen JC. 2003. Endothelial lipase: direct evidence for a role in HDL metabolism. J Clin Invest 111:318–321.
- Davidson JE, Lockhart A, Amos L, Stirnadel-Farrant HA, Mooser V, Sollberger M, et al. 2012. Plasma lipoprotein-associated phospholipase A2 activity in Alzheimer's disease, amnestic mild cognitive impairment, and cognitively healthy elderly subjects: a cross-sectional study. Alzheimers Res Ther 4:51–58.
- de Havenon A, Majersik JJ, Tirschwell DL, McNally JS, Stoddard G, Rost NS. 2019. Blood pressure, glycemic control, and white matter hyperintensity progression in type 2 diabetics. Neurology. 92:e1168–e1e75.
- Ederle J, Davagnanam I, van der Worp HB, Venables GS, Lyrer PA, Featherstone RL, et al. 2013. Effect of white-matter lesions on the risk of periprocedural stroke after carotid artery stenting versus endarterectomy in the International carotid stenting study (ICSS): a prespecified analysis of data from a randomised trial. Lancet Neurol 12:866–872.
- Gatz M, Reynolds CA, Finkel D, Pedersen NL, Walters E. 2010. Dementia in Swedish twins: predicting incident cases. Behav Genet 40:768–775.
- Goldberg CJ, Fogarty EE, Moore DP, Dowling FE. 1997. Fluctuating asymmetry and vertebral malformation. A study of palmar dermatoglyphics in congenital spinal deformities. Spine. 22:775–779. (Phila Pa 1976).
- Graff-Radford J, Arenaza-Urquijo EM, Knopman DS, Schwarz CG, Brown RD, Rabinstein AA, et al. 2019. White matter hyperintensities: relationship to amyloid and tau burden. Brain. 142:2483–2491.
- Guevarra AC, Ng SC, Saffari SE, Wong BYX, Chander RJ, Ng KP, et al. 2020. Age moderates Associations of hypertension, White matter hyperintensities, and cognition. J Alzheimer's Dis 75:1351–1360.
- Hainsworth AH, Minett T, Andoh J, Forster G, Bhide I, Barrick TR, et al. 2017. Neuropathology of White matter lesions, blood-brain barrier dysfunction, and dementia. Stroke. 48:2799–2804.
- Han L, Zhong C, Bu X, Xu T, Wang A, Peng Y, et al. 2017. Prognostic value of lipoprotein-associated phospholipase A2 mass for all-cause mortality and vascular events within one year after acute ischemic stroke. Atherosclerosis. 266:1–7.
- Hu HY, Ou YN, Shen XN, Qu Y, Ma YH, Wang ZT, et al. 2021. White matter hyperintensities and risks of cognitive impairment and dementia: A systematic review and meta-analysis of 36 prospective studies. Neurosci Biobehav Rev 120:16–27.
- Huang F, Wang K, Shen J. 2020. Lipoprotein-associated phospholipase A2: The story continues. Med Res Rev 40:79–134.
- Kalmijn S, Launer LJ, Lindemans J, Bots ML, Hofman A, Breteler MM. 1999. Total homocysteine and cognitive decline in a community-based sample of elderly subjects: the rotterdam study. Am J Epidemiol 150:283–289.
- Kim J, Kim H, Roh H, Kwon Y. 2018. Causes of hyperhomocysteinemia and its pathological significance. Arch Pharmacal Res 41:372–383.
- Kim M, Yoo HJ, Lee D, Lee JH. 2020. Oxidized LDL induces procoagulant profiles by increasing lysophosphatidylcholine levels, lysophosphatidylethanolamine levels, and Lp-PLA2 activity in borderline hypercholesterolemia. Nutr Metab Cardiovasc Dis 30:1137–1146.
- King E, O'Brien JT, Donaghy P, Morris C, Barnett N, Olsen K, et al. 2018. Peripheral inflammation in prodromal Alzheimer's and Lewy body dementias. J Neur Neurosurg Psychiatry 89:339–345.
- Lampe L, Kharabian-Masouleh S, Kynast J, Arelin K, Steele CJ, Loffler M, et al. 2019. Lesion location matters: The relationships between white matter hyperintensities on cognition in the healthy elderly. J Cereb Blood Flow Metab 39:36–43.
- Lee S, Viqar F, Zimmerman ME, Narkhede A, Tosto G, Benzinger TL, et al. 2016. White matter hyperintensities are a core feature of Alzheimer's disease: evidence from the dominantly inherited Alzheimer network. Ann Neurol 79:929–939.
- Lin J, Zheng H, Cucchiara BL, Li J, Zhao X, Liang X, et al. 2015. Association of Lp-PLA2-A and early recurrence of vascular events after TIA and minor stroke. Neurology. 85:1585–1591.
- Lovden M, Fratiglioni L, Glymour MM, Lindenberger U, Tucker-Drob EM. 2020. Education and cognitive functioning across the life span. Psychol Sci Public Interest 21:6–41.
- Lum H, Qiao J, Walter RJ, Huang F, Subbaiah PV, Kim KS, et al. 2003. Inflammatory stress increases receptor for lysophosphatidylcholine in human microvascular endothelial cells. Am J Physiol Heart Circ Physiol 285:H1786–9.
- Maher-Edwards G, De'Ath J, Barnett C, Lavrov A, Lockhart A. 2015. A 24-week study to evaluate the effect of rilapladib on cognition and cerebrospinal fluid biomarkers of Alzheimer's disease. Alzheimer's Dementia: Transl Res Clin Interventions. 1:131–140.
- McConnell JP, Hoefner DM. 2006. Lipoprotein-associated phospholipase A2. Clin Lab Med 26:679–697. vii.
- Merino JG. 2019. White matter hyperintensities on magnetic resonance imaging: What is a clinician to do? Mayo Clin Proc 94:380–382.
- Mielke MM, Montine T, Khachaturian AS. 2012. Vascular diseases: one pathway toward new conceptual models of dementia. Alzheimers Dement. 8:S69–S70.
- Miller JW, Green R, Ramos MI, Allen LH, Mungas DM, Jagust WJ, et al. 2003. Homocysteine and cognitive function in the Sacramento Area Latino Study on aging. Am J Clin Nutr 78:441–447.
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. 2005. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53:695–699.
- Nolze-Charron G, Mouiha A, Duchesne S, Bocti C. 2015. Alzheimer's Disease Neuroimaging I. White matter hyperintensities in mild cognitive impairment and lower risk of cognitive decline. J Alzheimer's Dis 46:855–862.
- Petersen RC. 2004. Mild cognitive impairment as a diagnostic entity. J Intern Med 256:183–194.
- Pokharel Y, Mouhanna F, Nambi V, Virani SS, Hoogeveen R, Alonso A, et al. 2019. Apob, small-dense LDL-C, Lp(a), LpPLA2 activity, and cognitive change. Neurology. 92:e2580–e2e93.
- Ravaglia G, Forti P, Maioli F, Muscari A, Sacchetti L, Arnone G, et al. 2003. Homocysteine and cognitive function in healthy elderly community dwellers in Italy. Am J Clin Nutr 77:668–673.
- Ravaglia G, Forti P, Maioli F, Zanardi V, Dalmonte E, Grossi G, et al. 2000. Blood homocysteine and vitamin B levels are not associated with cognitive skills in healthy normally ageing subjects. J Nutr Health Aging. 4:218–222.
- Roberts RO, Knopman DS, Przybelski SA, Mielke MM, Kantarci K, Preboske GM, et al. 2014. Association of type 2 diabetes with brain atrophy and cognitive impairment. Neurology. 82:1132–1141.
- Singh-Manoux A, Dugravot A, Brunner E, Kumari M, Shipley M, Elbaz A, et al. 2014. Interleukin-6 and C-reactive protein as predictors of cognitive decline in late midlife. Neurology. 83:486–493.
- Smith AD, Refsum H, Bottiglieri T, Fenech M, Hooshmand B, McCaddon A, et al. 2018. Homocysteine and dementia: An International consensus statement. J Alzheimer's Dis 62:561–570.
- Smith EE, Beaudin AE. 2018. New insights into cerebral small vessel disease and vascular cognitive impairment from MRI. Curr Opin Neurol 31:36–43.
- Stewart CR, Stringer MS, Shi Y, Thrippleton MJ, Wardlaw JM. 2021. Associations between White matter hyperintensity burden, cerebral blood flow and transit time in small vessel disease: An updated meta-analysis. Front Neurol 12:1–19.
- Strain WD, Paldanius PM. 2018. Diabetes, cardiovascular disease and the microcirculation. Cardiovasc Diabetol 17:1–10.
- Swardfager W, Yu D, Ramirez J, Cogo-Moreira H, Szilagyi G, Holmes MF, et al. 2017. Peripheral inflammatory markers indicate microstructural damage within periventricular white matter hyperintensities in Alzheimer's disease: A preliminary report. Alzheimer's Dementia: Diagnosis, Assessment Dis Monit 7:56–60.
- Tamura Y, Araki A. 2015. Diabetes mellitus and white matter hyperintensity. Geriatr Gerontol Int 15(Suppl 1):34–42.
- Teunissen CE, van Boxtel MP, Bosma H, Bosmans E, Delanghe J, De Bruijn C, et al. 2003. Inflammation markers in relation to cognition in a healthy aging population. J Neuroimmunol 134:142–150.
- Tozer DJ, Zeestraten E, Lawrence AJ, Barrick TR, Markus HS. 2018. Texture analysis of T1-weighted and fluid-attenuated inversion recovery images detects abnormalities that correlate With cognitive decline in small vessel disease. Stroke. 49:1656–1661.
- Tsimikas S, Tsironis LD, Tselepis AD. 2007. New insights into the role of lipoprotein(a)-associated lipoprotein-associated phospholipase A2 in atherosclerosis and cardiovascular disease. Arterioscler, Thromb, Vasc Biol 27:2094–2099.
- Tsironis LD, Mitsios JV, Milionis HJ, Elisaf M, Tselepis AD. 2004. Effect of lipoprotein (a) on platelet activation induced by platelet-activating factor: role of apolipoprotein (a) and endogenous PAF-acetylhydrolase. Cardiovasc Res 63:130–138.
- Tu WJ, Liu H, Liu Q, Cao JL, Guo M. 2017. Association between serum lipoprotein(a) and diabetic retinopathy in Han Chinese patients With type 2 diabetes. J Clin Endocrinol Metab 102:2525–2532.
- van den Berg E, Geerlings MI, Biessels GJ, Nederkoorn PJ, Kloppenborg RP. 2018. White matter hyperintensities and cognition in Mild cognitive impairment and Alzheimer's disease: A domain-specific meta-analysis. J Alzheimer's Dis 63:515–527.
- van Dijk JM, Willinsky RA. 2003. Venous congestive encephalopathy related to cranial dural arteriovenous fistulas. Neuroimaging Clin N Am 13:55–72.
- Venkatesan P, Balakrishnan R, Ramadoss K, Iyer RS. 2014. Heart appearance sign in pontine stroke: a result of bilateral infarction due to small vessel disease. Neurol India 62:115–116.
- Vipin A, Foo HJL, Lim JKW, Chander RJ, Yong TT, Ng ASL, et al. 2018. Regional white matter hyperintensity influences grey matter atrophy in mild cognitive impairment. J Alzheimer's Dis 66:533–549.
- Wang D, Guo X, Hou L, Cheng X, You T, Li H, et al. 2019. Measuring lipoprotein-associated phospholipase A2 activity in China: protocol comparison and recalibration. J Clin Lab Anal 33:1–13.
- Wardlaw JM, Valdes Hernandez MC, Munoz-Maniega S. 2015. What are white matter hyperintensities made of? relevance to vascular cognitive impairment. J Am Heart Assoc 4:1–32.
- Wilensky RL, Macphee CH. 2009. Lipoprotein-associated phospholipase A(2) and atherosclerosis. Curr Opin Lipidol 20:415–420.
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, et al. 2004. Mild cognitive impairment–beyond controversies, towards a consensus: report of the International working group on Mild cognitive impairment. J Intern Med 256:240–246.
- Zhang F, Guo J, Yang F, Zhou Y. 2021. Lp-PLA2 evaluates the severity of carotid artery stenosis and predicts the occurrence of cerebrovascular events in high stroke-risk populations. J Clin Lab Anal. 35:1–12.
- Zhang H, Zhang JY, Sun TW, Shen DL, He F, Dang YH, et al. 2013. Amelioration of atherosclerosis in apolipoprotein E-deficient mice by inhibition of lipoprotein-associated phospholipase A2. Clin Invest Med 36:32–41.
- Zhang Q, Rao P, Zhang G. 2018. Correlation between serum lipoprotein associated phosphatase A2 and vascular dementia induced by ischemic stroke. Nat Med J China. 15:1171–1175.