?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Nanoparticles open up new possibilities for treating skin problems. For the treatment of numerous skin problems, the topical route of administration has many advantages. This method avoids the hepatic first-pass impact, and many medications’ systemic availability is limited to skin cells like hair follicles, reducing undesired side effects and increasing localised therapeutic benefit. The skin's barrier function makes it difficult for nanoparticles to penetrate the tissue, even if the barrier is partially impaired in cases of damage or inflammation, such as in skin cancer. This could make nanoparticle penetration easier. Although a lot of work has gone into producing nanoparticles for topical distribution, there has been very little success in getting them to the clinic to treat skin malignancies. We review the various forms of skin malignancies and current clinical care strategies. Clinical therapy and management are also illustrated, explaining various skin cancer treatment options. In a brief manner, this study also emphasises the various nanoformulations that are being explored and reported, as well as the various types of nanoparticle systems.
Hghlights
Skin cancer is one of the widely occurring disease worldwide with varying mortality rates.
Clinical supervision of skin cancer is crucial as it may lead to some serious consequences.
Nanoparticle/nanovesicle systems would aid in better treatment and reduced adverse effects.
Different nano systems are currently under development for the topical treatment of skin cancer.
Penetration analysis of NPs is also important to develop a topical novel nanoformulation.
Abbreviations: NMSC, non-melanoma skin cancer; MSC, melanoma skin cancer; BCC, basal cell carcinoma; cSCC, cutaneous squamous cell carcinoma; SC, stratum corneum; BCS, biopharmaceutics classification system; UV, ultraviolet; WHO, World Health Organization; AK, actnic keratosis; PDT, photo dynamic therapy; 5-FU, 5-fluorouracil; nm, nanometre; mV, millivolts; FDA, Food and drug administration; API, active pharmaceutical ingredient; NPs, nanoparticles; SLNs, solid-lipid nanoaparticles; NLCs, nanostructured lipid carriers; AuNPs, gold nanoparticles; QDs, quantumn dots; CNTs, carbon nanotubes
Introduction
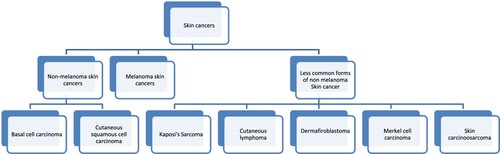
The prevalence of skin cancer has risen drastically in recent years (Adibzadeh et al. Citation2021; Elston Citation2009; Long et al. Citation2012). Skin cancers can be majorly categorised into two broad categories – melanoma skin cancer (MSC) and non-melanoma skin cancers (NMSC) (Aggarwal et al. Citation2019; Rahmati et al. Citation2020) (Figure ). MSCs are originated from melanocytes, whereas NMSCs originates from the epidermally linked cells (Radomska et al. Citation2019). NMSCs are further classified as basal cell carcinoma (BCC) and cutaneous squamous cell carcinoma (cSCC), about 99% of NMSCs are BCC or cSCC. MSC are the least occurring cancers, but they progress aggressively. NMSC are the most occurring skin cancers worldwide compared to melanoma skin cancer, but the treatments for these cancers are available and are easier to treat (Cohen and Lee Citation2016; Waldman and Grant-Kels Citation2021). Other than BCC and cSCC the other less common forms of NMSC are Kaposi's sarcoma, dermafibroblastoma, Merkel cell carcinoma, skin carcinosarcoma, cutaneous lymphomas (Cohen and Lee Citation2016). NMSCs have a great incidence of occurrence globally, and due to their high treatment costs, there is a greater public health concern and a major issue (Verkouteren et al. Citation2017).
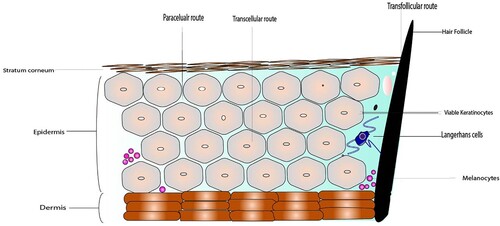
Figure 1. Structure of Skin membrane barrier showing different layers of skin tissue like stratum corneum followed by epidermis and lastly dermis along with different routes of permeation through skin.
Skin is the body's biggest organ, covering up to 16% of whole-body parts and acts as the first line of defence for the invading microorganisms, skin irritants, harmful chemicals, and Ultraviolet radiation (Bauhammer Citation2020). Apart from this, the skin acts as a thermo regulator of the body and maintains the salt water balance, i.e. overall homeostasis of the human body (Edwards Citation2011). The skin's protective mechanism is due to physical properties (pH, desquamation) and metabolic enzymes localised in the interstitial spaces of the viable epidermis and regions of dermal hair follicles (Kassem and Abd El-Alim Citation2021). The topical/dermal route of drug delivery through the upper-sectional layer of skin is very important. It helps localise the drug at the desired site, and the first-pass metabolism following oral delivery can be avoided (Tekade et al. Citation2017). Additionally, the topical route of drug delivery helps deliver the drug to skin lesions in skin cancer over the large surface area of the skin throughout the body (Desai et al. Citation2010).
In recent times there are many research going on to combat multiple cancer (Ang et al. Citation2021; Gao et al. Citation2021; Huang et al. Citation2021; Liu and Huang Citation2021; Lv et al. Citation2021; Wang et al. Citation2021; Ye et al. Citation2021; Zheng et al. Citation2021; Zhou et al. Citation2021; Zhou et al. Citation2020). In this context, there are various treatment options available for skin cancers. Options used for treatment are based on different aspects like the tumour's location, degree of progression, and the dimension of the tumour (Madan et al. Citation2010). The introduction of nanoparticles or nanotechnology has greatly changed drug delivery in every route of administration (Patra et al. Citation2018). Nanoparticles greatly enhance drug permeability and specificity while improving bio-availability, overall therapeutic efficacy, and increased patient consent to treatment therapy (Bhattacharya Citation2020; Sharma et al. Citation2019). Additionally, due to the nanoparticles’ adaptive properties, the retention time of drugs at the site of inflammation inside human skin increases. Still, virtually it is nearly impracticable to penetrate these nanoparticles to go across the skin (Amar Citation2007; Organization Citation2013). Various reports have shown and reported the delivery of nanoparticles via trans appendageal route (Desai et al. Citation2010). The trans appendageal route comprises sweat glands, hair follicles, sebaceous glands, and pilosebaceous glands (Verma et al. Citation2016). But this route covers not more than 0.2% of the overall skin area; therefore, it fails to enter the large complex molecules and the nanoparticles to the extensive deep layers of the skin tissue where the disease site is primarily located (Moosavi et al. Citation2018; Salvioni et al. Citation2021).
In this review article, we discuss different occurrences of skin cancer with its different types. The skin barrier properties are also emphasised along with present-day clinical management of skin cancer, focusing on non-surgical treatment therapy. This review also focuses on the different types and aspects of the nanoparticles used clinically for the topical/dermal delivery of the anti-neoplastic agents. Further, this review describes the interaction of nanoparticles with skin tissue, the different routes for drug delivery through the skin, and the detailed analysis of the topical penetration of the nanoparticles. This review explains the possible ways for topical/dermal delivery and provides guidelines for topical formulation development to aspiring researchers.
Skin: a challenging barrier
Skin is the outermost and wide-ranging organ of the body with a surface area of approximately 1.8m2 (Romes et al. Citation2021). The skin tissue comprises different layers consisting of hair follicles and sebaceous glands, and these layers, in general, are divided as subcutaneous tissue, dermis and epidermis (Cheng et al. Citation2021). The epidermis is the most outer layer. The stratum corneum is the peripheral part and is the potential barrier for delivery of anti-neoplastic agents at the diseased site, which consequently affects the overall efficiency of the topical and transdermal formulation (More et al. Citation2016). Usually, the SC consists of approximately 15–30 corneocyte layers inside human skin that impart a thickness of approximately 10–20 µm (Liu Citation2017). As a result of a process like epidermal maturation, keratinisation, and differentiation, these layers are formed. Followed by the stratum corneum, the viable layer is the epidermis (Chermnykh et al. Citation2020). The epidermis is the peripheral layer of the skin tissue. It consists primarily of keratinocytes at different maturation classes; it also consists of melanocytes, Langerhans and Merkel cells and ranges in thickness from 50 to 100 µm (Mahr Citation2017) (Figure ). The dermis is located beneath the epidermis, vascularised and responsible for nutrition to the epidermis (Khavkin and Ellis Citation2011). It originated from the mesoderm and holds cutaneous components like hair follicles, sebaceous glands, sweat glands, and nerves (Arda et al. Citation2014). Additionally, it also contains tremendous immune cells and fibroblasts responsible for the physiologic response of the skin. Nanoparticles that penetrate into the epidermis have access to viable and immunologically active cells and could transit into the lymph nodes (Krishnan and Mitragotri 2020).
Factors of nanoparticles that influence the delivery of drugs to skin cancer
The skin, the body's largest organ, is layered and complex. It can shield the body from the elements and regulate body temperature by regulating water and heat loss. Furthermore, many topical drugs come into contact with a very strong skin barrier (Borgheti-Cardoso et al. Citation2020). The stratum corneum, the skin's strongest barrier, prevents drugs from penetrating deeper into the layers of the skin. Drugs must also overcome the antimicrobial barrier, Langerhans cells in the epidermis, macrophages in the dermis, dendritic cells, and enzymatic systems, in addition to the stratum corneum. Drug molecule fates during skin penetration through the stratum corneum Nanoparticles have recently gained popularity as a permeation-enhancing strategy for overcoming the skin's various barrier properties. Targeting different skin organelles, such as the pilosebaceous gland, hair follicle, and dermis layer, has become increasingly popular in recent decades (Zhi et al. Citation2021). The use of nanotechnology in skin cancer research has resulted in a lot of effort being put into developing new imaging and therapeutic approaches; the main focus is on diagnosing and treating metastatic melanoma (Shah et al. Citation2021). Anticancer drugs with hydrophilic properties are known to have a low oil/water partition coefficient, high molecular weights, and ionic properties, making them difficult to penetrate the stratum corneum. Drug permeation through the stratum corneum is governed by Fick's second law (Equation (1)).
(1)
(1) where J is the flux, Dm is the drug's membrane diffusion coefficient, Cv is the drug concentration in the vehicle, P is the drug partition coefficient, and L is the thickness of the stratum corneum.
When nanoparticles are administered intravenously, the surface of the nanoparticles has a significant impact on how cytotoxic, active, and effective they are. As a result, a well-designed particle surface can aid nanoparticle entry and retention in the cells they're supposed to be in. By preventing agglomeration and flocculation, more electrostatic repulsion between particles on particle surfaces with either a positive or negative charge made nanosystems more stable. Furthermore, because cells’ plasma membranes are negatively charged, positively charged particles are more likely than negatively charged or neutral molecules to bind to target cells via electrostatic interactions. Phospholipids with a negative charge are also found in the membranes of tumour cells. Positive particle surfaces can also be designed to help nucleic acids form complexes with one another. This allows the nucleic acid to pass through the cell membrane and into the cytoplasm or nucleus more easily. PEGylation is a well-known surface modification that gives nanoparticles a longer half-life and greater overall effectiveness than non-stealthy nanoparticles (Dennison et al. Citation2022). This method reduces the likelihood of nanoparticles being taken up by the mononuclear phagocytic system, preventing them from being removed from the body and increasing the likelihood of cancer cells consuming them. Antibodies, proteins, aptamers, and folate, among other surface-modified NP strategies, guide nanoparticles to cancer cells with positive receptors. Following that, the nanoparticles enter the cells via receptor-mediated endocytosis. Nanoparticles, on the other hand, can be extremely beneficial if the therapeutic agent can reach the correct location in the cytoplasm, mitochondria, or nucleus without being destroyed by lysosomes.
Clinical treatments of skin cancers
Stress can build up in the body, resulting in skin carcinogenesis, which has three stages: initiation, promotion, and progression, all of which can be stopped with the use of bioactive agents (Figure ) (Hallan et al. Citation2020). Skin cancer, as stated earlier, has a various choice of treatment, including surgical procedures, chemotherapy, photodynamic therapy (PDT), etc. (Sidoroff and Thaler Citation2010). The surgical procedure includes cryosurgery, curettage and desiccation, laser therapy, dermabrasion, and Moh's micrographic surgery (Lagler and Freitag Citation2012). The drawback of surgical procedure is that their effectiveness is limited only for single and visible lesions and has some serious health complications (Wu et al. Citation2020). Radiotherapy is used mostly for the elderly population. It could significantly impact treatment efficacy and is mostly useful for treating lesions and tumours placed at a cumbersome place. Also, radiotherapy is done for adjuvant treatment, usually performed in a reoccurrence of cancer after surgery; it prevents the future risk of cancer (Colquhoun Citation2020).
When the lesions are advanced all over the body and the surface area for treatment is large, a non- surgical approach of treatment is considered. Photo dynamic therapy (PDT) involves photosensitising agents, and specific wavelengths of light are redirected to the tumour site along with photosensitising agents (Mfouo-Tynga et al. Citation2021). This specific light wavelength activates the compound and generates cytotoxic oxygen molecules, which oxidises and subsequently kills the tumour (Lucky et al. Citation2015). Electro chemotherapy is also important in treating NMSCs (Baltás et al. Citation2017). It is based on the principle of electroporation, in which penetration of drug inside cancerous cells is increased by the physical delivery system (Yarmush et al. Citation2014). It uses short and intense electric fields that increase the permeability of cell membrane and increases the permeability of poorly permeable drugs, and, therefore, increases their overall cytotoxicity (Poompavai and Gowri Sree Citation2021). In immunotherapy, the use of interferons is emphasised to strengthen the immune system to fight against cancer. Immunotherapy is useful for treating skin cancers advanced to the head and neck portion of the body (Economopoulou et al. Citation2016).
Topical therapy is generally intended where there are multiple lesions and treatment areas is vast. The development of surgical scars could be avoided with the treatment by topical therapy (Zagórska-Dziok and Sobczak Citation2020). Besides the cosmetic benefits, topical therapy has some limitations; topical formulations do not penetrate well into the skin. Hence, the cure rate by topical therapy is very much lower compared to surgical therapy. Topical therapy causes increased duration of the overall treatment (Tambunlertchai et al. Citation2021). Therefore, the patient adherence to the treatment lacks consistency and hence the risk of developing severe inflammation or systemic toxicity is more (Biswasroy et al. Citation2021). Some medications that are generally used for topical therapy are corticosteroids, Resiquimod, Imiquimod, 5-fluorouracil (Efudex), Diclofenac, Tazarotene, Bexarotene, Carmustine, Mechlorethamine, Ingenol mebutate, Retinoids and Photo dynamic therapy (PDT) (V. Krishnan and Mitragotri Citation2020). While these drugs are used for topical therapy, some of them, such as Ingenol mebutate, 5-fluorouracil (Efudex), and Imiquimod, have side effects that are primarily due to cytokine release, which causes skin irritation when used on larger areas (Cramer and Stockfleth Citation2020; Hengge et al. Citation2006). Topical therapy is limited due to severe pain, skin irritation, scarring, dermatitis, erythema, edoema, ulceration, pruritus, and a variety of other severe side effects (Haase et al. Citation2012).
Theranostic approach in skin cancer therapy
Researchers are looking for single or combination treatments that can be tested in vitro and in vivo before being tested on humans in clinical trials. Cancer-related immune checkpoints, as well as surgical procedures, adjuvant therapies, immunotherapy, targeted therapies, radiation therapy, and targeted therapies and theranostic approaches for other cancers, are all included in these treatment regimens. We anticipate that the theranostic approaches will not only improve treatment efficacy but will also prevent the emergence of drug resistance in melanoma, based on the recent development of clinical trials for triple therapy (combined BRAF/MEK inhibition with PD-1 blockade) and drug tolerance to target therapy in melanoma. Among the new diagnostic and therapeutic technologies being developed, the integration of imaging and treatment technology has recently received a lot of attention. This technology combines multi-modal imaging technology with treatment methods like photothermal therapy (PTT), photodynamic therapy (PDT), immunotherapy, and other new treatment methods. These studies have shown promising results in terms of reducing chemotherapeutic treatment side effects. Nano-drug delivery systems have the potential to reduce toxic side effects due to their ability to target and release drugs directly at the tumour site. The unique advantages of targeted nanocarriers loaded with drugs and imaging properties can significantly improve therapy effectiveness and specificity in difficult-to-treat cases, as well as overall treatment effectiveness. Nanotheranostics has a pivotal role in skin cancer therapy, according to Md. Habban Akhter et al., (Citation2020), because in recent years, functionalisation of liposomes, nanoshells, nanostructured lipid carriers, liposome, ethosome, bilosome, polymeric nanoparticle, nanosphere, dendrimers, carbon nanotubes, quantum dots, solid lipid nanoparticles, dendrimers, carbon nanotubes had extensively took place to kill cancerous cells, moreover to kill cancerous cells passive targeting of chemotherapeutic agents is also helpful in dealing with carcinoma.
Nanotechnology based therapeutic approach in skin cancer treatment
Nanoparticle based drug delivery is believed to be one of the best and attractive approaches for treating skin cancer because the efficiency and efficacy of therapy are boosted with the targeted delivery of the biomolecules with minimum toxic effects (Botella et al. Citation2011; Deaguero et al. Citation2020). Patient compliance also plays a pivotal role in formulating the nanoparticle-based formulation (Rizvi and Saleh Citation2018). These nanoparticles based formulations have been tried and tested both in-vitro and in-vivo (Anand et al. Citation2010). Despite this fact, nanoparticle-based therapies are not yet approved clinically for commercial use, and many studies are still at the preclinical level (Bobo et al. Citation2016). We have highlighted the works and research carried out for topical delivery of the anti-cancer drugs in this context. We have also emphasised different nanocarriers systems that have been tested and evaluated as topical medication and how some parameters define the optimisation of the topical formulation product.
Anti-cancer agents like Curcumin, Vismodegib, and 5-fluorouracil are formulated and characterised as a topical treatment in treating skin cancers (Goyal et al. Citation2017). But of the above-listed agents, 5-fluorouracil is the most commonly utilised in treating melanomas and non-melanomas (Ishioka et al. Citation2018). Owing to the adverse toxic effects of the drug, it is highly challenging for the patient to follow the treatment regimen strictly (Al-Harbi Citation2012). Adding to the above circumstances, drugs BCS class (Class III) and the hydrophilicity with a partition coefficient of -0.89 possess potential barrier to drugs for permeation through the skin (L. Zheng et al. Citation2020). But keeping aside all the above limitations, various research studies have been governed and evaluated for the nanoparticles based system of 5-flurouracil (Yassin et al. Citation2010). One such system was reported by Safwat et al. in which 5-FU loaded gold (Au) nanoparticles having a mean diameter size of 16.03 ± 0.20 nm with zeta potential having positive values (+47.80 ± 0.43 mV) was incorporated in Pluronic F127 gel and or cream base (vanishing) for topical/dermal application on cSCC tumour xenograft model. Increased drug flux within the peripheral epidermis and dermis was seen when ex-vivo studies skin permeation was performed on mice dorsal skin having full thickness (V. Krishnan and Mitragotri Citation2020; Safwat et al. Citation2016). Eighteen-fold reductions in tumour growth were seen when compared with this nanoparticle-based system with free 5- FU gel and unattended treatment (Safwat et al. Citation2016). In another study where the system was developed in a vesiculised form, enhanced drug retention was seen. In this study, different vesicle systems like liposomes (size = 120.2 ± 98 nm; zeta potential = −37.0 mV), niosomes (size = 250.40 ± 8.60 nm; zeta potential =−33.41 mV), and transferosomes (size = 153.4 ±10.5 nm; zeta potential = −39.5 mV) were developed (Mahira et al. Citation2019). Due to the higher lipidic (oily nature) content and elastic behaviour of the vesicles, increased permeation through the lipophilic nature of the stratum corneum is facilitated (Gupta et al. Citation2012). The nanovesicle system is an excellent and adjustable system in which various excipients can be combined and cross-combined to improve the efficacy of the overall system (Gao et al. Citation2018). Some researchers have suggested incorporating hydrogel viz hydroxypropyl methylcellulose, which contributes to the maximum deposition of a drug inside the skin (Mahant et al. Citation2020). The hydrogel vehicle can hydrate the stratum corneum and thereby disrupt it forming hydrophilic channels and delivering the drug within the skin (Kim et al. Citation2020). Other than the topical formulation, some locally acting radioactive formulation, i.e. radioactive bandages, have also been developed, which have some edge over topical systems like ease of application, high selectivity for skin cancer lesions, easy to store, and they are easily disposable (Ambekar and Kandasubramanian Citation2019). Table summarises and highlights different types of the formulation systems that are being researched, developed, characterised and evaluated for the effective treatment of the melanoma and non-melanoma skin cancer. The table also summarises the size characteristics of the formulation and what effect is exerted on the skin by the system is also emphasised (Table ).
Table 1. Different types of research studies showing performance of different nanoparticle system in skin cancer treatment with major emphasis given to 5-fluorouracil with some studies with different API.
As nanoparticle-based therapy is gaining a lot of recognition and attention for skin cancer treatment and the treatment of other diseases, these NP based formulations are yet to be approved by FDA for the topical/dermal therapy of skin cancer treatment (Zhou et al. Citation2021). The FDA approved a liposomal emulsion containing a bacterial repair enzyme called T4N5 in 2007 to treat hypersensitivity to light (photosensitivity) in patients with a condition called xeroderma pigmentosum (Akhtar and Khan Citation2016). The liposomal formulation has shown to prevent the actinic keratosis (AK) lesions in clinical studies when applied as a topical formulation for almost a year (Krutmann et al. Citation2015). One such formulation of 5-aminolevulinic acid (5-ALA), Ameluz® (BF- 200 ALA), is a nanosized lipid vesicle novel formulation (Schulten et al. Citation2012). This novel lipid vesicle formulation is in clinical trials and is intended to treat superficial basal cell carcinoma combined with photo thermal therapy (Bulbake et al. Citation2017). Nanobio Corporation developed a new nano-based topical antiviral emulsion – NB-001, which is in clinical trials for cold sores and Herpes Labialis (Patravale and Desai Citation2014). Nanoparticle-based formulation of paclitaxel for effective treatment of cutaneous melanomas under NMSC is also under clinical trials for the safety, tolerability and efficacy studies (Paolino et al. Citation2012).
Various types of physical methods like radiation therapy, microneedle technology, ultrasound, iontophoresis, and many more, it is easy for the penetration and or permeation of nanoparticles into the skin with the aid of such physical methods. These methods require a lot of equipment with unique features and therefore not compatible for patients always (Peto et al. Citation1976). The review highlights the delivery of drugs by the topical/dermal route without the aid of any physical equipment.
Different nanoparticle systems for topical/dermal delivery
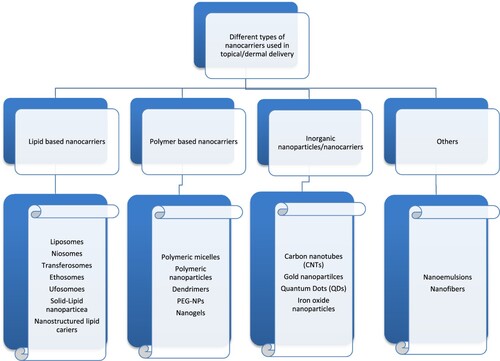
It is a well-known fact that nanotechnology is at a primary stage in skin cancer treatment (Goyal et al. Citation2017). Different varieties of nanoparticles are being researched and developed for skin cancer treatment (Gupta et al. Citation2012). Giving account to the various magnitude and classes of events, different barriers to skin cancer, different types of nanoparticles have been designed, fabricated and characterised for applications in the topical/dermal delivery of anti-cancer drugs. This includes lipid-based nanocarriers like liposomes, niosomes, ufasomes, transferosomes, ethosomes, solid lipid nanoparticles, dendrimers, nanoemulsions, metallic nanoparticles, polymeric nanoparticles, etc. (Bolla Citation2020). Further, the combination of various anti-cancer drugs with nanoparticles can be formulated in a suitable dosage form like cream, lotion, ointment, hydrogel, TD patches for topical/transdermal delivery (Das Kurmi et al. Citation2017). Several publications with vast literature are available for different nanoparticles that are used extensively. Here some of the nanoparticles intended for topical delivery are described in a brief manner (Figures and ).
Figure 4. Various nanocarriers of different classes – 2a- Lipid based nanocarriers (Liposome), 2b- Polymer based nanocarriers (Polymeric micelle) 2c- Inorganic nanocarriers (Gold nanoparticle)-1479 with permission from Springer Nature - License no: 5010250744068).
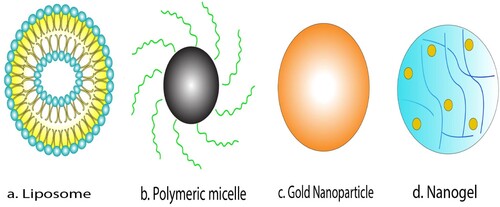
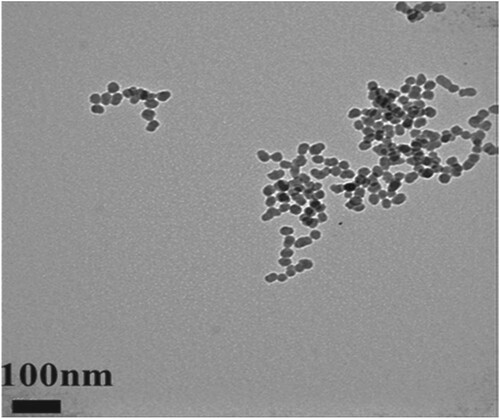
Figure 5. TEM images showing the uniform size of 20 nm of Silica NPs (Adapted from Lee et al. Citation2016. A quantitative study of nanoparticle skin penetration with interactive segmentation. Medical & biological engineering & computing, 54(10), pp.1469-1479 with permission from Springer Nature – License no: 5010250744068).
Lipid-based nanocarriers
Lipid-based nanocarriers or nanoparticle are one of the oldest nanoparticle systems. As shown in Figures and , it mainly consists of the vesicular system like liposomes, ethosomes, niososmes, ethosomes, etc. (Chuang et al. Citation2018). But additionally, it also consists of other lipid-based nanoparticles like solid lipid nanoparticles and nanostructured lipid carriers.
Talking about vesicular carriers’ liposomes is one of the conventional lipid vesicles primarily composed of phospholipids and cholesterol, which assembles into phospholipid bilayered vesicles that encapsulate the drug in the two regions having both aqueous core which favours hydrophilic moieties and hydrophobic entities outside the core (Barros et al. Citation2016). With decreased toxicity, liposomes can boost the medication's pharmacokinetics, specificity, and effectiveness (Kaminskas et al. Citation2012). The first instance where the liposomes were introduced as a topical delivery agent for skin diseases was Mezei and Gulasekharam in 1980 (Elsayed et al. Citation2007; Smith Jr. Citation2007). The rationale of liposomes in skin delivery is the lipoidal nature of liposomes allows their adsorption on the skin tissue and thus initiates the drug release by fusing with the stratum corneum (SC). Large liposomes are removed from deeper skin tissue layers. Small liposomes with a vesicular diameter of approximately 30–40 nm may be small and often monodisperse, acquired by a microfluidic approach to increase the capacity to penetrate the skin of these lipid NPs (Yang Citation2019). Furthermore, in in vitro studies, a blend of liposomes with biocompatible co-polymers resulted in the production of vesicles with an improved ability to penetrate deep skin layers (Carter et al. Citation2019; Love et al. Citation2009).
Niosomes are the lipid vesicles with non-ionic surfactants and in which cholesterol may be present or absent, but these are more stable and economically cheaper than conventional liposomes (Khan and Irchhaiya Citation2016). Also, niosomes are the first choice and extensively used for topical/dermal delivery of cosmetics and drugs like naftifine hydrochloride, fluconazole, and various other anticancer drugs also the overall effect of charge. Different surfactants like Brij 72, Span 40 and Span 60 have also been studied deeply in noisome preparation niosomes are the first choice and extensively used for topical/dermal delivery of cosmetics and drugs (Badenhorst Citation2015; Kumar and Rajeshwarrao Citation2011). Furthermore, for deeper penetration and better permeation into the skin tissue, a new vesicular system was introduced, and it was called transferosomes or deformable liposomes (Rajan et al. Citation2011). As conventional liposomes interact and exert their therapeutic action on the peripheral layer of the skin, we can say precisely as only with the stratum corneum its dermal use is very limited, and the new system was developed. Transferosomes are highly elastic and flexible with deformable properties, and Cevc and Blume first invented them in 1990 (Rai et al. Citation2017). The structural properties of transferosomes are similar to that of liposomes with aqueous (hydrophilic) core and phospholipid membrane and additional edge activator (Rajan et al. Citation2011). Edge activators are agents that provide flexibility and versatility by destabilisation of the lipid bilayer of the vesicle, decreasing the interfacial stress and increasing its structural deformability (Drashti Patel and Chatterjee Citation2020). Phospholipids widely used to produce these vesicles include, among others, Egg-phosphatidylcholine dipalmitoyl, Soyabean phosphatidylcholine or Distearoyl phosphatidylcholine (Li et al. Citation2015). Ethanol is used as a solvent in a larger proportion to prepare transferosomes to impart flexibility and increase the overall permeation across skin (Amin et al. Citation2013).
Similarly, another vesicular system where ethanol is used as a solvent in large proportion is Ethosomes (Hallan et al. Citation2020). Ethosomes are also elastic vesicles and was first studied by Touitou et al. As discussed earlier, ethanol imparts flexibility and aids in increased penetration and or permeation in the skin ethosomes delivers the formulation into the deeper layers of the skin or most probably into the systemic circulation (Ashtikar et al. Citation2016). One study demonstrated by Fang et al. suggested that 5-aminolevulinic acid (5-ALA) encapsulated ethosomes showed better results and an enhanced rate of skin penetration when compared to its conventional liposomal formulation. Another novel vesicular formulation for topical/dermal delivery is ufasomes (Bhattacharya Citation2021). These are bilayered structures that are made of unsaturated fatty acids (oleic acid, linoleic acid) and or their derivatives (oleates), otherwise called unsaturated fatty acid liposomes (Patel et al. Citation2011). They are fatty acid colloidal suspensions and ionised soaps that can shape lipids’ bilayers and trap active lipophilic drugs (Bolla et al. Citation2019).
As an enabling carrier system, in 1991, SLNs were launched, positioning themselves at the interface between the antecedent systems of lipids (emulsions and liposomes) and polymer-based polymeric nanoparticle systems (Thi et al. Citation2021). SLNs can combine engaging properties of the two earlier systems. SLNs are colloidal system in a size range of 50 nm to 1000 nm. SLNs have many benefits, such as biocompatibility, drug safety, controlled drug release. Due to the lipid (lipophilic) nature of skin drug carriers, SLNs are known to be very attractive (Surajit Das and Chaudhury Citation2011). Indeed, drug interactions between skin and lipid SLNs may increase skin permeation (Souto et al. Citation2020). Studies have emphasised the capability of SLNs in reducing oedema or delivering mRNA and antimicrobial drugs such as terbinafine, miconazole nitrate, econazole nitrate and itraconazole into the deeper layers of skin with improved retention and sustained release ability (V. Krishnan and Mitragotri Citation2020).
NLCs are the second class of nanoparticles with lipids (Naseri et al. Citation2015). The lipid matrix of NLCs consists of a mix of solid lipid moieties and liquid lipids (oils) with a standard ratio of 70:30–99.9:0.1, and this solid–liquid lipid does not possess an ideal crystalline structure (D’Souza and Shegokar Citation2020). NLCs, as SLNs, can provide an interesting controlled release for various active molecules, protecting vulnerable compounds from sunlight, hydrolysis or oxidation (Naseri et al. Citation2015). The water content of the NLC suspension is lesser than that of the SLN suspension, which allows lipid nanoparticles to be condensed and incorporated directly into dermal formulations (Tamjidi et al. Citation2013). In addition, NLCs demonstrate good biocompatibility, tolerability and ease to manufacture into their final formulation (Fang et al. Citation2013).
Polymer-based nanocarriers
Polymers from synthetic natural and origin are employed as carriers in a nanoparticle delivery system (Bhattacharya 2020; Bolhassani et al. Citation2014). Various nanoparticle systems have been developed from polymers-based carriers like nanospheres, polymeric nanocapsules, polymerososmes, dendrimers, etc. As drug delivery mechanisms, polymeric NPs have many desirable properties, such as ease of manipulation, the capacity for functionalisation and surface modification with various ligands, in vivo behaviour regulation, biodegradability and biocompatibility (Kamaly et al. Citation2016). Most commonly, polymeric NPs are given through the systemic intravenous (IV) route of administration. However, the delivery of such nanocarriers with many therapeutic agents that could be administered through biological membranes (i.e. topical route) is currently being researched and developed (Parveen et al. Citation2012). Polymeric nanoparticles; as stated earlier, can be of natural and synthetic origin in which natural-based polymers like chitosan and synthetic polymers like poly (lactide-co-glycolide) copolymer (PLGA), poly (ϵ-caprolactone) (PCL) and polylactic acid (PLA) have been used and studies for topical/ dermal delivery (Rai et al. Citation2019). Chitosan is a biodegradable natural polymer, and owing to its cationic nature, it strongly interacts with anionic, i.e. negatively charged skin tissue. Due to this property, chitosan nanoparticles have been very effective and have also increased solubility (Ahmad et al. Citation2022; Dash et al. Citation2011). Similar studies for synthetic nanoparticles have also been carried out by Sun et al. Curcumin loaded PLGA nanoparticles showed greater treatment efficacy than hydrogel of curcumin studied in a mouse model for imiquimod induced psoriasis (Moballegh Nasery et al. Citation2020). New topical gel formulations based on sodium alginate and hyaluronic acid-containing AS1411 aptamer-functionalised polymeric nanocapsules loaded with an antitumoural medication (5-Fluorouracil) have been developed as a unique method for skin cancer treatment (Chermnykh et al. Citation2020). To characterise these obtained topical gel formulations, rheological tests, permeation assays across Strat-M® artificial membrane, ex-vivo permeation assays across chicken skin membrane, haemolysis tests, skin irritation tests, in vitro cytotoxicity assay on human basal carcinoma cells, and in vivo tests were used. Rheological experiments on the examined samples revealed that as the shear rate increases, apparent viscosity decreases, indicating shear thinning behaviour. The low levels of heamolysis (between 0.03% and 0.55%) indicated that the formulations tested did not cause red blood cell lysis (Rata et al. Citation2021).
Nanocapsules carrying 5-FU were found to be non-irritant in gel formulations (Cadinoiu et al. Citation2021). Furthermore, it was demonstrated that nanoencapsulation improves the permeability properties of 5-FU by studying ex-vivo diffusion properties across the chicken skin membrane (Jhaveri et al. Citation2021) . In vitro cytotoxicity testing on the TE 354.T (ATCC® CRL-7762TM) human basal carcinoma cell line revealed that the 5-Fluorouracil-loaded formulations have a significant cytotoxic effect. Finally, the presence of Langerhans CD68 cells in the epidermis and epithelial sheath of dermal hair follicles shows that these cells are responsible for specific nanoparticle activation, migration, and retrieval (Rata et al. Citation2021).
Other polymer-based nanoparticles, such as polymersomes built-up of amphiphilic polymer liposomal membranes, have also received increased attention due to its structural heterogeneity of such membranes (Kassem and Abd El-Alim Citation2021). As a strategy for drug delivery via skin membranes, a composite polymeric NP/hydrogel system was successfully formulated and evaluated to permeate local anaesthetic benzocaine in vitro and for monitoring nanoparticles in the artificial membrane (Morantes et al. Citation2017). A solid-in-oil (S/O) nanodispersion was produced by Wakabayashi et al. to deliver hydrophilic molecules transcutaneously, which was an attractive device (Hardiningtyas et al. Citation2018).
Dendrimers are synthetic polymers that provide a spherical shape with symmetrically organised branching units. Dendrimers have a core–shell configuration (Mittal et al. Citation2021). With each synthetic cycle, the number of surface functional groups on dendrimers exponentially increases (Abbasi et al. Citation2014). Because of their monodispersity, high density of surface functional groups and (Mittal et al. Citation2021) nano-sized spherical architecture, dendrimers created greater interest as drug delivery systems (Abbasi et al. Citation2014). Dendrimers are commonly studied as enhancers of polymeric skin permeation that can accelerate drug transfer and penetration through the skin (Dave and Krishna Venuganti Citation2017).
Further dendrimers are used to fabricate three dimensional crosslinked hydrophilic and temperature-sensitive nanogels that potentially increase the penetration through the skin of small-scale and large scale moieties (Bamrungsap et al. Citation2012). In response to ionic strength, pH gradient of skin, these nanogels can be possibly designed to go through a physical transformation for improved skin penetration and payload release (Hajebi et al. Citation2019). The ability to penetrate drugs and carriers deep within the skin to treat inflammatory diseases like psoriasis has also been illustrated by chitin-based nanogels (Shah et al. Citation2020). In addition to the nanogels, PEG-NPs have also shown to increase permeation properties of the skin (Karakoti et al. Citation2011). PEG functionalised nanoparticles act on SC to solvate it and interact with keratin, thereby disrupting the SC by withdrawing the lipidic content (Pawar and Babu Citation2010). Functionalised Au NPs with PEG and PEG-oleylamine (OAm) can penetrate the SC and accumulate in vivo deeper into the subcutaneous adipose tissue (V. Krishnan and Mitragotri Citation2020). In another study, Mahmoud et al. showed the ability of phospholipid-PEG, and cholesterol-PEG functionalised Au nanorods to potentially accumulate within different skin membrane layers and their antibacterial activity induced by photothermal activity (Mahmoud et al. Citation2019). As per Mohammad Zaki Ahmad et al. (Citation2020), chitosan has significant importance in nano-based drug delivery for the treatment of cancer. Advancement in nanoscience provides the means of specific and selective targeting of cancer cells. The best part of chitosan is that it can encapsulate both hydrophilic and lipophilic chemotherapeutic drugs, proteins/peptides, and different genetic materials like DNA, miRNA, siRNA, etc. Chitosan drug delivery can improve pharmacokinetic drug profiling as well as it can deliver the drug passively and smartly can target cancer cells.
Inorganic nanoparticles/nanocarriers
Inorganic nanoparticles are generally intended and used for imaging and diagnosing purpose but also for the treatment purpose due to its improved drug loading capacity, greater surface area; also its biocompatibility is high with reduced side effects and with some metallic nanoparticles their magnetic properties also gives an edge over other nanoparticles (Badıllı et al. Citation2020; Riehemann et al. Citation2009). Metallic nanoparticles could be preferentially fabricated from different types of metals like gold (Au), silver (Ag) and different types of metallic oxides (titanium dioxide, magnesium dioxide) (Angélica Lizeth et al. Citation2022). Au NPs are being studied and reported since the nineteenth century. Au NPs are generally intended as drug carriers to deliver drugs, imaging and diagnosis, and the new cancer therapy (Aghebati-Maleki et al. Citation2020). The rationale for using Au NPs in cancer therapy is that they have specific characteristics that favour cancer therapy to accumulate deeper into the tumour (Zhang et al. Citation2019). One study has demonstrated gold nanoparticles covalently bounded to anticancer agent gemcitabine and cetuximab as targeting agent showed potential multifunctional results in pancreatic cancer (Patra et al. Citation2010). Different literature studies have shown that Au NPs and gold nanorods have shown effectiveness when employed in photothermal therapy (Parchur et al. Citation2018). Other than the Au NPs, other gold fabricated nanostructures like gold nanoshells, gold nanospheres and gold nanocages have also outlined and shown to be effective in photothermal therapy and anti-tumour therapy (Sharma et al. Citation2018).
Carbon nanotubes (CNTs) are carbon-based nanostructured synthetic materials that are generally known for their ideal and potent nature, favouring photodynamic therapy (Ni et al. Citation2020). Carbon nanotubes can be of different types like single-walled CNTs, double-walled CNTs and multi-walled CNTs (Ganesh Citation2013). When administered at the tumour site, they increase the overall temperature within the tumour cells (Madani et al. Citation2011). CNTs deliver the drugs directly within the tumour cells, and various in vitro and in vivo studies have been performed on antibody functionalised CNTs loaded with different anticancer agents (He et al. Citation2013). While CNTs as photosensitizers for PDT have been studied for topical use, new possibilities could be anticipated for their application in the future (Van Straten et al. Citation2017). However, for skin treatments, CNTs and fullerenes need to be further studied (Madani et al. Citation2011). Like other inorganic nanoparticles, quantum dots (QDs) are also another class of nanoparticles that are generally intended for therapeutic and diagnostic use (Erathodiyil and Ying Citation2011). Quantum dots give the upper hand as QDs have unique modifiable properties but cannot explore in-depth for dermal delivery (Dichello and Sarker Citation2017). Emphasising metal oxides, (TiO2) titanium oxide and (ZnO) zinc oxide have been employed as UV filters and sun screening agents in various sun skin preparations (Smijs and Pavel Citation2011). However, various concerns have been pointed out for the toxicological effects of metal oxides in sunscreens, particularly in cases where a damaged SC barrier such as skin lesions or sun burned areas can cause deeper skin penetration and can cause local toxicity (Miller et al. Citation2021). By proving the safety of using zinc oxide in sunscreens both on damaged and undamaged skin, Roberts and his colleagues answered these concerns (Angélica et al. Citation2022). Without inducing epidermal toxicity, the particles only collected on the upper surfaces of skin and inside the furrows.
Nanopharmaceutical sciences have enormous potential for improving cancer therapeutic pharmacokinetics, effectiveness, and safety through interdisciplinary applications (Abdel-Mageed et al. Citation2021). The utilisation of nanoplatforms has largely overcome the constraints of conventional therapeutic platforms used for skin cancer therapy (Cheng et al. Citation2021). For effective skin cancer therapy, different polymeric, lipidic, and inorganic nanoplatforms have been developed. The stimuli-responsive nanoplatforms, such as pH-responsive and temperature-responsive platforms, were also looked at (Chang et al. Citation2021). Different tactics for potentiating the application of nanoparticles for cancer therapy, such as surface engineering, drug conjugation, stimulus-responsive, and multimodal effect, have also been studied and compared to existing conventional treatments (Ang et al. Citation2021). As these discussed nanopharmaceutical systems are preliminary, various further research studies are needed in this area (Medici et al. Citation2021).
Phytochemical-based nanomedicine
A significant new strategic compilation of phytochemicals with potent antitumour properties has been made, with a particular focus on cell cycle arrest and apoptotic signalling mechanisms. The elimination of cancer cells, preferably via cell cycle arrest and programmed cell death with less harm to neighbouring normal cells, is a promising approach in tumour prevention. Cancer cells have a survival advantage because they can avoid apoptosis and divide incessantly to proliferate, speeding up the cell cycle. Because of their biocompatibility, low cytotoxicity, low resistance, and dynamic physiochemical properties distinguishing normal cells, the use of phytochemical-derived conjugated chemotherapeutic agents has increased dramatically in recent years in the treatment of various cancer types. As per Shadab, Md et al. (Citation2021) (α-mangostin-loaded polymeric nanoparticle gel (α-MNG-PLGA) formulation significantly impact B16-F10 cells. Plumbagin-Loaded Glycerosome Gel has a significant impact on Skin Cancer Therapy, according to Shadab Md et al., (Citation2021) In comparison to liposome gel and Plumbagin suspension, Glycerosome-loaded gel treated rat skin showed significantly (p < .05) higher drug accumulation in the dermis, higher cytotoxicity, and higher antioxidant activity. According to Kriti Soni et al. (Citation2020), ethosomal nanogels have a significant anti-cancer effect in the B16-F10 murine tumour cell line (p < .05) for effective skin cancer therapy. From the discussion mentioned above, it is understood that Phytochemical-Based Nanomedicine has a significant impact on skin cancer treatmen.
The challenge for nanoparticles in topical therapy of skin cancers
There are the different class of challenges in front of the topical delivery of nanoparticles in skin cancer treatment (Khan et al. Citation2019). It primarily includes the potential stratum corneum barrier, and different viable layers of the skin tissue discussed earlier. Other challenges include different physiological parameters like hair follicles, rate of sebum production, skin hydration, etc. (Matsui et al. Citation2016). These parameters limit the permeation of topically applied nano-formulation and reduce the overall efficacy of the treatment (Neves Borgheti-Cardoso et al. Citation2016). Apart from this, skin irritation due to nanoparticles can also pose a problem. A study performed to fasten the local therapeutic effect of 5-fluorouracil loaded poly butyl cyanoacrylate nanoparticles showed that these nanoparticles applied topically for 38–40 days with age ranging from 56 to 90 to treat superficial BCC. The system showed good results with complete eradication of tumour, but 95% of the patient skin became red with some irritation and sores (Choudhary et al. Citation2011; Hadjikirova et al. Citation2005). This system did not get a nod for clinical studies further. For any formulation to be ideal, it must surpass all these challenges. The drug-related physicochemical properties like molecular weight, charge, solubility, hydrophilicity, etc., must also be addressed to induce a greater therapeutic response (Liefeng et al. Citation2022). As per Habban Akhter et al. (Citation2021), receptor targeting using nanoengineering is a new area. However, the limitation of enhanced permeability and retention (EPR) effect has a pivotal role in delivering cancer targeting (Akhter et al. Citation2021). In another compilation, Habban Akhter et al. (Citation2018) discussed liposomes, dendrimer, quantum dots, carbon nanotubes, metallic nanoparticles, nano lipid carrier (NLC), etc., how they act proactively against multiple cancer (Akhter et al. Citation2018). Akhter et al. (Citation2018) dissected the significance of Epidermal growth factor in skin, breast, ovary, brain, lungs, pancreas, and gastric cancer. According to Javed Ahmad et al. (Citation2020) the significant importance of nanoparticle surface engineering and how it can improve drug bioavailability and target specificity in cancer tissue was discussed in their compilation (Ahmad et al. Citation2020).
Generally, the skin irritation or in-vivo and ex-vivo studies are performed on the animal skin, or cell line studies are employed. The animal commonly used for such studies is mice and mouse xenograft models. Biologically, there is a lot of difference between human skin and mouse skin (Onaciu et al. Citation2020). The later skin is loose with more hair follicle density and fragile epidermis compared to human skin epidermis (Rittié Citation2016). Therefore, the mouse skin acts differently and cannot simulate human skin, consequently impacting the end result (Rittié Citation2016). Thus, practically we can say that these mice models cannot predict and determine the exact results and there a lot of chance to question its viability. The nanoparticles must be 21 tried directly either on patients or biologically engineered skin equivalent for precise results (Dan-Lei et al. Citation2022).
Skin penetration analysis after topical delivery of nanoparticles
As evident, various routes are being proposed for the topical/dermal delivery of different nanoparticles, including intercellular, transcellular and trans follicular, and sweat glands (Jain et al. Citation2014). Each route has its pros and cons, whereas the penetration route via hair follicle is best compared to other routes. But the permeation across the skin can be done by a combination of more than two routes (Bolzinger et al. Citation2012). The rationale for developing an ideal topical nano formulation depends on various factors. The different formulations for different indications can be designed for (1) to accumulate deep inside the epidermis and other layers of skin for conditions like skin neoplasia. The drug retention would be maximum and with a limited amount of drug penetration (Pierre and Costa Citation2011). (2) drug to be retained superficially on the skin surface with less or no penetration for superficial skin lesions (Schäfer-Korting et al. Citation2007) and (3) drugs intended for transdermal delivery for delivering the active drug directly into the bloodstream, i.e. systemic circulation (Ahmad et al. Citation2019). Thus, the skin penetration of nanoparticles should be analysed and is an important concept for designing the ideal topical delivery system (Krishnan and Mitragotri Citation2020).
As discussed earlier (Barua and Mitragotri Citation2014), different routes are available, but the route is selected based on the different physicochemical properties of the formulation, skin condition, and characteristics of the formulation (Das Kurmi et al. Citation2017). Trans appendageal route consists of routes via sweat glands and hair follicles, and the overall surface area of appendages all over the body is merely 0.1%. Still, the contribution of these appendages in nanoparticle transport is very significant (Fang et al. Citation2014). The hair follicles loaded with lymphatic and blood vessels are potential targets for nanoparticle permeation and direct nanoparticles into the systemic circulation (Kalyane et al. Citation2019). A study proposed by Lagemann et al. shows faster clearance of coloured dye when compared with the nanoparticles loaded with the similar dye when applied topically and it passes through the hair follicles. So, the trans follicular route is conceptualised to be one of the widely used routes for the skin permeation of macromolecules or nanoparticles also (Rancan and Vogt Citation2014).
The intercellular and transcellular route is called a trans epidermal route of permeation through the skin (Ng and Lau Citation2015). In the transcellular pathway, the permeation of nanoparticles occurs through the matrix (cytoplasm) and the phospholipid bio membrane of dead keratinocytes. The permeation is assessed by highly hydrated keratin of corneocytes, and due to which this route creates a hydrophilic pathway and further prefers hydrophilic moieties for transfer (Nafisi and Maibach Citation2018). Therefore, the transcellular route is not considered the potential route for permeation as minimal drug permeation occurs via corneocytes, and a lot of partitioning takes place through SC (Rastogi and Yadav Citation2014). Penetration enhancer like urea, sulphides can aid in greater permeation of nanoparticles via the transcellular route. In contrast, in the intercellular pathway, nanoparticles traverse through the lipid matrix and between the small spaces of the cells. The intercellular pathway is preferred for the uncharged lipophilic particles and is regarded as the potential route for most nanoparticles (Stewart et al. Citation2018).
(Yu et al. Citation2021). One such factor is the particle size and surface morphology like particle charge; polarity is essential and affects the ability of skin for permeation across it (Labouta et al. Citation2011). The charge on the carrier barring the charge on the API also impacts the permeation (Neupane et al. Citation2020). While there are some reports of greater skin penetration of cationic (positively charged) micro molecules, some reports suggest enhanced skin permeation of anionic (negatively charged) nanoparticles, and this shows the diverse nature of effects of surface charge of these nanoparticles on the overall skin permeation (Gupta and Rai Citation2017). The study proposed by Gillet et al. in which liposomal formulation of betamethasone and betamethasone dipropionate reported that vesicles containing negative charge showed greater flux and permeation across the skin the positively charged particles with more drug accumulation on the superficial outer layer of the SC (Gillet et al. Citation2011). Another study performed by Piem et al., in which Econazole (ECZ) incorporated with deoxycholic acid (DCA) and stearyl amine to produce negative and positively charged microemulsions and further evaluated suggests that negatively charged had a massive impact on the permeation across the skin (Firooz et al. Citation2015). Kohli and Alpar demonstrated latex particles that were positivel and neutrally charged could not preferentially penetrate the skin at all size range from 50 to 500 nm with variable permeation of negatively charged particles with good permeation having a size of 50 nm and no penetration at all with the size 101–200 nm.
Regardless of the surface charge, particle size also plays a crucial role in the permeation across the skin. Quantum dots (QDs) with a size of 4–5 nm were unable to penetrate across the skin and remained at the top layer of the stratum corneum. Some sort of penetration was seen after it was physically forced with the techniques like tape stripping and massaging for 10 minutes. The study performed on QDs by Ryman Rasmussen demonstrated positively and negatively charged QDs dispersed in buffers at pH 8.3 and 9 showed that positively charged QDs showed better and extensive permeation. Negatively chargedQDs render more time than positive and neutrally charged particles (Ryman-Rasmussen et al. Citation2006). Similar results were also obtained by Prow et al., who showed that positively charged QDs showed potential penetration since positively charged QDs have stronger electrostatic interaction with the negatively charge of human skin (Liang et al. Citation2013). Size-dependent permeation of gold nanoparticles (Au NPs) was also seen. Au NPs having a size of 15 nm were seen to permeate at a greater extent into the rat skin and the human skin, whereas when the size reduced further at 6 nm, penetration was seen more into the deeper layers (dermis) of the skin (Gupta and Rai Citation2017). Various studies have been done, and further research is going on to understand the permeation of nanoparticles through human skin (Gupta and Rai Citation2017). Different metallic nanoparticles show little or no penetration through human or porcine skin in various studies. Metallic nanoparticles, including titanium dioxide and zinc, showed no penetration in the diverse size range of 20, 30, 100, 200 nm and these nanoparticles remained at the skin furrows only (Schilling et al. Citation2010). Silver nanoparticles with almost 25 nm showed little penetration and penetrated the upper layer of the epidermis of human skin. With such an extent of variation in permeation of nanoparticles through the skin, proper analysis of penetration and deposition of these nanoparticles inside the skin is critical (Bolzinger et al. Citation2012).
One such study was performed by Lee et al. (Citation2016) He did a quantitative analysis of the penetration of Silica nanoparticles with Transmission electron microscopy (TEM). The image (Figure ) shows that the silica nanoparticles have a uniform size of 20 nm and have a uniform spherical shape. Also, he captured and observed fluorescence images and confocal images and the degree of penetration of silica nanoparticles across SC. Different statistical measures like mean, kurtosis, skewness, integrated density, and area fraction were also studies additionally to image processing. Two charge groups, i.e. negative and positive charge groups, were prepared for the analysis, and both groups showed variation in the results. Statistical significance was observed earlier in negative charge groups after two days, whereas the positive charge groups showed effect after four days. Figure (a) and Figure (a) are original images of silica nanoparticles into peripheral stratum corneum, rotated, and shift values are applied. Figure (b) and Figure (b) are termed as window images extracted from original images by Otsu's method for accurate segmentation. This process is used as a window for extraction of only fluorescent silica from original images. Figure (c) and Figure (c) are called the 8-bit images for the same fluorescent silica obtained from authentic images with the help of window images (Figure (b) and Figure (b)).
Figure 6. Negative charge image (Adapted from Lee et al. Citation2016. A quantitative study of nanoparticle skin penetration with interactive segmentation. Medical & biological engineering & computing, 54(10), pp.1469-1479 with permission from Springer Nature - License no: 5010250744068)
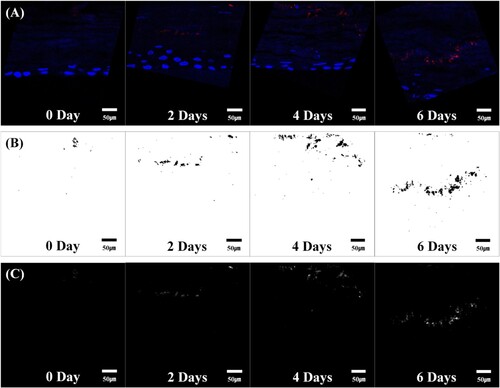
Figure 7. Positive charge image (Adapted from Lee et al. Citation2016. A quantitative study of nanoparticle skin penetration with interactive segmentation. Medical & biological engineering & computing, 54(10), pp.1469-1479 with permission from Springer Nature - License no: 5010250744068)
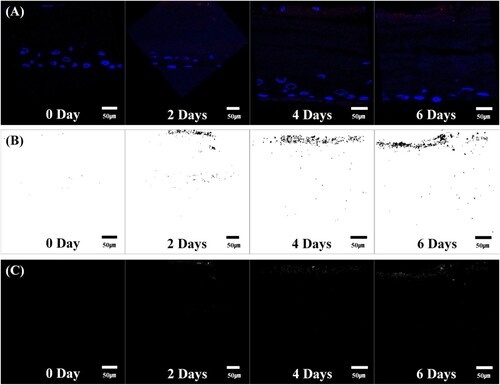
The pictures yielded by the negative charge group are shown in Figure . Fluorescent silica nanoparticles penetrated deep into 3D cells. As the time proceeds further, the number of nanoparticles was seen to be increased qualitatively and increased in the intensity at the areas where fluorescent silica is distributed. Similarly, the pictures yielded by positive charge groups are shown in Figure . Although penetration intensity of fluorescent silica nanoparticles of both charge groups is seen. But the intensity of penetration is significantly less as compared to the negative charge group results. Histogram symmetry or asymmetry is described by skewness. A decreasing positive value means that it increases the number of pixels with different intensities, and the histogram becomes symmetrical. In other words, this value is directly related to the fluorescent silica quantity, and as the positive value steadily decreases, the distribution area increases. In the positive charge group, the increase in the distribution area of fluorescent silica was significant from day four relative to day 0 as seen from Figure (c) and similarly in the negative charge group considerable increase in the distribution area of fluorescent silica was seen with day two, day four and day six gradually over time. The rate of increase in fluorescent silica was more in the negative charge group.
Figure 8. 7 Quantification results for each group. (A) Mean, (B) integrated density, (C) skewness, (D) kurtosis, (E) area fraction, (F) penetration depth. *vs. Day 0 Group, †vs. Day 2 Group, ‡vs. Day 4 Group (Student's t test, P < 0.05).
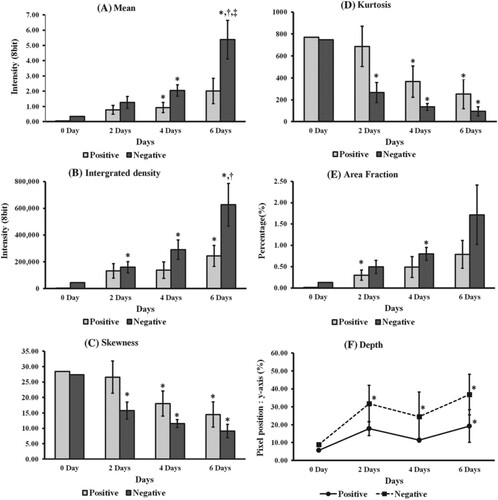
Kurtosis can be identified as a parameter that determines the intensity of fluorescent silica per unit area and determines the flatness or peakedness of the histogram. Kurtosis was seen at a later stage in the positive charge group (i.e. on day 4) compared to the negative charge group, where the kurtosis decreased significantly at day two, which can be seen in Figure (d).
The area fraction is the ratio of the fluorescent silica region to the entire image area. The negative group showed increased fraction but less statistical importance because all the ratio details were relatively closer, as seen in Figure (e). Figure (f) shows the penetration depth of the fluorescent silica, and both the groups showed penetration at day 0. Still, significant change was seen earlier in the negative group, i.e. at day two and a positive group showed somewhat later at about day 6 (Lee et al. Citation2016) (Figures ). A bundle of studies conducted for skin penetration analysis has reported the inability of these nanoparticles or nanovesicles to cross the stratum corneum. It points out that transcellular and intercellular pathways did not get involved in the picture. The only possible and convenient pathway for the permeation of nanoparticles into the deep skin layer is the trans follicular pathway (der Biologisch-Pharmazeutischen Citationn.d.). In addition to the permeation scenario, skin toxicity and safety studies of these nanoparticles are also needed. Such nanoparticles could accumulate into deep skin layers and possibly in the systemic or lymphatic circulation. One such study was performed by Kim et al. They showed the emigration of quantum dots (QDs) from epidermis to dermis to lymphatic system by Langerhans cells and macrophages of skin tissue. Also, metal oxides nanoparticles like titanium oxide (TiO2) may form free radicals and thus could cause damage to DNA which may lead to cell apoptosis via photo catalytic activity (Magdalane et al. Citation2016). Therefore, a thorough and complete analysis is essential to assess the safety and toxicity profile of any nanoparticle system intended to use as topically for the treatment of any skin related cancer (cSCC, BCC etc.).
Future challenges and conclusion
Nanotechnology for topical/dermal delivery has increased many advances in the upcoming days. It is an unconventional field of medical research with many things that are unknown to the researchers with the continuous research going on this topic. Nanotechnology offers a bundle of benefits over the old conventional ways to deliver the drug across the skin and treat or prevent skin-related lymphomas. The nanoformulations can be penetrated into deeper layers of skin without causing and other side effects to the skin tissue. These nanoformulations can be formulated with different excipients like skin penetration enhancers to aid and enhance drug permeation into different skin layers. Though nanoparticle drug delivery offers various supportive benefits over conventional therapies, there is a lack of in vitro and in vivo studies of many nanocarriers in topical/dermal delivery or skin cancer therapy.
The stratum corneum is the first barrier to transport in the skin; various strategies, including chemical enhancers, have been developed to overcome this barrier. However, these penetration enhancers have a number of disadvantages, including toxic side effects. In this context, nanomaterials research has developed new tools for extending the residence time of drugs by creating a reservoir, improving drug specificity and minimising adverse effects, and increasing the penetration of difficult-to-formulate drugs. Silica nanoparticles have been suggested as potential nanocarriers for skin delivery. Regrettably, the mechanisms governing the interaction, transport in the skin have not been thoroughly investigated.
Further studies are required for its efficient use. Various attempts have made in experimenting with the nanocarriers for dermal delivery, but in vivo – in vitro correlation is not established, so it needed further research for future development. Large scale formulation, stability, total efficacy and reproducibility are other challenges occurring while developing a nanoparticle-based drug delivery system. In conclusion, we can state that nanotechnology offers several strategies for combating skin cancer through dermal delivery. However, many efforts and research studies are still needed for the effective, safe and reproducible drug delivery system (DDS).
Acknowledgement
The authors are like to acknowledge Dr R.S. Gaud, Director, SVKM's NMIMS, Shirpur Campus, for providing necessary facilities and profound motivation while perusing this project.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Author contribution statement
Dnyanesh Saindane; Conceptualization; Writing-original draft; Writing - review & editing. Sankha Bhattacharya; Conceptualization, Writing Supervision, Validation. Prajapati BG: Concept design, analysis, interpretation, investigation, final approval of the version to be published and Rahul Shah: Design, writing, data analysis, Concept design, interpretation, final approval of the version to be published, investigation
Data availability statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.
References
- Abbasi E, Aval SF, Akbarzadeh A, Milani M, Tayefi Nasrabadi H, Woo Joo S, Hanifehpour Y, Nejati-Koshki K, Pashaei-As R. 2014. Dendrimers: synthesis, applications, and properties. Nanoscale Res Lett. 9:247. doi:10.1186/1556-276X-9-247.
- Abdel-Mageed HM, AbuelEzz NZ, Radwan RA, Mohamed SA. 2021. Nanoparticles in nanomedicine: a comprehensive updated review on current status, challenges and emerging opportunities. J Microencapsulation. 38(6):414–436. doi:10.1080/02652048.2021.1942275.
- Adibzadeh R, Golhin MS, Sari S, Mohammadpour H, Kheirbakhsh R, Muhammadnejad A, … Rahmati M. 2021. Combination therapy with TiO(2) nanoparticles and cisplatin enhances chemotherapy response in murine melanoma models. Clin Transl Oncol. 23(4):738–749. doi:10.1007/s12094-020-02463-y.
- Aggarwal A, Das N, Sreedevi I. 2019. Attention-guided deep convolutional neural networks for skin cancer classification. Paper presented at the 2019 Ninth International Conference on Image Processing Theory, Tools and Applications (IPTA).
- Aghebati-Maleki A, Dolati S, Ahmadi M, Baghbanzhadeh A, Asadi M, Fotouhi A, … Aghebati-Maleki L. 2020. Nanoparticles and cancer therapy: perspectives for application of nanoparticles in the treatment of cancers. J Cell Physiol. 235(3):1962–1972.
- Ahmad A, Mubharak N, Naseem K, Tabassum H, Rizwan M, Najda A, … Shaheen A. 2020. Recent advancement and development of chitin and chitosan-based nanocomposite for drug delivery: critical approach to clinical research. arab. J. Chem. 13:8935–8964.
- Ahmad J, Ameeduzzafar, Ahmad MZ, Akhter H. 2020. Surface-Engineered cancer nanomedicine: rational design and recent progress. Curr Pharm Des. 26(11):1181–1190. doi:10.2174/1381612826666200214110645.
- Ahmad MZ, Rizwanullah M, Ahmad J, Alasmary MY, Akhter MH, Abdel-Wahab BA, … Haque A. 2022. Progress in nanomedicine-based drug delivery in designing of chitosan nanoparticles for cancer therapy. International Journal of Polymeric Materials and Polymeric Biomaterials. 71(8):602–623. doi:10.1080/00914037.2020.1869737.
- Ahmad N, Ahmad FJ, Bedi S, Sharma S, Umar S, Ansari MA. 2019. A novel nanoformulation development of eugenol and their treatment in inflammation and periodontitis. Saudi Pharm J. 27(6):778–790.
- Akhtar N, Khan RA. 2016. Liposomal systems as viable drug delivery technology for skin cancer sites with an outlook on lipid-based delivery vehicles and diagnostic imaging inputs for skin conditions’. Prog Lipid Res. 64:192–230.
- Akhter MH, Ahsan MJ, Rahman M, Anwar S, Rizwanullah MJCN. 2020. Advancement in nanotheranostics for effective skin cancer therapy: state of the art. Current Nanomedicine. 10(2):90–104.
- Akhter MH, Beg S, Tarique M, Malik A, Afaq S, Choudhry H, Hosawi S. 2021. Receptor-based targeting of engineered nanocarrier against solid tumors: recent progress and challenges ahead. Biochim Biophys Acta Gen Subj. 1865(2):129777. doi:10.1016/j.bbagen.2020.129777.
- Akhter MH, Rizwanullah M, Ahmad J, Ahsan MJ, Mujtaba MA, Amin S. 2018. Nanocarriers in advanced drug targeting: setting novel paradigm in cancer therapeutics. Artif Cells Nanomed Biotechnol. 46(5):873–884. doi:10.1080/21691401.2017.1366333.
- Al-Harbi KS. 2012. Treatment-resistant depression: therapeutic trends, challenges, and future directions. Patient Prefer Adherence. 6:369.
- Amar A. 2007. Adaptation of the secretory machinery to pathophysiological conditions. In: R. Regazzi, editor. Molecular mechanisms of exocytosis. New York: Springer New York; p. 161–173.
- Ambekar RS, Kandasubramanian B. 2019. Advancements in nanofibers for wound dressing: A review. Eur Polym J. 117:304–336.
- Amin S, Sarfenejad A, Ahmad J, Kohli K, Mir SR. 2013. Nanovesicular transfersomes for enhanced systemic delivery of telmisartan. Adv Sci Eng Med. 5(4):299–308.
- Anand P, Nair HB, Sung B, Kunnumakkara AB, Yadav VR, Tekmal RR, Aggarwal BB. 2010. Design of curcumin-loaded PLGA nanoparticles formulation with enhanced cellular uptake, and increased bioactivity in vitro and superior bioavailability in vivo. Biochem Pharmacol. 79(3):330–338. doi:10.1016/j.bcp.2009.09.003.
- Ang MJY, Chan SY, Goh Y-Y, Luo Z, Lau JW, Liu X. 2021. Emerging strategies in developing multifunctional nanomaterials for cancer nanotheranostics. Adv Drug Delivery Rev. 178:113907. doi:10.1016/j.addr.2021.113907.
- Ang X, Xu Z, Zhou Q, Zhang Z, Ma L, Zhang X, … Chen W. 2021. PARGP1, a specific enhancer RNA associated with biochemical recurrence of prostate cancer. All Life. 14(1):774–781. doi:10.1080/26895293.2021.1969292.
- Angélica Lizeth, Sánchez-López, Perfecto-Avalos Y, Sanchez-Martinez A, Ceballos-Sanchez O, Sepulveda-Villegas M, Gabriel Rincón-Enríquez, Vicente Rodríguez-González, Rebeca Garcia-Varela, Marcelo Lozano L, Diego Eloyr Navarro-López, Gildardo Sanchez-Ante, Kaled Corona-Romero, Edgar R López-Mena. 2022. Influence of erbium doping on zinc oxide nanoparticles: structural, optical and antimicrobial activity. Appl Surf Sci. 575:151764. doi:10.1016/j.apsusc.2021.151764.
- Arda O, Göksügür N, Tüzün Y. 2014. Basic histological structure and functions of facial skin. Clin Dermatol. 32(1):3–13.
- Ashtikar M, Nagarsekar K, Fahr A. 2016. Transdermal delivery from liposomal formulations–evolution of the technology over the last three decades. J Controlled Release. 242:126–140.
- Badenhorst T. 2015. Dermal And cellular delivery Of An endogenous anti-ageing peptide using niosomes. Auckland: ResearchSpace@ Auckland.
- Badıllı U, Mollarasouli F, Bakirhan NK, Ozkan Y, Ozkan SA. 2020. Role of quantum dots in pharmaceutical and biomedical analysis, and its application in drug delivery. TrAC, Trends Anal Chem. 131:116013.
- Bagde A, Mondal A, Singh M. 2018. Drug delivery strategies for chemoprevention of UVB-induced skin cancer: A review. Photodermatol Photoimmunol Photomed. 34(1):60–68.
- Baltás E, Kis E, Nagy N, Sohár N, Varga E, Széll M, … Oláh J. 2017. Electrochemotherapy for non-melanoma skin cancer in a child with xeroderma pigmentosum. Acta Derm-Venereol. 97(8-9):962–964.
- Bamrungsap S, Zhao Z, Chen T, Wang L, Li C, Fu T, Tan W. 2012. Nanotechnology in therapeutics: a focus on nanoparticles as a drug delivery system. Nanomedicine. 7(8):1253–1271.
- Barros SM, Whitaker SK, Sukthankar P, Avila LA, Gudlur S, Warner M, … Tomich JM. 2016. A review of solute encapsulating nanoparticles used as delivery systems with emphasis on branched amphipathic peptide capsules. Arch Biochem Biophys. 596:22–42.
- Barua S, Mitragotri S. 2014. Challenges associated with penetration of nanoparticles across cell and tissue barriers: a review of current status and future prospects. Nano Today. 9(2):223–243.
- Bauhammer IAE. 2020. Establishment and validation of a viable in vitro skin model from different species for the evaluation of veterinary and human cutaneous diseases. lmu.
- Bhattacharya S. 2021. Preparation and characterizations of glyceryl oleate ufasomes of terbinafine hydrochloride: a novel approach to trigger candida albicans fungal infection. Futur J Pharm Sci. 7(3). doi:10.1186/s43094-020-00143-w.
- Bhattacharya S. 2020. Fabrication and characterization of chitosan-based polymeric nanoparticles of imatinib for colorectal cancer targeting application. Int J Biol Macromol. 151:104–115. doi:10.1016/j.ijbiomac.2020.02.151.
- Biswasroy P, Pradhan D, Kar B, Ghosh G, Rath G. 2021. Recent advancement in topical nanocarriers for the treatment of psoriasis. Aaps Pharmscitech. 22(5):1–27.
- Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR. 2016. Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm Res. 33(10):2373–2387.
- Bolhassani A, Javanzad S, Saleh T, Hashemi M, Aghasadeghi MR, Sadat SM. 2014. Polymeric nanoparticles: potent vectors for vaccine delivery targeting cancer and infectious diseases. Hum Vaccin Immunother. 10(2):321–332.
- Bolla PK. 2020. Formulation strategies To enhance solubility And permeability Of small molecules For drug delivery applications. El Paso: The University of Texas.
- Bolla PK, Meraz CA, Rodriguez VA, Deaguero I, Singh M, Yellepeddi VK, Renukuntla J. 2019. Clotrimazole loaded ufosomes for topical delivery: formulation development and in-vitro studies. Molecules. 24(17):3139. doi:10.3390/molecules24173139.
- Bolzinger M-A, Briançon S, Pelletier J, Chevalier Y. 2012. Penetration of drugs through skin, a complex rate-controlling membrane. Curr Opin Colloid Interface Sci. 17(3):156–165.
- Borgheti-Cardoso LN, Viegas JSR, Silvestrini AVP, Caron AL, Praça FG, Kravicz M, Bentley MVLB. 2020. Nanotechnology approaches in the current therapy of skin cancer. Adv Drug Delivery Rev. 153:109–136. doi:https://doi.org/10.1016/j.addr.2020.02.005.
- Botella P, Abasolo I, Fernández Y, Muniesa C, Miranda S, Quesada M, … Corma A. 2011. Surface-modified silica nanoparticles for tumor-targeted delivery of camptothecin and its biological evaluation. J Controlled Release. 156(2):246–257.
- Bulbake U, Doppalapudi S, Kommineni N, Khan W. 2017. Liposomal formulations in clinical use: an updated review. Pharmaceutics. 9(2):12. doi:10.3390/pharmaceutics9020012.
- Cadinoiu AN, Rata DM, Atanase LI, Mihai CT, Bacaita SE, Popa M. 2021. Formulations based on drug loaded aptamer-conjugated liposomes as a viable strategy for the topical treatment of basal cell carcinoma—In vitro tests. Pharmaceutics. 13(6):866. doi:10.3390/pharmaceutics13060866.
- Carter P, Narasimhan B, Wang Q. 2019. Biocompatible nanoparticles and vesicular systems in transdermal drug delivery for various skin diseases. Int J Pharm. 555:49–62.
- Chang D, Ma Y, Xu X, Xie J, Ju S. 2021. Stimuli-responsive polymeric nanoplatforms for cancer therapy. Front Bioeng Biotechnol. 9:707319. doi:10.3389/fbioe.2021.707319.
- Cheng B, Liu H, Li J, Fu X. 2021. Skin development and tissue repair and regeneration. In: Regenerative medicine in China. Springer; p. 119–138.
- Cheng Z, Li M, Dey R, Chen Y. 2021. Nanomaterials for cancer therapy: current progress and perspectives. J Hematol Oncol. 14(1):1–27.
- Chermnykh ES, Alpeeva EV, Vorotelyak EA. 2020. Transglutaminase 3: the involvement in epithelial differentiation and cancer. Cells. 9(9):1996. doi:10.3390/cells9091996.
- Choudhary S, Tang J, Elsaie ML, Nouri K. 2011. Lasers in the treatment of nonmelanoma skin cancer. Dermatol Surg. 37(4):409–425.
- Chuang S-Y, Lin C-H, Huang T-H, Fang J-Y. 2018. Lipid-based nanoparticles as a potential delivery approach in the treatment of rheumatoid arthritis. Nanomaterials. 8(1):42. doi:10.3390/nano8010042.
- Cohen DK, Lee PK. 2016. Photodynamic therapy for non-melanoma skin cancers. Cancers (Basel). 8(10):90. doi:10.3390/cancers8100090.
- Colquhoun SD. 2020. Hepatocellular carcinoma clinical update: current standards & therapeutic strategies. Liver Research. 4(4):180–190.
- Cramer P, Stockfleth E. 2020. Actinic keratosis: where do we stand and where is the future going to take US? Expert Opin Emerg Drugs. 25(1):49–58.
- Dan-Lei Yang, Fazal Faraz, Jie-Xin Wang, Norbert Radacsi. 2022. Combination of 3D Printing and Electrospinning Techniques for Biofabrication. Adv Mater Technol. 7:7. doi:10.1002/admt.202101309.
- Das S, Chaudhury A. 2011. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. Aaps Pharmscitech. 12(1):62–76.
- Das S, Das J, Samadder A, Paul A, Khuda-Bukhsh AR. 2013. Efficacy of PLGA-loaded apigenin nanoparticles in benzo [a] pyrene and ultraviolet-B induced skin cancer of mice: mitochondria mediated apoptotic signalling cascades. Food Chem Toxicol. 62:670–680.
- Dash M, Chiellini F, Ottenbrite RM, Chiellini E. 2011. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog Polym Sci. 36(8):981–1014.
- Das Kurmi B, Tekchandani P, Paliwal R, Rai Paliwal S. 2017. Transdermal drug delivery: opportunities and challenges for controlled delivery of therapeutic agents using nanocarriers. Curr Drug Metab. 18(5):481–495.
- Dave K, Krishna Venuganti VV. 2017. Dendritic polymers for dermal drug delivery. Ther Delivery. 8(12):1077–1096.
- Deaguero IG, Huda MN, Rodriguez V, Zicari J, Al-Hilal TA, Badruddoza AZM, Nurunnabi M. 2020. Nano-Vesicle based anti-fungal formulation shows higher stability, skin diffusion, biosafety and anti-fungal efficacy In vitro. Pharmaceutics. 12(6):516. doi:10.3390/pharmaceutics12060516.
- Dennison SR, Reddy SM, Morton LHG, Harris F, Badiani K, Phoenix DA. 2022. PEGylation enhances the antibacterial and therapeutic potential of amphibian host defence peptides. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1864(1):183806. doi:10.1016/j.bbamem.2021.183806.
- der Biologisch-Pharmazeutischen VdR. n.d. New Nanovesicles for Dermal Drug Delivery.
- Desai P, Patlolla RR, Singh M. 2010. Interaction of nanoparticles and cell-penetrating peptides with skin for transdermal drug delivery. Mol Membr Biol. 27(7):247–259.
- Dichello GA, Sarker DK. 2017. Encapsulation of lethal, functional, and therapeutic medicinal nanoparticles and quantum dots for the improved diagnosis and treatment of infection. In: Nanostructures for antimicrobial therapy. Elsevier; p. 597–622.
- D'Souza A, Shegokar R. 2020. Nanostructured lipid carriers (NLCs) for drug delivery: role of liquid lipid (Oil). Curr Drug Delivery. 18(3):249–270. doi:10.2174/1567201817666200423083807.
- Economopoulou P, Perisanidis C, Giotakis EI, Psyrri A. 2016. The emerging role of immunotherapy in head and neck squamous cell carcinoma (HNSCC): anti-tumor immunity and clinical applications. Ann Transl Med. 4(9):173. doi:10.21037/atm.2016.03.34.
- Edwards J. 2011. The skin is the largest organ or body system, accounting for 20% of total body weight. It functions as a barrier and first line of bodily defense against microorganisms, ultra-violet radiation, and physical forces. In addition, the skin. Pediatric Acute Care. 336.
- Elsayed MM, Abdallah OY, Naggar VF, Khalafallah NM. 2007. Lipid vesicles for skin delivery of drugs: reviewing three decades of research. Int J Pharm. 332(1-2):1–16.
- Elston DM. 2009. Topical antibiotics in dermatology: emerging patterns of resistance. Dermatol Clin. 27(1):25–31. doi:10.1016/j.det.2008.07.004.
- Erathodiyil N, Ying JY. 2011. Functionalization of inorganic nanoparticles for bioimaging applications. Acc Chem Res. 44(10):925–935.
- Fang C-L, A Al-Suwayeh S, Fang J-Y. 2013. Nanostructured lipid carriers (NLCs) for drug delivery and targeting. Recent Pat Nanotechnol. 7(1):41–55.
- Fang C-L, Aljuffali IA, Li Y-C, Fang J-Y. 2014. Delivery and targeting of nanoparticles into hair follicles. Ther Delivery. 5(9):991–1006.
- Firooz A, Nafisi S, Maibach HI. 2015. Novel drug delivery strategies for improving econazole antifungal action. Int J Pharm. 495(1):599–607.
- Ganesh E. 2013. Single walled and multi walled carbon nanotube structure, synthesis and applications. International Journal of Innovative Technology and Exploring Engineering. 2(4):311–320.
- Gao C, Bhattarai P, Chen M, Zhang N, Hameed S, Yue X, Dai Z. 2018. Amphiphilic drug conjugates as nanomedicines for combined cancer therapy. Bioconjugate Chem. 29(12):3967–3981.
- Gao P, Hao F, Zhang Q, Qiu Y. 2021. ROS mediated radiotherapy-induced protective autophagy in thyroid cancer. All Life. 14(1):58–65. doi:10.1080/26895293.2020.1862922.
- Gillet A, Compère P, Lecomte F, Hubert P, Ducat E, Evrard B, Piel G. 2011. Liposome surface charge influence on skin penetration behaviour. Int J Pharm. 411(1-2):223–231.
- Goyal N, Thatai P, Sapra B. 2017. Skin cancer: symptoms, mechanistic pathways and treatment rationale for therapeutic delivery. Ther Delivery. 8(5):265–287.
- Gupta M, Agrawal U, Vyas SP. 2012. Nanocarrier-based topical drug delivery for the treatment of skin diseases. Expert Opin Drug Delivery. 9(7):783–804.
- Gupta R, Rai B. 2017. Effect of size and surface charge of gold nanoparticles on their skin permeability: a molecular dynamics study. Sci Rep. 7(1):1–13.
- Haase A, Tentschert J, Luch A. 2012. Nanomaterials: a challenge for toxicological risk assessment? In: Molecular, clinical and environmental toxicology. Basel: Springer; p. 219–250.
- Habban Akhter M, Sateesh Madhav N, Ahmad J. 2018. Epidermal growth factor receptor based active targeting: a paradigm shift towards advance tumor therapy. Artif Cells Nanomed Biotechnol. 46(sup2):1188–1198. doi:10.1080/21691401.2018.1481863.
- Hadjikirova M, Troyanova P, Simeonova M. 2005. Nanoparticles as drug carrier system of 5-fluorouracil in local treatment of patients with superficial basal cell carcinoma. Journal-Balkan Union of Oncology. 10(4):517–521.
- Hajebi S, Rabiee N, Bagherzadeh M, Ahmadi S, Rabiee M, Roghani-Mamaqani H, … Hamblin MR. 2019. Stimulus-responsive polymeric nanogels as smart drug delivery systems. Acta Biomater. 92:1–18.
- Hallan SS, Sguizzato M, Mariani P, Cortesi R, Huang N, Simelière F, Marchetti N, Drechsler M, Ruzgas T, Esposito E. 2020. Design and characterization of ethosomes for transdermal delivery of caffeic acid. Pharmaceutics. 12(8):740. doi:10.3390/pharmaceutics12080740.
- Hardiningtyas SD, Wakabayashi R, Kitaoka M, Tahara Y, Minamihata K, Goto M, Kamiya N. 2018. Mechanistic investigation of transcutaneous protein delivery using solid-in-oil nanodispersion: A case study with phycocyanin. Eur J Pharm Biopharm. 127:44–50.
- He H, Pham-Huy LA, Dramou P, Xiao D, Zuo P, Pham-Huy C. 2013. Carbon nanotubes: applications in pharmacy and medicine. BioMed Res Int. 2013:578290. doi:10.1155/2013/578290.
- Hengge UR, Ruzicka T, Schwartz RA, Cork MJ. 2006. Adverse effects of topical glucocorticosteroids. J Am Acad Dermatol. 54(1):1–15.
- Huang H, Cao Y, Huang L, Lu R, Wang J, Zhou Y. 2021. Aloperine suppresses the proliferation, migration and invasion of human liver cancer cells via induction of G2/M cell cycle arrest and inhibition of GROα expression. All Life. 14(1):392–400. doi:10.1080/26895293.2021.1918583.
- Ishioka P, Maia M, Rodrigues S, Lellis R, Hirata S. 2018. In vivo confocal laser microscopy for monitoring of actinic keratosis treatment: a comparison with histopathologic assessment after treatment with topical 5% 5-fluorouracil. J Eur Acad Dermatol Venereol. 32(7):1155–1163.
- Jain A, Jain P, Kurmi J, Jain D, Jain R, Chandel S, … Jain A. 2014. Novel strategies for effective transdermal drug delivery: a review. Critical Reviews™ in Therapeutic Drug Carrier Systems. 31(3):219–272. doi:10.1615/critrevtherdrugcarriersyst.2014008126.
- Jhaveri J, Raichura Z, Khan T, Momin M, Omri A. 2021. Chitosan nanoparticles-insight into properties, functionalization and applications in drug delivery and theranostics. Molecules. 26(2):272. doi:10.3390/molecules26020272.
- Kalyane D, Raval N, Maheshwari R, Tambe V, Kalia K, Tekade RK. 2019. Employment of enhanced permeability and retention effect (EPR): nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Materials Science and Engineering: C. 98:1252–1276.
- Kamaly N, Yameen B, Wu J, Farokhzad OC. 2016. Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of controlling drug release. Chem Rev. 116(4):2602–2663.
- Kaminskas LM, McLeod VM, Kelly BD, Sberna G, Boyd BJ, Williamson M, … Porter CJ. 2012. A comparison of changes to doxorubicin pharmacokinetics, antitumor activity, and toxicity mediated by PEGylated dendrimer and PEGylated liposome drug delivery systems. Nanomed Nanotechnol Biol Med. 8(1):103–111.
- Karakoti AS, Das S, Thevuthasan S, Seal S. 2011. PEGylated inorganic nanoparticles. Angew Chem, Int Ed. 50(9):1980–1994.
- Kassem AA, El-Alim A, Hosam S. 2021. Vesicular Nanocarriers: a potential platform for dermal and transdermal drug delivery. In: Nanopharmaceuticals: Principles and Applications. Vol. 2. Cham: Springer; p. 155–209.
- Khan H, Ullah H, Martorell M, Valdes SE, Belwal T, Tejada S, … Kamal MA. 2019. Flavonoids nanoparticles in cancer: Treatment, prevention and clinical prospects. Paper presented at the Seminars in cancer biology.
- Khan R, Irchhaiya R. 2016. Niosomes: a potential tool for novel drug delivery. Journal of Pharmaceutical Investigation. 46(3):195–204.
- Khavkin J, Ellis DA. 2011. Aging skin: histology, physiology, and pathology. Facial Plastic Surgery Clinics. 19(2):229–234.
- Kim B, Cho H-E, Moon SH, Ahn H-J, Bae S, Cho H-D, An S. 2020. Transdermal delivery systems in cosmetics. Biomedical Dermatology. 4(1):1–12.
- Krishnan V, Mitragotri S. 2020. Nanoparticles for topical drug delivery: potential for skin cancer. Adv Drug Delivery Rev. 153:87–108. doi:10.1016/j.addr.2020.05.011.
- Krutmann J, Berking C, Berneburg M, Diepgen TL, Dirschka T, Szeimies M. 2015. New strategies in the prevention of actinic keratosis: a critical review. Skin Pharmacol Physiol. 28(6):281–289.
- Kumar GP, Rajeshwarrao P. 2011. Nonionic surfactant vesicular systems for effective drug delivery—an overview. Acta Pharmaceutica Sinica B. 1(4):208–219.
- Labouta HI, El-Khordagui LK, Kraus T, Schneider M. 2011. Mechanism and determinants of nanoparticle penetration through human skin. Nanoscale. 3(12):4989–4999.
- Lagler CNP, Freitag SK. 2012. Management of periocular actinic keratosis: a review of practice patterns among ophthalmic plastic surgeons. Ophthalmic Plastic & Reconstructive Surgery. 28(4):277–281.
- Lee O, Lee SH, Jeong SH, Kim J, Ryu HJ, Oh C, Son SW. 2016. A quantitative study of nanoparticle skin penetration with interactive segmentation. Med Biol Eng Comput. 54(10):1469–1479.
- Li J, Wang X, Zhang T, Wang C, Huang Z, Luo X, Deng Y. 2015. A review on phospholipids and their main applications in drug delivery systems. Asian J Pharm Sci. 10(2):81–98.
- Li X, Xie QR, Zhang J, Xia W, Gu H. 2011. The packaging of siRNA within the mesoporous structure of silica nanoparticles. Biomaterials. 32(35):9546–9556.
- Liang XW, Xu ZP, Grice J, Zvyagin AV, Roberts MS, Liu X. 2013. Penetration of nanoparticles into human skin. Curr Pharm Des. 19(35):6353–6366.
- Liefeng Hu, Chuxiao Xiong, Gaohui Wei, Yunhao Yu, Sihui Li, Xiaoxing Xiong, Jun-Jie Zou, Jian Tian. 2022. Stimuli-responsive charge-reversal MOF@polymer hybrid nanocomposites for enhanced co-delivery of chemotherapeutics towards combination therapy of multidrug-resistant cancer. J Colloid Interface Sci. 608:1882–1893. doi:10.1016/j.jcis.2021.10.070.
- Liu G, Huang X. 2021. Circ_0000518 contributes to breast cancer development depending on the regulation of miR-1258/ZEB1 axis. All Life. 14(1):195–208. doi:10.1080/26895293.2021.1890643.
- Liu X. 2017. Biomechanics of the human skin barrier. Doctoral dissertation, State University of New York at Binghamton.
- Long MD, Martin CF, Pipkin CA, Herfarth HH, Sandler RS, Kappelman MD. 2012. Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology. 143(2):390–399. e391.
- Love WE, Bernhard JD, Bordeaux JS. 2009. Topical imiquimod or fluorouracil therapy for basal and squamous cell carcinoma: a systematic review. Arch Dermatol. 145(12):1431–1438. doi:10.1001/archdermatol.2009.291.
- Lucky SS, Soo KC, Zhang Y. 2015. Nanoparticles in photodynamic therapy. Chem Rev. 115(4):1990–2042.
- Lv L, Zhu W, Chen J, Gou X, Xu J, Zhu W, … Shen X. 2021. Transcriptome analysis of FuZheng XiaoJi prescription inhibiting the proliferation of colorectal cancer. All Life. 14(1):719–729. doi:10.1080/26895293.2021.1963325.
- Madan V, Lear JT, Szeimies R-M. 2010. Non-melanoma skin cancer. The Lancet. 375(9715):673–685.
- Madani SY, Naderi N, Dissanayake O, Tan A, Seifalian AM. 2011. A new era of cancer treatment: carbon nanotubes as drug delivery tools. Int J Nanomed. 6:2963–2979. doi:10.2147/IJN.S16923.
- Magdalane CM, Kaviyarasu K, Vijaya JJ, Siddhardha B, Jeyaraj B. 2016. Photocatalytic activity of binary metal oxide nanocomposites of CeO2/CdO nanospheres: investigation of optical and antimicrobial activity. J Photochem Photobiol, B. 163:77–86.
- Mahant S, Kumar S, Nanda S, Rao R. 2020. Microsponges for dermatological applications: perspectives and challenges. Asian J Pharm Sci. 15(3):273–291.
- Mahira S, Kommineni N, Doppalapudi S, Khan W. 2019. Edge activated ultradeformable liposomes of psoralen and its derivatives: development and comparative evaluation for vitiligo therapy. J Drug Deliv Sci Technol. 52:83–95.
- Mahmoud NN, Alhusban AA, Ali JI, Al-Bakri AG, Hamed R, Khalil EA. 2019. Preferential accumulation of phospholipid-PEG and cholesterol-PEG decorated gold nanorods into human skin layers and their photothermal-based antibacterial activity. Sci Rep. 9(1):1–15.
- Mahr D. 2017. Winning the Race: Bacterial Growth versus Dynamic Dosage of Antimicrobial Compounds.
- Matsui MS, Pelle E, Dong K, Pernodet N. 2016. Biological rhythms in the skin. Int J Mol Sci. 17(6):801. doi:10.3390/ijms17060801.
- Md S, Alhakamy NA, Aldawsari HM, Husain M, Khan N, Alfaleh MA, … Akhter MH. 2021. Plumbagin-Loaded glycerosome Gel as topical delivery system for skin cancer therapy. Polymers (Basel). 13(6):923. doi:10.3390/polym13060923.
- Md S, Alhakamy NA, Neamatallah T, Alshehri S, Mujtaba MA, Riadi Y, … Akhter MH. 2021. Development, characterization, and evaluation of α-mangostin-loaded polymeric nanoparticle gel for topical therapy in skin cancer. Gels. 7(4). doi:10.3390/gels7040230
- Medici S, Peana M, Pelucelli A, Zoroddu MA. 2021. An updated overview on metal nanoparticles toxicity. Paper presented at the Seminars in Cancer Biology.
- Menezes AC, Campos PM, Euletério C, Simões S, Praça FSG, Bentley MVLB, Ascenso A. 2016. Development and characterization of novel 1-(1-naphthyl) piperazine-loaded lipid vesicles for prevention of UV-induced skin inflammation. Eur J Pharm Biopharm. 104:101–109.
- Mfouo-Tynga IS, Dias LD, Inada NM, Kurachi C. 2021. Biophysical and biological features of third generation photosensitizers used in anticancer photodynamic therapy. Photodiagn Photodyn Ther. 102091. doi:10.1016/j.pdpdt.2020.102091.
- Miller IB, Pawlowski S, Kellermann MY, Petersen-Thiery M, Moeller M, Nietzer S, Schupp PJ. 2021. Toxic effects of UV filters from sunscreens on coral reefs revisited: regulatory aspects for “reef safe” products. Environmental Sciences Europe. 33(1):1–13.
- Mittal P, Saharan A, Verma R, Altalbawy F, Alfaidi MA, Batiha GE, Akter W, Gautam RK, Uddin MS, Rahman MS. 2021. Dendrimers: a new race of pharmaceutical nanocarriers. BioMed Res Int. 2021:8844030. doi:10.1155/2021/8844030.
- Moballegh Nasery M, Abadi B, Poormoghadam D, Zarrabi A, Keyhanvar P, Khanbabaei H, Ashrafizadeh M, Mohammadinejad R, Tavakol S, Sethi G. 2020. Curcumin delivery mediated by bio-based nanoparticles: A review. Molecules. 25(3):689. doi:10.3390/molecules25030689.
- Mohammad O, Faisal SM, Ahmad N, Rauf MA, Umar MS, Mujeeb AA, … Ajmal M. 2019. Bio-mediated synthesis of 5-FU based nanoparticles employing orange fruit juice: a novel drug delivery system to treat skin fibrosarcoma in model animals. Sci Rep. 9(1):1–14.
- Moosavi MA, Haghi A, Rahmati M, Taniguchi H, Mocan A, Echeverría J, … Atanasov AG. 2018. Phytochemicals as potent modulators of autophagy for cancer therapy. Cancer Lett. 424:46–69. doi:https://doi.org/10.1016/j.canlet.2018.02.030.
- Morantes SJ, Buitrago DM, Ibla JF, García YM, Lafaurie GI, Parraga JE. 2017. Composites of hydrogels and nanoparticles: A potential solution to current challenges in buccal drug delivery. In: Biopolymer-Based composites. Elsevier; p. 107–138.
- More SB, Nandgude TD, Poddar SS. 2016. Vesicles as a tool for enhanced topical drug delivery. Asian J Pharm. 10(3):S196–S208.
- Munaweera I, Levesque-Bishop D, Shi Y, Di Pasqua AJ, Balkus Jr KJ. 2014. Radiotherapeutic bandage based on electrospun polyacrylonitrile containing holmium-166 iron garnet nanoparticles for the treatment of skin cancer. ACS Appl Mater Interfaces. 6(24):22250–22256.
- Nafisi S, Maibach HI. 2018. Skin penetration of nanoparticles. In: R. Shegokar, E. B. Souto, editors. Emerging nanotechnologies in immunology. Boston: Elsevier; p. 47–88.
- Naseri N, Valizadeh H, Zakeri-Milani P. 2015. Solid lipid nanoparticles and nanostructured lipid carriers: structure, preparation and application. Adv Pharm Bull. 5(3):305–313. doi:10.15171/apb.2015.043.
- Neupane R, Boddu SH, Renukuntla J, Babu RJ, Tiwari AK. 2020. Alternatives to biological skin in permeation studies: current trends and possibilities. Pharmaceutics. 12(2):152. doi:10.3390/pharmaceutics12020152.
- Neves Borgheti-Cardoso L, M Gelfuso G, FV Lopez R, Gratieri T. 2016. Topical and transdermal delivery of drug-loaded nano/microsystems with application of physical enhancement techniques. Curr Drug Targets. 17(13):1545–1559.
- Ng KW, Lau WM. 2015. Skin deep: the basics of human skin structure and drug penetration. In: N. Dragicevic, H. Maibach, editors. Percutaneous penetration enhancers chemical methods in penetration enhancement. Berlin, Heidelberg: Springer; p. 3–11.
- Ni Y, Wang R, Zhang W, Shi S, Zhu W, Liu M, Yang C, Xie X, Wang J. 2020. Graphitic carbon nitride (g-C3N4)-based nanostructured materials for photodynamic inactivation: synthesis, efficacy and mechanism. Chem Eng J. 404:126528.
- Niu J, Chu Y, Huang Y-F, Chong Y-S, Jiang Z-H, Mao Z-W, … Gao J-Q. 2017. Transdermal gene delivery by functional peptide-conjugated cationic gold nanoparticle reverses the progression and metastasis of cutaneous melanoma. ACS Appl Mater Interfaces. 9(11):9388–9401.
- Onaciu A, Munteanu R, Munteanu VC, Gulei D, Raduly L, Feder R-I, … Irimie A. 2020. Spontaneous and induced animal models for cancer research. Diagnostics. 10(9):660. doi:10.3390/diagnostics10090660.
- Organization WH. 2013. Nanotechnology and human health: Scientific evidence and risk governance.
- Paolino D, Celia C, Trapasso E, Cilurzo F, Fresta M. 2012. Paclitaxel-loaded ethosomes®: potential treatment of squamous cell carcinoma, a malignant transformation of actinic keratoses. Eur J Pharm Biopharm. 81(1):102–112.
- Parchur AK, Sharma G, Jagtap JM, Gogineni VR, LaViolette PS, Flister MJ, … Joshi A. 2018. Vascular interventional radiology-guided photothermal therapy of colorectal cancer liver metastasis with theranostic gold nanorods. ACS Nano. 12(7):6597–6611.
- Parveen S, Misra R, Sahoo SK. 2012. Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging. Nanomed Nanotechnol Biol Med. 8(2):147–166.
- Patel D, Chatterjee B. 2020. Identifying underlying issues related to the inactive excipients of transfersomes based drug delivery system. Curr Pharm Des. 27(7):971–980. doi:10.2174/1381612826666201016144354.
- Patel D, Jani R, Patel C. 2011. Ufasomes: a vesicular drug delivery. Systematic Reviews in Pharmacy. 2(2):72.
- Patra CR, Bhattacharya R, Mukhopadhyay D, Mukherjee P. 2010. Fabrication of gold nanoparticles for targeted therapy in pancreatic cancer. Adv Drug Delivery Rev. 62(3):346–361.
- Patra JK, Das G, Fraceto LF, Campos EVR, del Pilar Rodriguez-Torres M, Acosta-Torres LS, … Sharma S. 2018. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol. 16(1):1–33.
- Patravale VB, Desai PP. 2014. Topical nanointerventions for therapeutic and cosmeceutical applications. In: Focal controlled drug delivery. Boston, MA: Springer; p. 535–560.
- Pawar KR, Babu RJ. 2010. Polymeric and lipid-based materials for topical nanoparticle delivery systems. Critical Reviews™ in Therapeutic Drug Carrier Systems. 27(5).
- Peto R, Pike M, Armitage P, Breslow N, Cox D, Howard SV, … Smith P. 1976. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. introduction and design. Br J Cancer. 34(6):585–612.
- Pierre MBR, Costa IdSM. 2011. Liposomal systems as drug delivery vehicles for dermal and transdermal applications. Arch Dermatol Res. 303(9):607–621.
- Poompavai S, Gowri Sree V. 2021. Synergy of electrical pulses and black pepper (piper nigrum) extracts for effective breast cancer treatment: An in vitro model study. IETE J Res. doi:10.1080/03772063.2021.1896977.
- Radomska KJ, Coulpier F, Gresset A, Schmitt A, Debbiche A, Lemoine S, … Topilko P. 2019. Cellular origin, tumor progression, and pathogenic mechanisms of cutaneous neurofibromas revealed by mice with Nf1 knockout in boundary cap cells. Cancer Discov. 9(1):130–147.
- Rahmati M, Ebrahim S, Hashemi S, Motamedi M, Moosavi MA. 2020. New insights on the role of autophagy in the pathogenesis and treatment of melanoma. Mol Biol Rep. 47(11):9021–9032. doi:10.1007/s11033-020-05886-6.
- Rai R, Alwani S, Badea I. 2019. Polymeric nanoparticles in gene therapy: New avenues of design and optimization for delivery applications. Polymers (Basel). 11(4):745. doi:10.3390/polym11040745.
- Rai S, Pandey V, Rai G. 2017. Transfersomes as versatile and flexible nano-vesicular carriers in skin cancer therapy: The state of the art. Nano Reviews & Experiments. 8(1):1325708. doi:10.1080/20022727.2017.1325708.
- Rajan R, Jose S, Mukund VB, Vasudevan DT. 2011. Transferosomes-A vesicular transdermal delivery system for enhanced drug permeation. J Adv Pharm Technol Res. 2(3):138–143. doi:10.4103/2231-4040.85524.
- Rancan F, Vogt A. 2014. Getting under the skin: what is the potential of the transfollicular route in drug delivery? Ther Delivery. 5(8):875–877.
- Rastogi V, Yadav P. 2014. Transdermal drug delivery system: An overview. Asian J Pharm. 6(3).
- Rata DM, Cadinoiu AN, Atanase LI, Popa M, Mihai C-T, Solcan C, … Vochita G. 2021. Topical formulations containing aptamer-functionalized nanocapsules loaded with 5-fluorouracil-An innovative concept for the skin cancer therapy. Materials Science and Engineering: C. 119:111591. doi:10.1016/j.msec.2020.111591.
- Riehemann K, Schneider SW, Luger TA, Godin B, Ferrari M, Fuchs H. 2009. Nanomedicine—challenge and perspectives. Angew Chem, Int Ed. 48(5):872–897.
- Rittié L. 2016. Cellular mechanisms of skin repair in humans and other mammals. J Cell Commun Signal. 10(2):103–120.
- Rizvi SA, Saleh AM. 2018. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm J. 26(1):64–70.
- Romes NB, Abdul Wahab R, Abdul Hamid M. 2021. The role of bioactive phytoconstituents-loaded nanoemulsions for skin improvement: a review. Biotechnology & Biotechnological Equipment. 35(1):711–729.
- Ryman-Rasmussen JP, Riviere JE, Monteiro-Riviere NA. 2006. Penetration of intact skin by quantum dots with diverse physicochemical properties. Toxicol Sci. 91(1):159–165.
- Safwat MA, Soliman GM, Sayed D, Attia MA. 2016. Gold nanoparticles enhance 5-fluorouracil anticancer efficacy against colorectal cancer cells. Int J Pharm. 513(1-2):648–658.
- Salvioni L, Morelli L, Ochoa E, Labra M, Fiandra L, Palugan L, … Colombo M. 2021. The emerging role of nanotechnology in skincare. Adv Colloid Interface Sci. 293:102437. doi:10.1016/j.cis.2021.102437.
- Schäfer-Korting M, Mehnert W, Korting H-C. 2007. Lipid nanoparticles for improved topical application of drugs for skin diseases. Adv Drug Delivery Rev. 59(6):427–443.
- Schilling K, Bradford B, Castelli D, Dufour E, Nash JF, Pape W, … Schellauf F. 2010. Human safety review of “nano” titanium dioxide and zinc oxide. Photochem Photobiol Sci. 9(4):495–509.
- Schulten R, Novak B, Schmitz B, Lübbert H. 2012. Comparison of the uptake of 5-aminolevulinic acid and its methyl ester in keratinocytes and skin. Naunyn-Schmiedeberg's Arch Pharmacol. 385(10):969–979.
- Shah S, Rangaraj N, Laxmikeshav K, Sampathi S. 2020. Nanogels as drug carriers–introduction, chemical aspects, release mechanisms and potential applications. Int J Pharm. 581:119268. doi:10.1016/j.ijpharm.2020.119268.
- Shah S, Rangaraj N, Singh SB, Srivastava S. 2021. Exploring the unexplored avenues of surface charge in nano-medicine. Colloid and Interface Science Communications. 42:100406. doi:https://doi.org/10.1016/j.colcom.2021.100406.
- Sharma A, Goyal AK, Rath G. 2018. Recent advances in metal nanoparticles in cancer therapy. J Drug Targeting. 26(8):617–632.
- Sharma P, Mehta M, Dhanjal DS, Kaur S, Gupta G, Singh H, … Bakshi HA. 2019. Emerging trends in the novel drug delivery approaches for the treatment of lung cancer. Chem-Biol Interact. 309:108720.
- Siddalingam R, Chidambaram K. 2016. Topical nano-delivery of 5-fluorouracil: preparation and characterization of water-in-oil nanoemulsion. Tropical Journal of Pharmaceutical Research. 15(11):2311–2319.
- Sidoroff A, Thaler P. 2010. Taking treatment decisions in non-melanoma skin cancer—The place for topical photodynamic therapy (PDT). Photodiagn Photodyn Ther. 7(1):24–32.
- Smijs TG, Pavel S. 2011. Titanium dioxide and zinc oxide nanoparticles in sunscreens: focus on their safety and effectiveness. Nanotechnol, Sci Appl. 4:95–112. doi:10.2147/NSA.S19419.
- Smith Jr. JG. 2007. Burden of skin disease. J Am Acad Dermatol. 56(4):706. doi:10.1016/j.jaad.2006.09.008.
- Soni K, Mujtaba A, Akhter MH, Zafar A, Kohli K. 2020. Optimisation of ethosomal nanogel for topical nano-CUR and sulphoraphane delivery in effective skin cancer therapy. J Microencapsul. 37(2):91–108. doi:10.1080/02652048.2019.1701114.
- Souto EB, Baldim I, Oliveira WP, Rao R, Yadav N, Gama FM, Mahant S. 2020. SLN and NLC for topical, dermal, and transdermal drug delivery. Expert Opin Drug Delivery. 17(3):357–377.
- Stewart MP, Langer R, Jensen KF. 2018. Intracellular delivery by membrane disruption: mechanisms, strategies, and concepts. Chem Rev. 118(16):7409–7531.
- Tambunlertchai S, Geary SM, Salem AK. 2021. Skin penetration enhancement strategies used in the development of melanoma topical treatments. AAPS J. 23(1):1–16.
- Tamjidi F, Shahedi M, Varshosaz J, Nasirpour A. 2013. Nanostructured lipid carriers (NLC): A potential delivery system for bioactive food molecules. Innovative Food Science & Emerging Technologies. 19:29–43.
- Tekade RK, Maheshwari R, Tekade M. 2017. Biopolymer-based nanocomposites for transdermal drug delivery. In: Biopolymer-based composites. Woodhead Publishing; p. 81–106.
- Thi TTH, Suys EJ, Lee JS, Nguyen DH, Park KD, Truong NP. 2021. Lipid-Based nanoparticles in the clinic and clinical trials: from cancer nanomedicine to COVID-19 vaccines. Vaccines (Basel). 9(4):359. doi:10.3390/vaccines9040359.
- Torin Huzil J, Sivaloganathan S, Kohandel M, Foldvari M. 2011. Drug delivery through the skin: molecular simulations of barrier lipids to design more effective noninvasive dermal and transdermal delivery systems for small molecules, biologics, and cosmetics. Wiley Interdiscip Rev: Nanomed Nanobiotechnol. 3(5):449–462.
- Van Straten D, Mashayekhi V, De Bruijn HS, Oliveira S, Robinson DJ. 2017. Oncologic photodynamic therapy: basic principles, current clinical status and future directions. Cancers (Basel). 9(2):19. doi:10.3390/cancers9020019.
- Venuganti VVK, Perumal OP. 2009. Poly (amidoamine) dendrimers as skin penetration enhancers: influence of charge, generation, and concentration. J Pharm Sci. 98(7):2345–2356.
- Verkouteren J, Ramdas K, Wakkee M, Nijsten T. 2017. Epidemiology of basal cell carcinoma: scholarly review. Br J Dermatol. 177(2):359–372.
- Verma A, Jain A, Hurkat P, Jain SK. 2016. Transfollicular drug delivery: current perspectives. Research and Reports in Transdermal Drug Delivery. 5:1–17.
- Waldman RA, Grant-Kels JM. 2021. Dermatology patients on biologics and certain other systemic therapies should receive a “booster” messenger RNA COVID-19 vaccine dose: a critical appraisal of recent food and drug administration and advisory committee on immunization practices recommendations. J Am Acad Dermatol. 85(5):1113–1116. doi:10.1016/j.jaad.2021.08.031.
- Wang R, Hu H, Meng F, Wu Q. 2021. Expression and significance of N-MID, PAI-1, and RBM5 in patients with brain metastases of lung cancer. All Life. 14(1):365–374. doi:10.1080/26895293.2021.1917458.
- Wu M-h, Xiao L-f, Zhang C, Lei J, Deng Z-m. 2020. The minimally invasive endoscopic technique for the treatment of symptomatic benign bone lesions: preliminary results from a retrospective study. J Bone Oncol. 24:100313. doi:10.1016/j.jbo.2020.100313.
- Yang Y. 2019. Development of a mesoporous silica based nanosystem for safe and efficient therapeutic delivery.
- Yarmush ML, Golberg A, Serša G, Kotnik T, Miklavčič D. 2014. Electroporation-based technologies for medicine: principles, applications, and challenges. Annu Rev Biomed Eng. 16:295–320.
- Yassin AEB, Anwer MK, Mowafy HA, El-Bagory IM, Bayomi MA, Alsarra IA. 2010. Optimization of 5-flurouracil solid-lipid nanoparticles: a preliminary study to treat colon cancer. Int J Med Sci. 7(6):398–408. doi:10.7150/ijms.7.398.
- Ye Z-q, Dong X-b, Chen H-b, Gu D-n, Qiu Z-j. 2021. Circular RNA CDR1as-induced autophagy regulates the proliferation and migration of CD44+/CD24- phenotype breast cancer stem cells in vitro. All Life. 14(1):569–576. doi:10.1080/26895293.2021.1934575.
- Yu Y-Q, Yang X, Wu X-F, Fan Y-B. 2021. Enhancing permeation of drug molecules across the skin via delivery in nanocarriers: novel strategies for effective transdermal applications. Front Bioeng Biotechnol. 9:646554. doi:10.3389/fbioe.2021.646554.
- Zagórska-Dziok M, Sobczak M. 2020. Hydrogel-based active substance release systems for cosmetology and dermatology application: a review. Pharmaceutics. 12(5):396. doi:10.3390/pharmaceutics12050396.
- Zhang YR, Lin R, Li HJ, He Wl, Du JZ, Wang J. 2019. Strategies to improve tumor penetration of nanomedicines through nanoparticle design. Wiley Interdiscip Rev: Nanomed Nanobiotechnol. 11(1):e1519. doi:10.1002/wnan.1519.
- Zheng L, Zhao Z, Yang Y, Li Y, Wang C. 2020. Novel skin permeation enhancers based on amino acid ester ionic liquid: design and permeation mechanism. Int J Pharm. 576:119031. doi:10.1016/j.ijpharm.2020.119031.
- Zheng S, Fu G-B, Shen H-C, Liu Q, Wang W-B. 2021. Inhibitory effects of combinations of trastuzumab and cytotoxic chemotherapy drugs in HER2-positive gastric cancer. All Life. 14(1):226–235. doi:10.1080/26895293.2021.1906330.
- Zhi D, Yang T, Zhang T, Yang M, Zhang S, Donnelly RF. 2021. Microneedles for gene and drug delivery in skin cancer therapy. J Controlled Release. 335:158–177. doi:https://doi.org/10.1016/j.jconrel.2021.05.009.
- Zhou Y, Wang D, Liu C, Yan T, Li C, Yang Q, … Güngör C. 2021. Nomograms predicting overall survival and cancer-specific survival for patients with appendiceal cancer after surgery. All Life. 14(1):428–440. doi:10.1080/26895293.2021.1926342.
- Zhou Y, Xu G, Fang M, Wu S, Tan Z, Quan R, … Li X. 2020. LncRNA SNHG14 promotes non-small cell lung cancer progression by sponging miR-382-5p. All Life. 13(1):386–396. doi:10.1080/26895293.2020.1790431.
- Zhou Z, Sun T, Jiang C. 2021. Recent advances on drug delivery nanocarriers for cerebral disorders. Biomed Mater. 16(2):024104. doi:10.1088/1748-605X/abdc97.