Abstract
Therapy-related myeloid neoplasms (t-MNs) are associated with chemotherapy, radiotherapy, or autologous hematopoietic stem cell transplantation (auto-HSCT). Clonal abnormalities after auto-HSCT are not rare and do not always indicate t-MNs. We describe a rare case of transient clonal chromosomal abnormality after auto-HSCT for acute myeloid leukemia (AML). In this case, an extra copy of the Y chromosome accompanied by slight bone marrow dysplasia lasted for approximately 11 months and disappeared without therapy. Genetic testing of 236 hematologic malignancies showed no somatic mutations. From previously reported literature, 10 cases with the appearance of clonal chromosomal abnormalities after auto-HSCT for AML were analyzed. Six of these cases presented with persistent chromosomal abnormalities that did not develop t-MNs, one presented with inv(16) associated with therapy-related AML, and the remaining three presented with transient abnormalities. Most persistent or transient clonal chromosomal abnormalities occurring after auto-HSCT for AML could indicate a good prognosis.
Introduction
Therapy-related myeloid neoplasms (t-MNs) are a group of clonal hematopoietic stem cell disorders, comprising myelodysplastic syndrome (MDS), myelodysplastic/myeloproliferative neoplasms (MDS/MPN), and acute myeloid leukemia (AML; Singh et al. Citation2007). These neoplasms are directly related to previous chemotherapy, radiotherapy, or autologous hematopoietic stem cell transplantation (auto-HSCT) for Hodgkin’s and non-Hodgkin’s lymphomas, acute lymphoblastic leukemia, sarcoma, ovarian cancer, testicular cancer, and AML (Laurenti et al. Citation2002; Bhatia Citation2013; Shao et al. Citation2017; Rady et al. Citation2018; Rogers et al. Citation2020).
The prognosis of most patients with t-MNs is poor. However, patients who have clonal chromosomal abnormalities after anti-tumor therapy but do not develop MDS or AML may have better outcomes, even with abnormal clones accompanied by chromosomal abnormalities. Clonal chromosomal abnormalities after auto-HSCT are usually persistent and occasionally transient even without treatment (Martinez-climent et al. Citation2000; Pedersen-Bjergaard et al. Citation2000; Nishikii et al. Citation2019; Kato et al. Citation2020). A previous study indicated that half of the clonal chromosomal abnormalities after auto-HSCT for lymphoma developed t-MNs (Traweek et al. Citation1994). However, the outcome of clonal chromosomal abnormalities following auto-HSCT for AML remains unclear. We report an interesting case of transient clonal chromosomal abnormality after auto-peripheral blood SCT for t(8;21) AML and present a brief literature review.
Clinical presentation
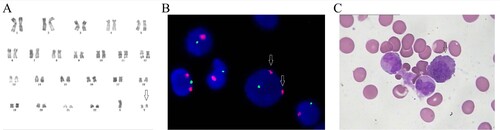
A 47-year-old man was hospitalized for fever and bleeding gums. His white blood cell (WBC) count, platelet count and hemoglobin level at admission were 4.3×109/L, 13×109/L, and 74 g/L, respectively. Bone marrow aspirate was hypercellular, comprising 25% myeloblasts and 17.5% monoblasts. Flow cytometric analysis indicated that blast cells were positive for CD19, CD33, CD34, CD38 and CD117. Cytogenetic analysis revealed 45,X,-Y,t(8;21)(q22;q22)[9]/46,XY[1]. In addition, the AML1-ETO fusion gene was positive without FLT3-ITD and c-KIT mutation by polymerase chain reaction (PCR) and sequencing method. Based on these results, the patient was diagnosed with AML1-ETO-positive AML. He began undergoing induction chemotherapy with idarubicin (10mg on days 1-4) and ara-C (150mg on days 1-7) and achieved complete remission (CR). Subsequently, the patient received intermediate-dose ara-C as consolidation therapy and underwent auto-SCT six months later. Karyotypic analysis conducted 45 months after the transplant revealed 47,XY,+Y[3]/46,XY[20] (Figure (A)). Fluorescent in situ hybridization revealed that 20% of the bone marrow contained an additional chromosome Y (Figure (B)). This abnormal clonal karyotype persisted for approximately 1 year before disappearing without any anti-leukemia therapy. Slight myeloid and erythroid dysplasia were observed in the bone marrow aspirate smear (Figure (C)) when the patient harbored the extra chromosome Y. However, his bone marrow aspirate smear exhibited CR after auto-HSCT (Supplementary Table 1). Two hundred thirty-six hematological malignancies-associated gene mutations were sequenced by next generation sequencing (NGS) at initial diagnosis, +Y clone occurrence, and disappearance of the extra Y chromosome. Gene mutations such as SBDS, KIT, MSH6, PCLO, RUNX1, and USH2A mutations were detected. However, these mutations were considered as germline or tumor-unrelated because they appeared at different periods. Currently, 114 months after auto-HSCT, the patient remains in CR without relapse or anti-tumor therapy.
Figure 1. Karyotype, FISH, and bone marrow analysis during the appearance of the extra chromosome Y. (A) The karyotype analysis showed 47,XY,+Y. (B) FISH analysis showed that the centromere of Y (red signals) was copied while the centromere of X (green signals) was normal. (C) Bone marrow aspirate smear analysis showed slight dysplasia of the erythroid cells.
Discussion
Chromosomal abnormalities are one of the characteristics of t-MNs, such as del(5q), -7/del(7q), complex karyotype, abnormalities in chromosome 12, and translocations to chromosome bands 11q23 and 21q22 (Akhtari et al. Citation2013; Mcnerney et al. Citation2017). Ten cases of chromosomal abnormalities after auto-HSCT for AML have been previously reported (Shao et al. Citation2017; Imrie et al. Citation1998; Lambertenghi Deliliers et al. Citation1999; Table ). Of these 10 cases, only one patient with the appearance of inv(16) after auto-HSCT developed therapy-related AML (Shao et al. Citation2017). The other cases had clonal chromosomal abnormalities that were persistent or transient but did not progress to t-MNs. Interestingly, our case exhibited a loss of chromosome Y in the primary neoplasm and a gain of Y post-HSCT. However, the extra copy of the Y chromosome was transient. According to previous reports, clonal chromosomal abnormalities appeared ranging from 3 to 36 months after auto-HSCT. In our case, the patient acquired transient chromosomal abnormality 11 months after auto-HSCT. The follow-up time was 120 months. As presented in Table , one patient showed 46,XX,-7,t(14;15)(q11.2;q26),+mar karyotype, developed ringed sideroblasts (10%) in the marrow, but without progression to MDS or AML (Imrie et al. Citation1998). However, Traweek et al. (Traweek et al. Citation1994) reported that the risk of developing chromosomal abnormalities after auto-HSCT for lymphoma, such as diffuse large B-cell lymphoma(DLBCL), Burkitt lymphoma, T-cell large cell lymphoma(TLCL), and follicular lymphoma(FL), is nearly 9% at 3 years, and half of the cohort developed t-MNs. By comparison, clonal chromosomal abnormalities following auto-HSCT for AML could indicate a favorable prognosis.
Table 1. Genetic and survival summary of the reported cases of chromosome abnormalities after auto-HSCT for AML.
Recently, Kato et al. reported a multicenter clinical study on chromosomal abnormalities after auto-HSCT (Kato et al. Citation2020). In their study, most chromosomal abnormalities were observed in a single metaphase, and the chromosomal abnormality patterns changed inconsistently and randomly at each test, which might be caused by nearly all of their cases receiving irradiation prior to transplantation. In our case, an extra copy of the Y chromosome was observed in multiple metaphases in the sequential tests. In previous studies, acquired mutations were not described for chromosomal abnormalities after auto-HSCT. We sequenced 236 target genes associated with hematological malignancies using NGS. However, no acquired mutations were found, indicating that this patient had a good prognosis. In general, these transient clones without acquired mutations are not involved in the gain or loss of driver or fusion genes that contribute to tumor progression. Moreover, a possible explanation for the transient phenomenon is that these cytogenetic abnormalities without driver mutations could not cause an excessive expansion of aberrant clones, and they disappeared in a timeline coinciding with full immune reconstitution (Ogonek et al. Citation2016).
In summary, the appearance of persistent or transient clonal chromosomal abnormalities after auto-HSCT for AML is not rare, and a majority of these are persistent or transient and do not develop t-MNs. Unlike lymphoma, most patients with chromosomal abnormalities after auto-HSCT for AML had a good prognosis. However, it is necessary to monitor bone marrow morphology and cytogenetics dynamically. We suggest to monitor the bone marrow morphology and cytogenetics in these patients every 3-6 months until the clones disappear. In the future, multicenter studies are needed to better understand the prognostic importance of clonal chromosomal abnormalities after auto-HSCT for AML.
Supplemental Material
Download MS Word (29.2 KB)Acknowledgement
The authors thank Dr Heesun J. Rogers (Department of Laboratory Medicine, Cleveland Clinic) for a critical suggestion of the manuscript. Ying Chen and Mu Qitian were involved in the conception and design. Data acquisition was performed by Ying Wu and Zhaoyi Zhang. Analysis and interpretation of the data were performed by Duobin Zou and Tingting Niu. The drafting of the paper was revised critically for intellectual content by Kaihong Xu, Yi Zhang and Guifang Ouyang. All authors agree to be accountable for all aspects of the work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available in the figshare repository at https://doi.org/10.6084/m9.figshare.19233795.
Consent for publication
Written informed consent was obtained from the patient to publish of this case report.
Additional information
Funding
References
- Akhtari M, Bhatt VR, Tandra PK, Krishnamurthy J, Horstman H, Dreessen A, Chen PX, Armitage JO. 2013. Therapy-related myeloid neoplasms after autologous hematopoietic stem cell transplantation in lymphoma patients. Cancer Biol Ther. 14:1077–88.
- Bhatia S. 2013. Therapy-related myelodysplasia and acute myeloid leukemia. Semin Oncol. 40:666–75.
- Imrie KR, Dube I, Prince HM, Girouard C, Crump M, Keating A. 1998. New clonal karyotypic abnormalities acquired following autologous bone marrow transplantation for acute myeloid leukemia do not appear to confer an adverse prognosis. Bone Marrow Transpl. 21:395–9.
- Kato M, Nakasone H, Nakano N, Fuji S, Shinohara A, Yokoyama H, Sakashita K, Hori T, Takahashi S, Nara M, et al. 2020. Clinical course of autologous recovery with chromosomal abnormalities after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transpl. 55:1023–1028.
- Lambertenghi Deliliers G, Annaloro C, Pozzoli E, Oriani A, Dellavolpe A, Soligo D, Lambertenghi Deliliers D, Tagliaferri E, Bertolli V, Romitti L. 1999. Cytogenetic and myelodysplastic alterations after autologous hemopoietic stem cell transplantation. Leuk Res. 23:291–7.
- Laurenti L, Chiusolo P, Garzia MG, Zini G, Sora F, Piccirillo N, Piccioni P, Zollino M, Leone G, Sica S. 2002. Periodic morphologic, cytogenetic and clonality evaluation after autologous peripheral blood progenitor cell transplantation in patients with lymphoproliferative malignancies. Haematologica. 87:59–66.
- Martinez-climent JA, Comes AM, Vizcarra E, Benet I, Arbona C, Prosper F, Solano C, Garcia Clavel B, Marugan I, Lluch A, Garcia-Conde J. 2000. Chromosomal abnormalities in women with breast cancer after autologous stem cell transplantation are infrequent and may not predict development of therapy-related leukemia or myelodysplastic syndrome. Bone Marrow Transpl. 25:1203–8.
- Mcnerney ME, Godley LA, Le Beau MM. 2017. Therapy-related myeloid neoplasms: when genetics and environment collide. Nat Rev Cancer. 17:513–527.
- Nishikii H, Kurita N, Shinagawa A, Sakamoto T, Kusakabe M, Yokoyama Y, Kato T, Sakata-Yanagimoto M, Obara N, Hasegawa Y, et al. 2019. Durable Leukemic Remission and Autologous Marrow Recovery with Random Chromosomal Abnormalities after Allogeneic Hematopoietic Stem Cell Transplantation for Chronic Lymphocytic Leukemia. Case Rep Hematol. 2019:9710790.
- Ogonek J, Kralj Juric M, Ghimire S, Varanasi PR, Holler E, Greinix H, Weissinger E. 2016. Immune Reconstitution after Allogeneic Hematopoietic Stem Cell Transplantation. Front Immunol. 7:507.
- Pedersen-Bjergaard J, Andersen MK, Christiansen DH. 2000. Therapy-related acute myeloid leukemia and myelodysplasia after high-dose chemotherapy and autologous stem cell transplantation. Blood. 95:3273–9.
- Rady K, Blombery P, Westerman DA, Wall M, Curtis M, Campbell LJ, Seymour FJ. 2018. “Reversible” myelodysplastic syndrome or ineffectual clonal haematopoiesis? – add. (6p.) myeloid neoplasm with a spontaneous cytogenetic remission. Leuk Res. 73:1–4.
- Rogers HJ, Wang X, Xie Y, Davis AR, Thakral B, Wang SA, Borthakur G, Cantu MD, Margolskee EM, Philip JKS, et al. 2020. Comparison of therapy-related and de novo core binding factor acute myeloid leukemia: A bone marrow pathology group study. Am J Hematol. 95:799–808.
- Shao H, Yang Q, Wu C, Cen J, Chen S, Pan J. 2017. Therapy-related acute myeloid leukemia with inv(16) after successful therapy for de novo acute myeloid leukemia with t(8;21). Ann Hematol. 96:2127–2129.
- Singh ZN, Huo D, Anastasi J, Smith SM, Karrison T, Le Beau MM, Larson RA, Vardiman WJ. 2007. Therapy-related myelodysplastic syndrome: morphologic subclassification may not be clinically relevant. Am J Clin Pathol. 127:197–205.
- Traweek ST, Slovak ML, Nademanee AP, Brynes RK, Niland JC, Forman J S. 1994. Clonal karyotypic hematopoietic cell abnormalities occurring after autologous bone marrow transplantation for Hodgkin's disease and non-Hodgkin's lymphoma. Blood. 84:957–63.
