Abstract
We retrospectively collected drug susceptibility data of Klebsiella pneumoniae from six tertiary hospitals in Shaoxing City in 2019 and performed a comparative analysis of drug resistance among different hospitals, sexes, ages, and specimens. In total, 1954 strains were identified. The antibiotic resistance rate varied from 4.42% to 36.18%. Most K. pneumoniae were still susceptible to carbapenems and tigecycline, with resistance rates of less than 10%. Drug resistance was relatively mild in Shaoxing Traditional Chinese Medicine Hospital and Shaoxing Maternal and Children Health Hospital, but most severe in Affiliated Hospital of Shaoxing University. Specimens were primarily obtained from elderly and male patients, and the resistance rate increased with age. The specimens were mostly collected from the respiratory and urinary tracts. No carbapenem-resistant strains were collected in 112 isolates from patients under 20 years of age. The ratio of carbapenem-resistant K. pneumoniae isolates was highest in blood-isolated strains (23.86%), and that of extended-spectrum beta-lactamase positive strains was highest in non-blood-sterile body fluids (37.37%). The resistance spectrum of K. pneumoniae varied between hospitals in the same area. Elderly and male patients, non-sterile body fluids, and blood source strains should be seriously considered in empirical treatment.
Highlights
Prevention and control should be strengthened in hospitals with high rate of drug resistance.
Strengthen screening of drug-resistant bacteria in specific populations.
Transferred patients should be alerted to the bacterial drug resistance status.
Introduction
Klebsiella pneumoniae is one of the major and challenging pathogens associated with community- and hospital-acquired infections (Tang et al. Citation2020). The threat of growing antibiotic resistance caused by K. pneumoniae, particularly carbapenem-resistant K. pneumoniae (CRKP), is concerning (Chuang et al. Citation2020). A multicentre epidemiological investigation of CRKP indicated that it also increases health expenditures and mortality rates (Zhen et al. Citation2020). CRKP is recognised as a critical pathogen by the World Health Organization (WHO) (World Health Organization (WHO), Citation2017).
The mechanisms of antibiotic resistance in K. pneumoniae involve the expression of β-lactamases, efflux, outer protein membrane, or porin reduction (Durante-Mangoni et al. Citation2019). The SHV, CTX-M, and TEM β-lactamase families produced by K. pneumoniae are becoming more prevalent globally (Adler et al. Citation2016). During the last decade, CRKP has spread worldwide, and become a matter of great concern. The prevailing genes are KPC, NDM, and OXA-48(Chang et al. Citation2021). In addition, polymyxin and ceftazidime-avibactam-resistant CRKP strains have also appeared in recent years, mutations in mgrB gene and in the Ω loop of KPC-2 are main drug resistance mechanism (Di Tella et al. Citation2019; Galani et al. Citation2021). Plasmids encoding resistance genes are commonly associated with genes mediating resistance to other antibiotics including fluoroquinolones and aminoglycosides, which facilitates the dissemination of resistant strains in hospital settings (San Millan Citation2018).
Data from the China Carbapenem-Resistant Enterobacteriaceae (CRE) Network indicate that 73.9% of CRE strains are CRKP (Zhang et al. Citation2018). According to the China Antimicrobial Surveillance Network (CHINET), the resistance rates of K. pneumoniae to imipenem and meropenem fluctuated around 18.6% and 64.1%, respectively (Hu et al. Citation2019a). CHINET data suggested that the CRKP isolation rate in 2018 was 53.3% in Zhejiang Province (http://www.chinets.com/Chinet), which primarily included large tertiary teaching hospitals in Hangzhou. According to an epidemiological study in Zhejiang Province, 36–95 tertiary and secondary hospitals were included, and a significant increase in CRKP prevalence was observed from 2.5% in 2008–15.8% in 2018 (Hu et al. Citation2020). This study also revealed that resistant strains were significantly more prevalent in tertiary hospitals in Hangzhou than in other hospitals, and sputum accounted for the majority of samples collected (approximately 75%) (Hu et al. Citation2020).
The above research shows that the distribution of K. pneumoniae antibiotic resistance rates varies across different regions and hospitals. Thus, empiric antibiotic treatment should be determined based on the local antibiotic susceptibility data. The aim of this study was to collect drug susceptibility data of K. pneumoniae from six tertiary hospitals in Shaoxing City in 2019 and analyzed the drug resistance of drug resistance among different hospitals, populations and specimens, and to monitor the isolation of extended -spectrum beta-lactamases and CRKP to effectively prevent and control the spread of multidrug-resistant K. pneumoniae.
Materials and methods
Sample size and source
We analysed 1954 consecutively and non-duplicated isolates from six tertiary hospitals in Shaoxing City, Zhejiang province in 2019, including Shaoxing people’s hospital (SPH), Shaoxing second hospital (2ndH), Shaoxing seventh hospital (7thH), The affiliated hospital of Shaoxing University (SUH), Shaoxing traditional Chinese medicine hospital (TCM), and Shaoxing Maternal and Child Health Hospital (SMCH). All hospitals involved in this study were accredited for pathogen identification and antimicrobial susceptibility testing. Electronic notes such as age, sex, and medical department, were also collected. The age range was 1 d to 99 years (mean age of 61.85 ± 22.17 years), and we divided the samples into six age groups: ≤20, 21–40, 41–60, 61–80, and ≥80 years.
Antimicrobial susceptibility testing
Isolate identification was performed using a Vitek-2 compact system (bioMerieux, France) according to the manufacturer’s instructions. Antibiotic susceptibility testing was performed using AST-GN13 plate (bioMerieux) by SUH and 2ndH, and AST-GN16 plate (bioMerieux) used by SPH, 7thH, SMCH and TCM. Minimum inhibitory concentration (MIC) of meropenem (10 µg; Oxoid Ltd., UK), ceftazidime (30 µg; Oxoid Ltd.), cefoperazone/sulbactam (105 µg; Oxoid Ltd.) were obtained with K-B disc diffusion method. Susceptibility interpretations were carried out in accordance with CLSI clinical breakpoints (Clinical and Laboratory Standards Institute(CLSI), Citation2019). Those K. pneumoniae strains which were resistant to one or more carbapenems (ertapenem, imipenem, and meropenem) were defined as CRKP(Centers for Disease Control and Prevention Facility Citation2015). The extended-spectrum β-lactamase K. pneumoniae (ESBL-KP) strain was determined via the standard double-disc synergy test. In brief, the disk cefotaxime (30 µg; Oxoid Ltd.) and ceftazidime (30 µg; Oxoid Ltd.) alone and in combination with clavulanic acid (10 µg; Oxoid Ltd.) was used to test inhibition zone diameter in accordance with CLSI (Clinical and Laboratory Standards Institute(CLSI), Citation2019). CRKP strains were excluded from the ESBL strains during statistical analysis. E. coli American type culture collection (ATCC) 25922 (negative control) and K. pneumoniae ATCC 700603 (positive ESBL control) were used as quality control (QC) strains. Data were only included when the QC test results were within acceptable ranges.
Statistical analysis
Data were collected using WHONET5.6 software (http://www.whonet.org.cn/news/19_509.html). Statistical software (SPSS 17.0, IBM, Armonk, NY, USA) was used for data analysis after data were exported and verified. Data were expressed as percentages. Measurement data are expressed as the mean ± standard deviation. Categorical variables were compared using χ2 or Fisher’s exact test. The statistical significance level was set at p < 0.05.
Results
Distribution of K. pneumoniae strains
A total of 1954 non-repetitive K. pneumoniae strains were collected. The number of isolates from SPH, 2ndH, SMCH, SUH, TCM and 7thH were 870 (44.52%), 289 (14.79%), 289 (14.79%), 261 (13.36), 166 (8.50%) and 79 (4.04%), respectively (Table ). There were 1,149(58.80%) and 805(41.20%) strains isolated from male and female patients, respectively (Table ). The proportion of male patients was higher than that of female patients in all hospitals, except for SMCH, and was highest in TCM (71.08%).
Table 1. The general characteristics and K. pneumoniae strains in various hospitals.
The proportions of specimens isolated from the respiratory tract samples(RTS), non-sterile body fluids(nSBF), sterile body fluids(SBF), urine, and blood were 52.61%, 21.39%, 15.51%, 5.99%, and 4.50%, respectively (Table ). nSBF included lochia and cervical secretions (n = 175), wound or infection site secretions (n = 99), pus (n = 85), drainage (n = 25), tissue removed during operation (n = 22), pharyngeal and ear swabs (n = 9), and others (n = 3). SBF included bile (n = 85), ascites (n = 10), venous catheter (n = 9), pleural fluid (n = 5), puncture fluid (n = 5), cerebrospinal fluid (n = 1), peritoneal dialysis solution (n = 1), and prostatic fluid (n = 1). The monthly distribution of number of isolated strains, ESBL-KP and CR-KP was shown in Figure S1.
K. pneumoniae sensitivity results in various hospitals
K. pneumoniae had the lowest resistance to amikacin (4.42%); meanwhile, the resistance to carbapenems and tigecycline was less than 10%, and the average resistance rate to β-lactam-β-lactamase inhibitor combinations ranged from 9.95% to 16.92% (Table ). The rates of resistance to cephalosporins, aztreonam, cefoxitin, ciprofloxacin, nitrofurantoin, and sulfamethoxazole ranged from 12.63% to 28.72% (Table ). Nitrofurantoin had the highest drug resistance rate (36.18%) in SPH, whereas the other antibiotics had the highest drug resistance rate in SUH (Table ). Significant differences in resistance rates were detected among the hospitals (p < 0.05).
Table 2. Comparison of the antibiotic resistance rate (%) of K. pneumoniae in various hospitals in Shaoxing city.
Antibiotic susceptibility among different patients’ groups
Among the 1,954 isolated strains, ≤20, 21–40, 41–60, 61–80, and ≥80-year groups accounted for 5.73%, 9.67%, 22.52%, 41.35%, and 20.73%, respectively. Resistance rates to cefazolin, ceftriaxone, and sulfamethoxazole/trimethoprim were higher in the ≤20-year group, most of which were greater than 20% (Table ). Notably, the meropenem resistance rate in the 21–40 year age group was 16.67% (Table ). The overall drug resistance rate in the >60 years age groups was higher than that in the younger age groups (Table ).
Table 3. Comparison of antibiotic resistance rates (%) of K. pneumoniae among age groups.
There was no significant difference in the drug resistance rates of K. pneumoniae isolated from male and female patients for ceftazidime, cefoperazone/sulbactam, meropenem and sulfamethoxazole/trimethoprim, with p values of 0.03, 0.11, 0.04, and 0.88, respectively. The resistance rate to other antibiotics in male patients was significantly higher than that in female patients (p < 0.05).
Antibiotic susceptibility of K. pneumoniae isolated from different specimens
K. pneumoniae resistance rates in samples obtained from blood and urine were higher than those from the other specimens (Table ). Notably, the resistance rates of K. pneumoniae in blood samples to ertapenem, imipenem, piperacillin/tazobactam, aztreonam, and cefepime were 22.09%, 22.73%, 20.45%, 40.91%, and 27.27%, respectively (Table ). K. pneumoniae isolated from the urine was significantly resistant to levofloxacin, ciprofloxacin, cefoperazone/sulbactam, piperacillin/tazobactam, amikacin, and gentamicin (p < 0.05) (Table ). In addition to meropenem, the antibiotic resistance rates were significantly different among the various specimens (p < 0.05)(Table ).
Table 4. Comparison of antibiotic resistance rate (%) of K. pneumoniae in different specimens.
ESBL-producing and carbapenem-resistant K. pneumoniae
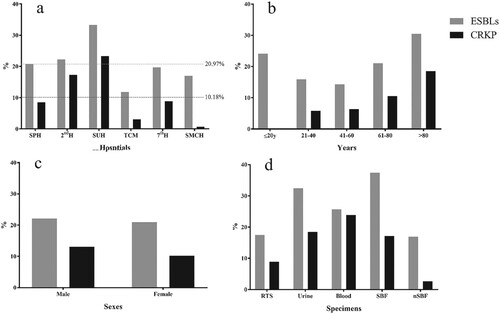
The average proportions of CRKP and ESBL-KP isolates were 10.18% and 20.97%, respectively (Figure ). There were significant differences in the proportion of ESBL-KP and CRKP isolates among the hospitals (ESBL: χ2 = 30.04, p = 0.00; CRKP: χ2 = 106.26, p = 0.00) (Figure (a)). With increasing age, the proportion of CRKP increased significantly (χ2 = 54.511, p = 0.00) (Figure (b)). There was no statistically significant difference in the ESBL-KP rates between male (22.10%) and female (20.92%) patients. The proportion of CRKP isolated from male patients was 13.05%, which was significantly higher than that isolated from female patients (10.18%) (χ2 = 25.13, p = 0.00), (Figure (c)). The rate of ESBL-KP from nSBF (37.37%) and CRKP from blood(23.86%) was highest among the source of specimens. There were significant differences in the proportions of ESBL-KP and CRKP among the specimens (ESBL: χ2 = 48.04, p = 0.00; CRKP: χ2 = 74.98, p = 0.00) (Figure (d)).
Figure 1. The ratio of ESBL-KP and CRKP strains. ESBL-KP rate (grey) and CRKP rate (black) for all strains. (a) ESBL-KP rate and CRKP rate of strains isolated from six hospitals (grey dotted line: average isolated rate of ESBL-KP; black dotted line: average isolated rate of CRKP). (b) ESBL-KP rate and CRKP rate of strains from patients with different age groups. (c) ESBL-KP rate and CRKP rate of strains from different sexes. d, ESBL-KP rate and CRKP rate of strains from different specimens. Abbreviations: RTS, respiratory tract samples; SBF, sterile body fluids; nSBF, non-sterile body fluids.
Discussion
K. pneumoniae is an opportunistic pathogen that causes various infections particularly in immunocompromised individuals (Effah et al. Citation2020). This study demonstrated that K. pneumoniae was primarily isolated from male patients, most of whom were middle-aged or elderly, with higher resistance severity discovered in isolated strains. Demographic epidemiology-based research is important for monitoring the emergence and trends of antimicrobial resistance and it should contribute to correct antibiotic choices during therapy.
EARSNet results showed increasing antibiotic resistance of K. pneumoniae, particularly with third-generation cephalosporins, fluoroquinolones, and aminoglycosides (Yang et al. Citation2019). A meta-analysis of multiple studies in Asia revealed that the overall resistance rate to amikacin was 40.8% (Effah et al. Citation2020), which was predominantly higher than the resistance rate in our study (4.42%). Amikacin can be used in combination with empirical therapies in this region. The drug resistance of strains from different clinical sources was different. Ballén et al. found that urine strains had the highest antibiotic resistance rate compared to blood or respiratory strains as well as the highest ESBL production(Ballen et al. Citation2021). Our results showed that urine-isolated K. pneumoniae resistance to ciprofloxacin and levofloxacin was considerably higher than those isolated from other specimens. This may be because fluoroquinolones are the first-line antibiotics used in the empirical treatment of urinary tract infections (Schaeffer and Nicolle Citation2016; Caron et al. Citation2018).
The ESBL-KP rate in our study was similar to that of an epidemiological survey in Zhejiang Province (Hu et al. Citation2020). However, the ESBL-KP rates varied among hospitals. The proportion of ESBL-KP was the lowest in TCM. It is unknown whether the patients admitted had less severe infections or if the focus on traditional Chinese medicine treatment unintentionally resulted in less use of antibiotics. In this study, SUH had the highest proportion of ESBL-KP isolates. Notably, SUH was converted from an infectious disease hospital to a general hospital in July 2018, and patients with hepatic failure in Shaoxing were still primarily admitted to the hospital. The prolonged hospital stay and recurrent hospitalisation history of these patients may contribute to severe antibiotic resistance (Liu et al. Citation2020). Considering the type of hospital is important while choosing empirical antibiotics in clinical practice. K. pneumoniae may have become an important pathogen in childhood infections and a vital cause of mortality (Hu et al. Citation2019b). A systematic review suggested that K. pneumoniae is the most frequent cause of community-acquired pneumonia among children in China (Ning et al. Citation2017). Children with bloodstream infections have the highest isolation rate of K. pneumoniae, particularly ICU patients (Li et al. Citation2019). In this study, the youngest group had a high rate of ESBL-KP isolation, which requires further epidemiological investigation.
In particular, the high rate of resistance of K. pneumoniae to carbapenems in blood samples should be concerned. Yang et al. found that K. pneumoniae was the second leading cause of blood infections, with an increasing trend of imipenem resistance between 2012 and 2017 (Yang et al. Citation2019). The increasing trend of carbapenem resistance makes clinical treatments of such infectious difficult, while bloodstream infections caused by K. pneumoniae are of the greatest concern owing to their high mortality (Maraolo et al. Citation2021). Currently, therapeutic options for CRKP infections are limited. Infections by hypervirulent K. pneumoniae (hvKp) are increasingly recognised worldwide, and have been magnified by increasing the descriptions of the evolution of carbapenem-resistant hypervirulent K. pneumoniae (CR-hvKP) strains(Thomas and Candace Citation2019). According to a multicentre epidemiological analysis in China, the proportion of CR-hvKP was approximately 5.23% (Zhang et al. Citation2020). Polymyxins and tigecycline are considered ‘last-resort’ antibiotics; however, there have been reports of the evolution of colistin and tigecycline resistance following treatment (Chen et al. Citation2021). These strains carry virulence plasmids that confer a serious threat to anti-infective therapy (Chen et al. Citation2021). Multiple mechanisms are involved in the emergence and global dissemination of CR-hvKP. Thus, we need to strengthen the monitoring of carbapenem-resistant strains and the emergence of CR-hvKP, and the administration of antibiotics should be strictly controlled.
The main strength of our study was the analysis of the epidemiology, of ESBL-producing, and carbapenase strains of K. pneumoniae. However, this study has several limitations. First, we did not preserve KP strains and did not conduct resistance mechanism and virulence gene analyses. Second, we did not obtain clinical information, and therefore, we could not distinguish between colonisation and infection. Third, for resistant strains, particularly CRKP, susceptibility to polymyxin and ceftazidime-avibactam were not detected, and their level of resistance to these drugs is unclear.
Conclusions
The resistance spectrum of K. pneumoniae varies among different hospitals in the same area, among different populations, and among specimen sources. Surveillance systems are vital for monitoring the emergence and trends of antimicrobial resistance, and appropriate recommendations and instructions for empirical antibiotic choice are important. When treating infections caused by K. pneumoniae, clinicians should consider factors such as sex, age, source of specimens, and cross-hospital visits. In addition, studying the mechanisms of K. pneumoniae resistance is necessary to effectively prevent and control the spread of multidrug-resistant K. pneumoniae.
Author contributions
Meichun Liang, Guofeng Mao and Yiqing Zhou conceived and designed the study. Xiaojiao Zhang, Qiuli He, Qunhua Ying, Sheliang Wang, Faxiang Jin, Su Dong, Xiuqin Lin, and Li Lv performed the susceptibility testing and collected data. Yongchun Ruan and Minghui Li analyzed the data. Meichun Liang wrote the manuscript. Yiqing Zhou critically reviewed and edited the manuscript. All coauthors have read, commented, and approved the final version of the article to be published.
Ethics approval
Ethical approval was approved by the Academic Ethics Committee of Shaoxing People’s Hospital (Ethics Clearance No.104), and waived the requirement for written informed consent due to the study’s retrospective nature.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article. The raw data can be obtained by https://figshare.com/articles/dataset/2019KP_xls/17808266. The dataset was fully anonymous that obviously presents minimal risk to confidentiality of study participants.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Adler A, Katz DE, Marchaim D. 2016. The continuing plague of extended-spectrum beta-lactamase-producing enterobacteriaceae infections. Infect Dis Clin North Am. 30(2):347–375. doi:10.1016/j.idc.2016.02.003.
- Ballen V, Gabasa Y, Ratia C, Ortega R, Tejero M, Soto S. 2021. Antibiotic resistance and virulence profiles of Klebsiella pneumoniae strains isolated from different clinical sources. Front Cell Infect Microbiol. 11:738223. doi:10.3389/fcimb.2021.738223.
- Caron F, Galperine T, Flateau C, Azria R, Bonacorsi S, Bruyere F, Cariou G, Clouqueur E, Cohen R, Doco-Lecompte T, et al. 2018. Practice guidelines for the management of adult community-acquired urinary tract infections. Med Mal Infect. 48(5):327–358. doi:10.1016/j.medmal.2018.03.005.
- Centers for Disease Control and Prevention Facility. 2015. Guidance for control of carbapenem-resistant Enterobacteriaceae (CRE)—November 2015 Update CRE Toolkit.
- Chang SL, Dela Cruz CS, Zhang D. 2021. Clinical epidemiology, risk factors, and control strategies of Klebsiella pneumoniae infection. Front Microbiol. 12:750662. doi:10.3389/fmicb.2021.750662.
- Chen JW, Zeng Y, Zhang R, Cai JC. 2021. In vivo emergence of colistin and tigecycline resistance in carbapenem-resistant hypervirulent Klebsiella pneumoniae during antibiotics treatment. Front Microbiol. 12:702956. doi:10.3389/fmicb.2021.702956.
- Chuang C, Su CF, Lin JC, Lu PL, Huang CT, Wang JT, Chuang YC, Siu LK, Fung CP, Lin YT. 2020. Does antimicrobial therapy affect mortality of patients with carbapenem-resistant Klebsiella pneumoniae bacteriuria? A nationwide multicenter study in Taiwan. Microorganisms. 8(12):2035. doi:10.3390/microorganisms8122035.
- Clinical and Laboratory Standards Institute(CLSI). 2019. Performance standards for antimicrobial susceptibility testing, M100 29th ed. Wayne, PA: Clinical and Laboratory Standards Institute.
- Di Tella D, Tamburro M, Guerrizio G, Fanelli I, Sammarco ML, Ripabelli G. 2019. Molecular epidemiological insights into colistin-resistant and carbapenemases-producing clinical Klebsiella pneumoniae isolates. Infect Drug Resist. 12:3783–3795. doi:10.2147/IDR.S226416.
- Durante-Mangoni E, Andini R, Zampino R. 2019. Management of carbapenem-resistant enterobacteriaceae infections. Clin Microbiol Infect. 25(8):943–950. doi:10.1016/j.cmi.2019.04.013.
- Effah CY, Sun TW, Liu SH, Wu YJ. 2020. Klebsiella pneumoniae: an increasing threat to public health. Ann Clin Microbiol Antimicrob. 19(1):1. doi:10.1186/s12941-019-0343-8.
- Galani I, Karaiskos I, Angelidis E, Papoutsaki V, Galani L, Souli M, Antoniadou A, Giamarellou H. 2021. Emergence of ceftazidime-avibactam resistance through distinct genomic adaptations in KPC-2-producing Klebsiella pneumoniae of sequence type 39 during treatment. Eur J Clin Microbiol Infect Dis. 40(1):219–224. doi:10.1007/s10096-020-04000-9.
- Hu FP, Guo Y, Yang Y, Zheng YG, Wu S, Jiang XF, Zhu DM, Wang F. 2019a. Resistance reported from China antimicrobial surveillance network (CHINET) in 2018. Eur J Clin Microbiol Infect Dis. 38(12):2275–2281. doi:10.1007/s10096-019-03673-1.
- Hu Y, Liu C, Shen Z, Zhou H, Cao J, Chen S, Lv HY, Zhou MM, Wang Q, Sun L, et al. 2020. Prevalence, risk factors and molecular epidemiology of carbapenem-resistant Klebsiella pneumoniae in patients from Zhejiang, China, 2008-2018. Emerg Microbes Infect. 9(1):1771–1779. doi:10.1080/22221751.2020.1799721.
- Hu YJ, Ogyu A, Cowling BJ, Fukuda K, Pang HH. 2019b. Available evidence of antibiotic resistance from extended-spectrum beta-lactamase-producing enterobacteriaceae in paediatric patients in 20 countries: a systematic review and meta-analysis. Bull World Health Organ. 97(7):486–501B. doi:10.2471/BLT.18.225698.
- Li X, Ding X, Shi P, Zhu Y, Huang Y, Li Q, Lu JM, Li ZP, Zhu L. 2019. Clinical features and antimicrobial susceptibility profiles of culture-proven neonatal sepsis in a tertiary children's hospital, 2013 to 2017. Medicine (Baltimore). 98(12): e14686. doi:10.1097/MD.0000000000014686.
- Liu J, Gao Y, Wang X, Qian Z, Chen J, Huang Y, Meng ZJ, Lu XB, Deng GH, Liu F, et al. 2020. Culture-positive spontaneous ascitic infection in patients with acute decompensated cirrhosis: multidrug-resistant pathogens and antibiotic strategies. Yonsei Med J. 61(2):145–153. doi:10.3349/ymj.2020.61.2.145.
- Maraolo AE, Corcione S, Grossi A, Signori A, Alicino C, Hussein K, Trecarichi EM, Viale P, Timsit JF, Veeraraghavan B, et al. 2021. The impact of carbapenem resistance on mortality in patients with Klebsiella pneumoniae bloodstream infection: an individual patient data meta-analysis of 1952 patients. Infect Dis Ther. 10(1):541–558. doi:10.1007/s40121-021-00408-8.
- Ning GJ, Wang XX, Wu D, Yin ZD, Li YX, Wang HQ, Yang WZ. 2017. The etiology of community-acquired pneumonia among children under 5 years of age in mainland China, 2001-2015: a systematic review. Hum Vaccin Immunother. 13(11):2742–2750. doi:10.1080/21645515.2017.1371381.
- San Millan A. 2018. Evolution of plasmid-mediated antibiotic resistance in the clinical context. Trends Microbiol. 26(12):978–985. doi:10.1016/j.tim.2018.06.007.
- Schaeffer AJ, Nicolle LE. 2016. Clinical practice. Urinary tract infections in older men. N Engl J Med. 374(6):562–571. doi:10.1056/NEJMcp1503950.
- Tang M, Kong X, Hao JC, Liu JB. 2020. Epidemiological characteristics and formation mechanisms of multidrug-resistant hypervirulent Klebsiella pneumoniae. Front Microbiol. 11:581543. doi:10.3389/fmicb.2020.581543.
- Thomas AR, Candace MM. 2019. Hypervirulent Klebsiella pneumoniae. Clin Microbiol Rev. 32(3):e00001–e00019. doi:10.1128/CMR.00001-19.
- World Health Organization (WHO). 2017. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. World Health Organization. 2017. Accessed on 1 Jan 2022. Available online: http://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf.
- Yang SS, Xu HF, Sun JD, Sun S. 2019. Shifting trends and age distribution of ESKAPEEc resistance in bloodstream infection, southwest China, 2012-2017. Antimicrob Resist Infect Control. 8:61. doi:10.1186/s13756-019-0499-1.
- Zhang YW, Jin LY, Ouyang PW, Wang Q, Wang RB, Wang J, Gao H, Wang XJ, Wang H. 2020. Evolution of hypervirulence in carbapenem-resistant Klebsiella pneumoniae in China: a multicentre, molecular epidemiological analysis. J Antimicrob Chemother. 75(2):327–336. doi:10.1093/jac/dkz446.
- Zhang YW, Wang Q, Yin YY, Chen HB, Jin LY, Gu B, Xie LY, Yang CX, Ma XB, Li HY, et al. 2018. Epidemiology of carbapenem-resistant enterobacteriaceae infections: report from the China CRE network. Antimicrob Agents Chemother. 62(2):e01882–e01817. doi:10.1128/AAC.01882-17.
- Zhen XM, Stalsby Lundborg C, Sun XS, Gu SY, Dong HJ. 2020. Clinical and economic burden of carbapenem-resistant infection or colonization caused by Klebsiella pneumoniae, Pseudomonas aeruginosa. Acinetobacter Baumannii: A Multicenter Study in China. Antibiotics (Basel). 9(8):514. doi:10.3390/antibiotics9080514.
