Abstract
Submergence stress in plants subjected to flooding is a significant issue in agriculture. Decades of previous research have elucidated many molecular and physiological responses of plants to this stress. Since Arabidopsis thaliana has been established as a model organism in this area of research, in this study, we started by replicating published results of seven sensitive and tolerant ecotypes of Arabidopsis to dark-submergence stress to assess its generalizability and consistency, adapted a leaf-damage index to quantify the progression of stress over time, and measured the recalcitrance of all those ecotypes to Agrobacterium transformation. By using photographic comparisons, median lethal time (LT50), and leaf-damage index, we ascertained that the order of tolerance reported by previous studies is robust enough to allow independent replications (Cvi-0 < Ita-0 < Bay-0 < Col-0 < Kin-0 < Lp2-6 < C24), supporting their consistency. We continued by testing the transformation recalcitrance of all seven ecotypes to three different Agrobacterium strains and identified C24 and Cvi-0 as the most suitable ecotypes useful in a transformation platform despite their low transformation frequencies (<0.1%). The results from this study can be useful in developing transgenic methodologies that enhance plant responses to stress and transitioning these methodologies from the laboratory to the field.
Introduction
The unpredictability and intensity of flooding increase in close association with climate change (Bailey-Serres et al., Citation2019). Flooding events cause submergence stress in plants resulting in decreases in crop productivity; this stress is rated along with drought-stress as the two top causes of annual economic damage in the United States (NAS, Citation2019). The seriousness of this issue has led to a substantial amount of research being generated in this field over the past three decades. These efforts have led to the discovery of metabolites and macromolecules now known to be involved in plant signaling, transcription, biochemical homeostasis and stress recovery processes (Sasidharan et al., Citation2018; Fukao et al., Citation2019).
Currently, large datasets of Quantitative Trait Loci (QTLs), transcriptomes, translatomes, and metabolomes are accessible for plant biotechnologists and breeders to develop methodologies that can enhance the response of crops to this stress (Mustroph, Citation2018; Fukao et al., Citation2019). This knowledge can be linked to strategies aimed at accelerating the transition of laboratory discoveries to real-world implementation in the field, such as germplasm collections, near-isogenic lines and transgenic testing in plants (Mickelbart et al., Citation2015; Bailey-Serres et al., Citation2019).
Despite these advances, only two improved cultivars – SUB1A rice and PRT6-RNAi barley – are currently available for farmers looking for genetic resilience in crops potentially vulnerable to submergence stress (Singh et al., Citation2013; Mendiondo et al., Citation2016).
Arabidopsis thaliana is a model plant that has been central in submergence stress research. However, most of what is currently known has been discovered in the Col-0 ecotype, which is tolerant to stress and can survive for up to seven days under dark-submergence stress and up to two weeks under illuminated-submergence stress (Vashisht et al., Citation2011). A consequence of this innate tolerance may be why some studies have found little or no improvement in submergence tolerance when testing transgenes or knock-out genes in this ecotype (Lee et al., Citation2011; Peña-Castro et al., Citation2011).
Almost a decade ago a seminal work on the submergence tolerance of 86 Arabidopsis ecotypes demonstrated the existence of genetic diversity in this species (Vashisht et al Citation2011). This knowledge has been used to contrast transcriptomes during stress (van Veen et al., Citation2016), tolerance mechanisms related to post-submergence survival (Yeung et al., Citation2018), mitochondrial signaling during submergence (Meng et al., Citation2020) and sequential stress effects (Morales et al., Citation2022). Here, we originally propose to use the work of Vashisht et al. (Citation2011) in the field of biotechnology by developing a transformation platform that uses diverse genetic backgrounds with differential submergence tolerance to test genes of interest, promoters, mutations, and other mechanisms by transgenics.
For that goal, we considered that submergence stress is a compound event with multiple and complex variables in its experimental setting. The main difficulties when applying submergence stress to plants are the diffusion of gases like CO2, ethylene, and specially O2, which is produced and consumed by the plant and soil microorganisms (Sasidharan et al., Citation2017). The absence of O2 is one of the main causes of stress during submergence and related stresses like hypoxia/anoxia and waterlogging (Fukao et al., Citation2019). Recently, the National Academies recommended that when researching complex systems with multiple variables of changing stability across time, it is important to independently scrutinize and report replicability of previously published results (NASEM, Citation2019). In return for the replication effort, the community receives increments in the confidence of discoveries and helps to overcome the inherent uncertainty of such complex systems (Nosek and Errington, Citation2020). This is the aim that led us in this work to start the build-up of information for the proposed transformation platform by testing and reporting our independent replication of Arabidopsis ecotypes tolerance.
Another shortcoming in this area of research has been scoring the effects of submergence stress on Arabidopsis by survival or dry weight, especially when younger leaves tend to survive intermediate stresses (Giuntoli et al., Citation2014; Tsai et al., Citation2016) and dead leaves remain attached to the plant (Licausi et al., Citation2011; Peña-Castro et al., Citation2011; Weits et al., Citation2014). In this study, we tested the use of alternative stress-scoring systems.
Finally, we obtained information on the transformation recalcitrance of the tested ecotypes to contribute to the development of transgenic platforms that can lead to the development of submergence tolerance of plant crops overall.
Materials and methods
Plant material and submergence stress
Seeds of ecotypes Cvi-0, Ita-0, Bay-0, Col-0, Kin-0, Lp2-6, and C24 were obtained from ABRC (arabidopsis.org) and propagated. Seeds were then surface disinfected and stratified in water for six days at 4 °C, germinated on Murashige & Skoog (MS) agar medium (0.5% w/v MS salts, pH 5.7, 1% w/v sucrose) in vertically oriented plates in a growth room limited to short-day conditions to prevent premature flowering (23 °C, 8 h light/16 h dark, 150 µE m−2 sec−1 PAR, 60% humidity). After ten days of growth, the seedlings were transferred to pots containing autoclaved soil (Sunshine Mix #3, 1:4 v/v perlite:substrate, and 2% w/w Nitrofoska NPK 12-12-17 after 2 h of cooling) and weighted down with gravel at the bottom. A fine sand layer was added to the soil surface to prevent it from floating away, causing water turbidity.
When plants were twenty-four days old (the 10-leaf stage) they were subjected to submergence stress. This consisted of submerging plants in 30 cm deep columns of filtered water inside disinfected plastic tanks with opaque walls where it was dark (dark-submergence). All ecotypes were submerged simultaneously, side-by-side in a randomized array (36 pots with 4–5 plants each). Plants that were subjected to dark-only stress without submergence (controls) were grown in the same type of plastic tanks but without water. Submergence stress was applied starting at Zeitgeber time 07 (ZT07; 1 h before night) and plants (both submerged and controls) were carefully removed every 24 h from the tanks at the indicated time points (Figure ) under green light in a predefined randomized array.
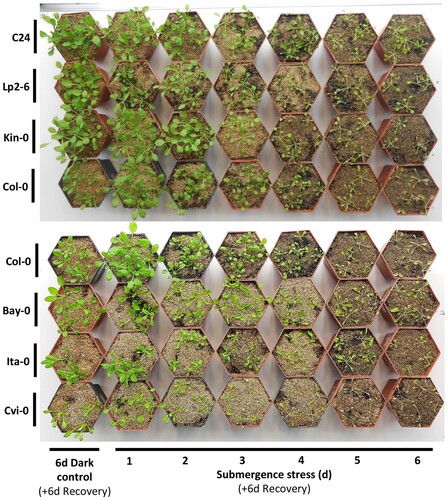
Figure 1. Photographic comparisons of sensitive and tolerant Arabidopsis ecotypes after submergence stress. Representative 34-day-old plants of each ecotype pictured six days after being removed from the indicated time of submergence stress. The tolerant group consists of C24, Lp2-6 and Kin-0 while the sensitive group consists of Bay-0, Ita-0 and Cvi-0. Col-0 is considered the standard ecotype with intermediate tolerance.
Stress damage quantification
A plant was recorded as surviving if it had remained green six days after being removed from stress. These data were used to calculate the median lethal time (LT50) using the online tools IC50 (http://ic50.tk) and Quest Graph (AAT Bioquest). We also adapted and recorded a damage index proposed by Tsai et al. (Citation2016), whereby leaves of each plant (dark-submerged and control) are categorized as turgent, chlorotic, or senescent immediately after being removed from stress as well as six days after being removed. Data were analyzed using Minitab 8.0 by one-way ANOVA followed by Tukey's HSD.
Floral dip transformation of ecotypes
To test the transformation frequencies of all ecotypes used, the plasmid pCAMBIA1105.1 – which confers spectinomycin resistance in Agrobacterium – and hygromycin in Arabidopsis was utilized.
Three strains of Agrobacterium tumefaciens (GV3101, GV2260, and EHA105) were donated by the group of Professor Alejandra Covarrubias (IBt-UNAM). All three strains were electroporated with pCAMBIA1105.1, selected in LB media with 50 µg/mL spectinomycin (selection antibiotic), and used to transform all ecotypes of Arabidopsis using the floral dip method as described by Zhang et al. (Citation2006). After transformation, approximately 3,000 seeds (calculated by weight) from the T0 generation were placed on MS plates with 25 µg/ml of hygromycin (selection antibiotic) and 50 µg/ml cefotaxime (Agrobacterium inhibitory concentration). Seedlings showing resistance to hygromycin were counted as transformants and used to calculate transformation frequency using the following formula: (number of hygromycin-resistant plants/number of seeds tested)x100. The results of six independent transformation experiments were graphed as boxplots using the online tool BoxPlotR (boxplot.tyerslab.com; Spitzer et al., Citation2014). Data were analyzed using Minitab 8.0 by two-way ANOVA followed by Tukey's multiple comparison test.
Results and discussion
The submergence stress response of Arabidopsis plants has been investigated under normal light cycles as well as continuous darkness previously by Vashisht et al. (Citation2011). However, dark-submergence has been the most commonly employed experimental condition for plants such as Col-0 that can tolerate submergence for multiple days. This is because the application of experimental light cycles for six or more days necessitates the use of additional implements (e.g. UV light filters) to control for variables such as microbial growth or microalgae shading, which can impact experiments requiring measurements over two or more weeks (Sasidharan et al., Citation2017). To avoid this pitfall, we employed the dark-submergence method because it has been demonstrated to quickly separate sensitive and tolerant ecotypes of Arabidopsis (Vashisht et al., Citation2011).
Testing Arabidopsis ecotypes with contrasting submergence sensitivity and tolerance
We used the findings of Vashisht et al. (Citation2011) to select our ecotypes for this experiment. Vashisht et al. (Citation2011) subjected eighty-six Arabidopsis ecotypes to dark-submergence and detected tolerant, intermediate, and sensitive ecotypes, scored survival rates, and calculated LT50. We selected the most extreme tolerant and sensitive ecotypes, as well as the standard Col-0 ecotype and then replicated the experiment of Vashisht et al. (Citation2011).
Our results aligned with those of Vashisht et al. (Citation2011), with a visual examination of our plants resulting in the same order of tolerance with regard to ecotype: Cvi-0 < Ita-0 < Bay-0 < Col-0 < Kin-0 < Lp2-6 < C24 (Figure , Supplemental Figure 1).
Our LT50 results revealed shorter survival rates than those reported by Vashisht et al. (Citation2011) (Table , Supplemental Figure 2). These differences could be explained by divergent experimental conditions such as water quality, the density of potted plants, or different temperature controls. However, it is notable that the order of tolerance –as measured both visually and that scored using the LT50 measurement– were similar. This points to the robustness of previous results that have measured the responses of these ecotypes to stress. Although the identicality of outcomes was not achieved, as expected by the complexity of submergence stress testing, we support its generalizability and replicability.
Table 1. Median lethal time (LT50) of seven submergence tolerant and sensitive Arabidopsis ecotypes tested in this work. The data from Vashisht et al., (Citation2011) are added for comparison purposes.
Scoring submergence sensitivity and tolerance of Arabidopsis ecotypes
Sasidharan et al. (Citation2017) has suggested that submergence related experiments in the laboratory might have more than twenty variables to control for. The impact of these variables can be exacerbated if results obtained consist of a simple ‘yes/no’ survival measurement such as LT50. The fact that some plants survive in spite of being heavily damaged compromises the results further (e.g. Bay-0, Figure ). Additionally, it has recently been documented that the response of Arabidopsis to submergence is dependent on plant age and leaf maturity (Guintoli et al., Citation2014; Bui et al., Citation2020).
To address these issues, we adapted a leaf-damage index proposed by Tsai et al. (Citation2016) to categorize leaves as turgent, chlorotic, or senescent. We then quantified the number of leaves in each category and calculated the percentage distributions of leaf damage over the entire recovered population. This method can characterize seedlings or mature plants and allows for a quantitative treatment of stress complementary to the LT50 measurement which can overlook the impacts of leaf-damage on the overall population's stress response.
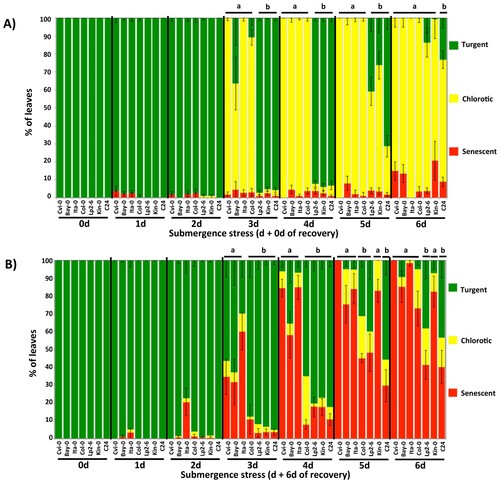
Applying the leaf-damage index to our dark-submerged plants resulted in the same order of tolerance for all ecotypes. Additionally, we were able to separate the effects of stress over time for the sensitive and tolerant groups, as well as identify Cvi-0 and C24 as the extreme ecotypes (Figure ). When scoring was applied immediately after removal from stress, chlorotic leaves were the predominant leaf-damage category (Figure A, Supplemental Figure 1), whereas six days after removal senescent leaves were the predominant leaf-damage category (Figure , Figure B).
Figure 2. Leaf damage measurements of sensitive and tolerant Arabidopsis ecotypes after submergence stress. Quantification of damage was measured based on the number of turgent, chlorotic or senescent leaves per plant either: A) immediately after being removed or; B) six days after being removed from the indicated time of submergence. The results are mean values (± S.E.) of three independent experiments (n = 12-15, each). Letters denote significant differences (P < 0.05, ANOVA and paired Student's t-test) for: A) chlorotic/turgent or; B) senescent/turgent leaves on plants from the same rescue day.
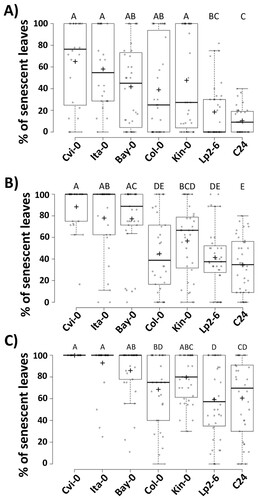
We observed that the differences among ecotypes were more significant from day 3 to day 5 (Figure ). For this time period, we recorded the percentage of senescent leaves from each individual of the population for each ecotype and used box-plots to analyze these values. As reported by previous studies (Licausi et al., Citation2011; Tsai et al., Citation2016; Yeung et al., Citation2018; Meng et al., Citation2020), we confirmed that hypoxic and submergence stress damage is not a discrete measurement but rather a continuum in the tested population, even at individual level (Figure ). These ecotypes provide a statistically verifiable platform that may be useful for exploring different biotechnologies since they show a robust response in the extremes of tolerance, particularly Cvi-0 and Ita-0 (sensitive) and Lp2-6 and C24 (tolerant).
Figure 3. Percent of senescent leaves of sensitive and tolerant Arabidopsis ecotypes after submergence stress. Quantification of senescent leaves was measured six days after being removed from stress at A) 3 d, B) 4 d and C) 5 d. Center lines show the medians; box limits indicate the 25th and 75th percentiles; whiskers extend 1.5 times the interquartile range from the 25th and 75th percentiles; crosses represent sample means; data points are plotted as open circles. The results are from three independent experiments (n = 23-34). Letters indicate significant differences (P < 0.05, one-way ANOVA followed by Tukey HSD).
Additionally, since originally reported (Vashisht et al., Citation2011), these ecotypes have been used by van Veen et al. (Citation2016) to study their transcriptomes (except Kin-0) under dark-submergence stress and control conditions. Yeung et al. (Citation2018) performed transcriptomic studies using the contrasting ecotypes Bay-0 and Lp2-6 in the recovery phase after submergence stress. Meng et al. (Citation2020) studied Bay-0 and Lp2-6 (in addition to other ecotypes) to conduct different physiological and molecular studies leading to the characterization of the role of ROS under submergence. All this molecular information is available for further use in biotechnological screenings.
Transformation frequency of Arabidopsis ecotypes with different Agrobacterium strains
The earliest attempts of transforming Arabidopsis using the floral dip method noticed that not all ecotypes have the same susceptibility to transformation as Col-0 (Clough and Bent, Citation1998). Furthermore, different genotypes of the transformation vector Agrobacterium can vary in their effectiveness by up to three orders of magnitude (Oltmanns et al., Citation2010).
First, we tested if the ecotypes showed natural tolerance to hygromycin. All controls performed did not show any hygromycin tolerant plant in the wild-type population of all ecotypes before transformation (Supplemental Figure 3). To ascertain which of these ecotypes were suitable for a transformation platform, we tested the recalcitrance of each of them to genetic transformation under three different Agrobacterium genotypes (Figure ). Within the sensitive ecotype group, our results indicated that only Ita-0 was highly recalcitrant to transformation, whereas Cvi-0 and Bay-0 were transformable ecotypes. In the tolerant ecotype group, C24 was the most recalcitrant ecotype, which is consistent with the findings of Ghedira et al. (Citation2013). More transformants can be obtained for transgenic studies through larger screenings of seeds or more plants being subjected to floral dipping.
Figure 4. Transformation frequencies of submergence sensitive and tolerant Arabidopsis ecotypes with different Agrobacterium genotypes. Center lines show medians of the quantification of plants tolerant to hygromycin in MS media obtained after transformation through the floral dip method of six independent experiments; box limits indicate the 25th and 75th percentiles and whiskers extend 1.5 times the interquartile range from the 25th and 75th percentiles; data points are plotted as circles; letters indicate significant differences (P < 0.05, two-way ANOVA followed by Tukey's multiple comparison test).
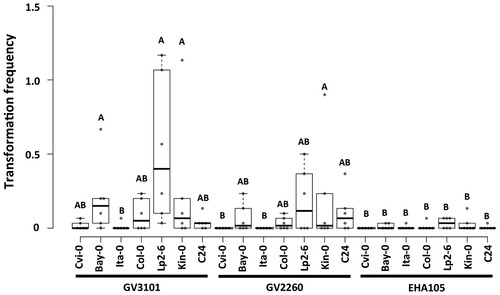
Regarding Agrobacterium, we observed that EHA105 was an ineffective transformation vector for the Arabidopsis ecotypes tested in this study. The strain GV3101 had the highest frequencies for transformation, followed by GV2260 (Figure ). These results match those of Oltmanns et al. (Citation2010), whom also discovered GV3101 to be the most effective of all tested strains. EHA105, GV3101, and GV2260 are considered similar in their genetic background (Nonaka et al. Citation2019). Nevertheless, significant differences in transformation frequencies were observed in this study. Although GV3101 strain has been mainly used for Arabidopsis transformation (Oltmanns et al. Citation2010; Ghedira et al. Citation2013), GV2260 remains a candidate to improve over transformation efficiency in other Arabidopsis ecotypes.
The availability of both tolerant and sensitive Arabidopsis ecotypes that can be transformed by Agrobacterium may allow for the testing of different promoters, genes or constructs and their effects in genotypes that are increasingly being studied because of their diverse molecular strategies of survival to submergence stress.
Conclusion
In this study, we report on research aimed to develop an original Arabidopsis-based transformation platform with different genetic backgrounds that can be used in the further development of transgenic submergence-tolerant plants. To achieve this, we replicated previous research investigating the response of tolerant and sensitive Arabidopsis ecotypes to dark-submergence stress. We also demonstrated the utility of a scoring system that quantifies the degree of leaf damage across an entire population. Finally, we reported the recalcitrance to transformation of these ecotypes with three different Agrobacterium strains. The results of our independent replication and scoring system support the generalization and trans-laboratory consistency of the reported order of tolerance of different Arabidopsis ecotypes to submergence stress. We conclude that Cvi-0 and C24 were the most contrasting ecotypes with respect to submergence tolerance, could be differentiated using an individual leaf-damage score, and could be effectively transformed by the GV3101 strain of Agrobacterium.
Acknowledgments
We thank Dr. Alejandro Aparicio, Dr. José Abad, and Dra. Jacqueline Capataz (UNPA) for sharing equipment, the research group of Dra. Alejandra Covarrubias (IBt-UNAM) for the generous gift of the Agrobacterium strains, and María Hernández and Jeziel Pacheco (UNPA) for administrative assistance. We acknowledge CONACyT-México for M.Sc. scholarships awarded to MS-V (Beca Nacional 726896; Mujeres Indígenas 942242), and Secretaría del Trabajo - México for fellowships awarded to EO-L and AF-M (Jóvenes Construyendo el Futuro).
Author contribution statement
MSV, EOL and AFM carried out the laboratory work and data analysis; BEBF and EGL contributed with materials and equipment and planning of experiments; JMPC designed the experiments and wrote the manuscript. All authors revised the manuscript for critical content, approved the version to be published and agree to be accountable for all aspects of the work.
Data availability
The data that support the findings of this study are openly available in Figshare at: https://figshare.com/s/f9f3cd8198b890c08408
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- AAT Bioquest. Quest Graph™ LC50 Calculator. 18 Aug. 2021, https://www.aatbio.com/tools/lc50-calculator.
- Bailey-Serres J, Parker JE, Ainsworth EA, Oldroyd GED, Schroeder JI. 2019. Genetic strategies for improving crop yields. Nature. 575 (7781):109-118. doi:10.1038/s41586-019-1679-0.
- Bui LT, Shukla V, Giorgi FM, Trivellini A, Perata P, Licausi F, Giuntoli B. 2020. Differential submergence tolerance between juvenile and adult arabidopsis plants involves the ANAC017 transcription factor. Plant J. 104(4):979–994. doi: 10.1111/tpj.14975.
- Clough SJ, Bent AF. 1998. Floral dip: a simplified method forAgrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16(6):735–743. doi: 10.1046/j.1365-313x.1998.00343.x.
- Fukao T, Barrera-Figueroa BE, Juntawong P, Peña-Castro JM. 2019. Submergence and waterlogging stress in plants: A review highlighting research opportunities and understudied aspects. Front Plant Sci. 10(340). doi: 10.3389/fpls.2019.00340.
- Ghedira R, De Buck S, Nolf J, Depicker A. 2013. The efficiency of arabidopsis thaliana floral Dip transformation Is determined Not only by the agrobacterium strain used but also by the physiology and the ecotype of the dipped plant. Mol Plant Microbe Interact. 26(7):823–832. doi: 10.1094/MPMI-11-12-0267-R.
- Giuntoli B, Lee SC, Licausi F, Kosmacz M, Oosumi T, van Dongen JT, Bailey-Serres J, Perata P. 2014. A trihelix DNA binding protein counterbalances hypoxia-responsive transcriptional activation in arabidopsis. PLoS Biol. 12(9):e1001950. doi: 10.1371/journal.pbio.1001950.
- Lee SC, Mustroph A, Sasidharan R, Vashisht D, Pedersen O, Oosumi T, Voesenek LA, Bailey-Serres J. 2011. Molecular characterization of the submergence response of the arabidopsis thaliana ecotype columbia. New Phytol. 190(2):457–471. doi:10.1111/j.1469-8137.2010.03590.x.
- Licausi F, Kosmacz M, Weits DA, Giuntoli B, Giorgi FM, Voesenek LA, Perata P, van Dongen JT. 2011. Oxygen sensing in plants is mediated by an N-end rule pathway for protein destabilization. Nature. 479(7373):419–422.
- Mendiondo GM, Gibbs DJ, Szurman-Zubrzycka M, Korn A, Marquez J, Szarejko I, Maluszynski M, King J, Axcell B, Smart K, et al. 2016. Enhanced waterlogging tolerance in barley by manipulation of expression of the N-end rule pathway E3 ligase PROTEOLYSIS6. Plant Biotechnol J. 14(1):40–50. doi: 10.1111/pbi.12334.
- Meng X, Li L, Narsai R, De Clercq I, Whelan J, Berkowitz O. 2020. Mitochondrial signalling is critical for acclimation and adaptation to flooding in arabidopsis thaliana. Plant J. 103(1):227–247. doi: 10.1111/tpj.14724.
- Mickelbart MV, Hasegawa PM, Bailey-Serres J. 2015. Genetic mechanisms of abiotic stress tolerance that translate to crop yield stability. Nat Rev Genet. 16(4):237–251. doi: 10.1038/nrg3901.
- Morales A, de Boer HJ, Douma JC, Elsen S, Engels S, Glimmerveen T, Sajeev N, Huber M, Luimes M, Luitjens E, et al. 2022. Effects of sub-lethal single, simultaneous, and sequential abiotic stresses on phenotypic traits of arabidopsis thaliana. AoB Plants. 14(4):plac029. doi:10.1101/2021.12.10.472073.
- Mustroph A. 2018. Improving flooding tolerance of crop plants. Agronomy. 8(9):160. doi:10.3390/agronomy8090160.
- National Academies of Sciences, Engineering, and Medicine. 2019. Reproducibility and replicability in science. Washington, DC: The National Academies Press.
- National Academies of Sciences, Engineering, and Medicine. 2019. Science breakthroughs to advance food and agricultural research by 2030. Washington, DC: The National Academies Press.
- Nonaka S, Someya T, Kadota Y, Nakamura K, Ezura H. 2019. Super-Agrobacterium ver. 4: improving the transformation frequencies and genetic engineering possibilities for crop plants. Front Plant Sci. 10:1204. doi:10.3389/fpls.2019.01204.
- Nosek BA, Errington TM. 2020. What is replication? PLoS Biol. 18(3):e3000691. doi:10.1371/journal.pbio.3000691.
- Oltmanns H, Frame B, Lee LY, Johnson S, Li B, Wang K, Gelvin SB. 2010. Generation of backbone-free, Low transgene copy plants by launching T-DNA from the agrobacterium chromosome. Plant Physiol. 152(3):1158–1166. doi:10.1104/pp.109.148585.
- Peña-Castro JM, van Zanten M, Lee SC, Patel MR, Voesenek LA, Fukao T, Bailey-Serres J. 2011. Expression of rice SUB1A and SUB1C transcription factors in arabidopsis uncovers flowering inhibition as a submergence tolerance mechanism. Plant J. 67; 67(3):434-446. doi:10.1111/j.1365-313X.2011.04605.x.
- Sasidharan R, Bailey-Serres J, Ashikari M, Atwell BJ, Colmer TD, Fagerstedt K, Fukao T, Geigenberger P, Hebelstrup KH, Hill RD, et al. 2017. Community recommendations on terminology and procedures used in flooding and low oxygen stress research. New Phytol. 214(4):1403–1407. doi:10.1111/nph.14519.
- Sasidharan R, Hartman S, Liu Z, Martopawiro S, Sajeev N, van Veen H, Yeung E, Voesenek LACJ. 2018. Signal dynamics and interactions during flooding stress. Plant Physiol. 176(2):1106–1117. doi: 10.1104/pp.17.01232.
- Singh US, Dar MH, Singh S, Zaidi NW, Bari MA, Mackill DJ, Collard BCY, Singh VN, Singh JP, Reddy JN, et al. 2013. Field performance, dissemination, impact and tracking of submergence tolerant (Sub1) rice varieties in south Asia. SABRAO J Breed Genet. 45(1):112–131.
- Spitzer M, Wildenhain J, Rappsilber J, Tyers M. 2014. Boxplotr: a web tool for generation of box plots. Nat Methods. 11(2):121–122. doi: 10.1038/nmeth.2811.
- Tsai KJ, Lin CY, Ting CY, Shih MC. 2016. Ethylene-regulated glutamate dehydrogenase fine-tunes metabolism during anoxia-reoxygenation. Plant Physiol. 172(3):1548–1562. doi: 10.1104/pp.16.00985.
- van Veen H, Vashisht D, Akman M, Girke T, Mustroph A, Reinen E, Hartman S, Kooiker M, van Tienderen P, Schranz ME, et al. 2016. Transcriptomes of eight arabidopsis thaliana accessions reveal core conserved, genotype- and organ-specific responses to flooding stress. Plant Physiol. 172(2):pp.00472.2016–689. doi:10.1104/pp.16.00472.
- Vashisht D, Hesselink A, Pierik R, Ammerlaan JM, Bailey-Serres J, Visser EJ, Pedersen O, van Zanten M, Vreugdenhil D, Jamar DC, et al. 2011. Natural variation of submergence tolerance among arabidopsis thaliana accessions. New Phytol. 190(2):299–310. doi:10.1111/j.1469-8137.2010.03552.x.
- Weits DA, Giuntoli B, Kosmacz M, Parlanti S, Hubberten HM, Riegler H, Hoefgen R, Perata P, van Dongen JT, Licausi F. 2014. Plant cysteine oxidases control the oxygen-dependent branch of the N-end-rule pathway. Nat Commun. 5:3425. doi: 10.1038/ncomms4425.
- Yeung E, van Veen H, Vashisht D, Sobral Paiva AL, Hummel M, Rankenberg T, Steffens B, Steffen-Heins A, Sauter M, de Vries M, et al. 2018. A stress recovery signaling network for enhanced flooding tolerance in arabidopsis thaliana. Proc Natl Acad Sci U S A. 115(26):E6085–E6094. doi:10.1073/pnas.1803841115.
- Zhang X, Henriques R, Lin SS, Niu QW, Chua NH. 2006. Agrobacterium-mediated transformation of arabidopsis thaliana using the floral dip method. Nat Protoc. 1(2):641–646. doi: 10.1038/nprot.2006.97.
