Abstract
Immunoglobulin A nephropathy (IgAN), the most common primary renal disease, is characterized by abnormal IgA1 deposition. IgAN is caused by NOD-like receptor pyrin domain-containing-3 (NLRP3), which regulates innate immunity. Gubentongluo (GB) decoction, a traditional Chinese medicine, is an effective clinical treatment for IgAN in China. However, little is known about the underlying mechanisms. In this study, IgA1, which is separated from patients with IgAN, together with GB decoction was applied to treat human mesangial cells (HMCs). CCK-8 and the enzyme-linked immunosorbent assay were used to assess cell proliferation and the production of interleukin-1β (IL-1β), respectively. qPCR and Western blot were applied to detect the expression levels of NLRP3, IL-1β, and caspase-1. Our results showed that GB decoction inhibited IgA1-induced cell proliferation and IL-1β production. Furthermore, IgA1-induced upregulation of IL-1β, NLRP3, and caspase-1 was reduced after treatment with GB. Interestingly, MCC950, an NLRP3 inhibitor, showed similar effect as GB decoction. Additionally, NLRP3 overexpression stimulated cell proliferation, IL-1β secretion, and the expressions of IL-1β and caspase-1 in HMCs. But these effects could be alleviated by GB decoction. In conclusion, GB may play a protective role in IgAN cell model by inhibiting NLRP3.
KEYWORDS:
Introduction
Immunoglobulin A nephropathy (IgAN) is the most common primary glomerulonephritis, which is a type of disease related to end-stage renal disease (ESRD) (Moroni et al. Citation2019). It is estimated that 30–40% of patients with IgAN progress to ESRD after 20–30 years. IgAN is characterized by circulating galactose-deficient IgA1 immune complex which consists of abnormally synthesized IgA1 and specific autoantibodies. This complex which deposits in glomeruli often stimulates matrix expansion, mesangial cell proliferation, and secretion of profibrotic and proinflammatory cytokines (Chan et al. Citation2004). In addition, the activation of innate immunity has been considered as the leading cause of IgAN (Coppo et al. Citation2010). However, the exact pathogenic mechanisms underlying IgAN are not well understood. Although kidney transplantation is the most effective IgAN treatment, it is a huge financial burden (Shao et al. Citation2017). Therefore, it is urgent to develop a novel efficient treatment for IgAN.
NOD-like receptor pyrin domain-containing-3 (NLRP3) plays a key role in the innate immunity. The NLRP3 inflammasome, which connects innate and adaptive immunity, regulates caspase-1 activation, which is essential for the maturation and secretion of IL-1β and IL-18 (Tschopp and Schroder Citation2010). High levels of IL-1β and IL-18 have been found in the patients with IgAN (Hahn et al. Citation2009; Shi et al. Citation2012). Furthermore, highly expressed NLRP3 is observed in renal tubular epithelial cells in the patients with IgAN, and NLRP3 expression level is correlated with tubular injury (Hua et al. Citation2013). Several studies have demonstrated that various renal diseases are associated with increased NLRP3 expression and activation of its downstream signaling proteins (Mulay et al. Citation2014; Xiong et al. Citation2015). Therefore, it is hypothesized that the NLRP3 inflammasome contributes to the pathogenesis of IgAN by regulating inflammation.
For many years, traditional Chinese medicine (TCM) has been widely used in maintaining health and treating various diseases in China. Gubentongluo (GB) decoction, a classic TCM, contains nine herbs, including Rhizoma imperatae (baimaogen in Chinese), Rumex madaio Makino (todahuang in Chinese), Astragali radix (huangqi in Chinese), Radix salvia miltiorrhizae (dansen in Chinese), Ramulus euonymi (guijianyu in Chinese), Fructus ligustri lucidi (nvzhenzi in Chinese), Yerbadetajo herb (hanliancao in Chinese), Semen persicae (taoren in Chinese), and Herba lycopi (zelanye in Chinese). Rhizoma imperatae, a primary herb of GB, has anti-inflammatory effect in RAW264.7 macrophages (An et al. Citation2015; Park et al. Citation2016). Astragali radix has been found to possess antioxidant property (Shahzad et al. Citation2016). In addition, GB decoction is suggested to suppress IgA deposition in the glomerular mesangial area of mice with IgAN (Shen and He Citation2016). However, the underlying mechanism of GB decoction in the treatment of IgAN is still unknown. The aim of this study was to explore the effect and the underlying mechanisms of GB on IgAN cell model. Our results indicated that GB decoction played a protective role on an IgAN cell model and would be a good alternative for treating IgAN.
Materials and methods
Isolation of serum IgA1
All the patients were enrolled at Shuguang Hospital (Shanghai, China), and their written informed consent were obtained. This study was approved by the Shuguang Hospital Affiliated to Shanghai University of TCM Research Ethics Committee (2019-703-58-01) and met the declaration of Helsinki. Jacalin affinity chromatography was used to isolate IgA1 from the serum of patients with IgAN (Reinhart et al. Citation2012). Briefly, 5 mL of whole blood was used to separate serum samples, which were then filtered through a 0.2 µm Corning syringe filter (Millipore/Sigma, St. Louis, MO, USA). The columns were prepared using jacalin immobilized agarose (Pierce, Rockford, IL, USA) and then washed with phosphate-buffered saline (PBS, pH 7.0) until the optical density (OD) value at 280 nm was less than 0.1. Next, the serum samples were diluted 1:1 with PBS (pH 7.0) and applied to the column. After that, PBS (pH 7.0) was used to wash the column to remove all the unattached proteins. When the OD value at 280 nm returned to 0.1, the attached IgA1 was then eluted with 0.1 M melibiose (Sigma). The eluted fractions were pooled, concentrated, and applied to a Sephacryl HiPrep S-200 gel filtration column (GE Healthcare/Sigma). The concentrated IgA1 sample was verified by using Western blot, and the concentrations of purified IgA1 were measured using IgA1-specific enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN, USA).
Cells, cell culture, and treatments
Human mesangial cells (HMCs) were obtained from ScienCell Research Laboratories (Carlsbad, CA, USA) and cultured in mesangial cell medium (ScienCell Research Laboratories) containing 10% fetal bovine serum (FBS) in an environment with 37°C and 5% CO2.
For the measurement, HMCs were harvested 48 h after being treated with various concentrations of IgA1 (0, 100, 250, and 500 µg/mL). HMCs were also treated with 250 µg/mL of IgA1 together with different concentrations of GB (0.01, 0.02, 0.05, 0.1, and 0.2 mg/mL) or 1 µM of MCC950 (Selleck, Radnor, PA, USA), an NLRP3 inhibitor.
Production of GB
The GB decoction was combined at a ratio of 6:6:3:3:3:3:3:2:2 with Rhizoma imperatae, Rumex madaio Makino, Astragali radix, Radix salvia miltiorrhizae, Ramulus euonymi, Fructus ligustri lucidi, Yerbadetajo herb, Semen persicae, and Herba lycopi. The decoction was then produced as a freeze-dried powder.
Cell proliferation
Cell proliferation of HMCs was measured using CCK-8 assay (APExBIO, Houston, TX, USA). Briefly, a total of 1 × 105 of HMC were seeded into a 96-well plate and cultured overnight. The cells were then stimulated with various treatments for a specific amount of time. Next, the culture medium was replaced with CCK-8 working solution (10% CCK-8 and 90% culture medium without FBS) and incubated for 1 h. Lastly, cell proliferation was analyzed by measuring the absorbance at 450 nm.
RNA isolation and real-time quantitative PCR (qPCR)
Total cellular RNAs from HMC were isolated using TRIZOL buffer (Invitrogen, Carlsbad, CA, USA). To measure the mRNA expression level of NLRP3, M-MLV reverse transcriptase kit (Thermo Fisher Scientific, Inc., Grand Island, NY, USA) was used to convert total RNAs to cDNA. qPCR was performed in triplicate by using SYBR Green Master kit (Thermo Fisher Scientific, Inc.) on an ABI7400 system (Applied Biosystems, Foster City, CA, USA). In addition, the relative gene expression was normalized to GAPDH. The primers used were listed below:
Western blot analysis
The total treated HMCs were harvested using RIPA buffer (Thermo Fisher Scientific, Inc.), which contains a phosphatase and protease inhibitor cocktail (Thermo Fisher Scientific, Inc.). An equal amount of each sample (30 µg) was separated using the SDS-PAGE gel and then transferred to PVDF membranes (Millipore, Bedford, MA, USA). Then, the blots were blocked with 5% nonfat milk and then incubated with primary and secondary antibodies. Finally, the brands were visualized using enhanced chemiluminescence kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and collected by a scanner (Bio-Rad Laboratories, Inc.).
The primary antibodies and ladders used were listed below:
Anti-NLRP3, Ab214185, dilution, 1:1000, Abcam, St. Louis, MO, USA
Anti-IL-1β, Ab2105, dilution, 1:1000, Abcam
Anti-pro IL-1β, NB600-633, dilution, 1:1000, NOVUS, Centennial, CO, USA
Anti-caspase-1, Ab74279, dilution, 1:1000, Abcam
Anti-pro caspase-1, Ab179515, dilution, 1:1000, Abcam
Anti-GAPDH, 60004-1-1G, dilution, 1:2500, Proteintech, Rosemont, IL, USA
The ladder which was used in Figure (D) was purchased from Bio-Rad (Cat 1610374) and the one used in Figures (D) and (D) was purchased from Thermo Fisher (Cat 26617).
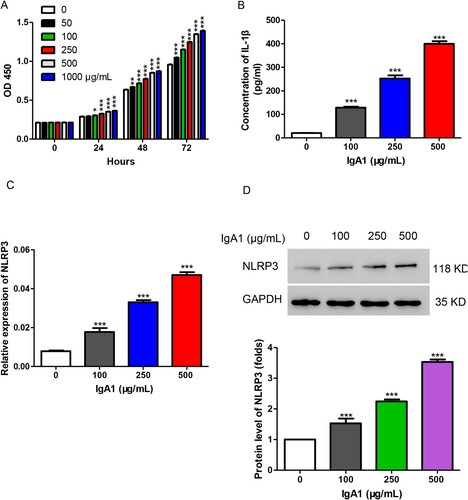
Figure 1. IgA1 increased cell proliferation and the levels of IL-1β and NLRP3 in HMCs. (A) HMCs were stimulated with different concentrations of IgA1 (0, 50, 100, 250, 500, and 1000 µg/mL). Cell proliferation was measured by CCK-8 assay. (B) The production of IL-1β was detected by ELISA after treatment with IgA1 (0, 100, 250, and 500 µg/mL). (C, D) The expression level of NLRP3 was detected by qPCR (C) and Western blot (D) after treatment with IgA1 (0, 100, 250, and 500 µg/mL). *P < 0.05; **P < 0.01; ***P < 0.001 vs. 0 µg/mL of IgA1.
Analysis of the secretion of IL-1β
The ELISA kit to detect IL-1β was purchased from R&D Systems. The secretion of IL-1β was measured according to the manufacturer’s instructions.
Plasmid
NLRP3 overexpression plasmid was obtained from Shanghai GeneChem Company Co., Ltd. (Shanghai, China). The overexpression efficiency was measured by qPCR and Western blot.
Statistic
In three separate experiments, all data were presented as mean ± SD. Statistical analyses between the two groups were analyzed using two-tailed Student’s t-test. Two-way analysis of variance was used to evaluate the difference between the three groups. P values of <0.05 were considered statistically significant.
Results
IgA1 increased cell proliferation, the levels of IL-1β and NLRP3 in HMC
As previously described, IgA1 was isolated from the patients with IgAN and then was used to treat HMCs at various concentrations (0, 50, 100, 250, 500, and 1000 µg/mL). The results showed that IgA1 significantly increased cell proliferation in a dose-dependent manner in HMC (Figure (A)). Then, we measured the IL-1β secretion level upon treatment with IgA1 (0, 100, 250, and 500 µg/mL) and found that IgA1 markedly stimulated IL-1β secretion in HMC (Figure (B)). As reported, NLRP3 affects IgAN by regulating inflammation (Tschopp and Schroder Citation2010), and we measured the expression level of NLRP3 after treatment with IgA1 (0, 100, 250, and 500 µg/mL). As shown in Figure (C,D), the mRNA and protein levels of NLRP3 were significantly increased upon treatment with IgA1. IgA1 at 250 µg/mL showed an almost fourfold increase in NLRP3 and an eightfold increase in IL-1β. In order to avoid the cytotoxicity caused by high IgA1 concentration, 250 µg/mL of IgA1 was used to treat cells in the following studies. Taken together, IgA1 increased cell proliferation and the levels of IL-1β and NLRP3 in HMC.
GB suppressed proinflammatory cytokine production in IgAN cell model
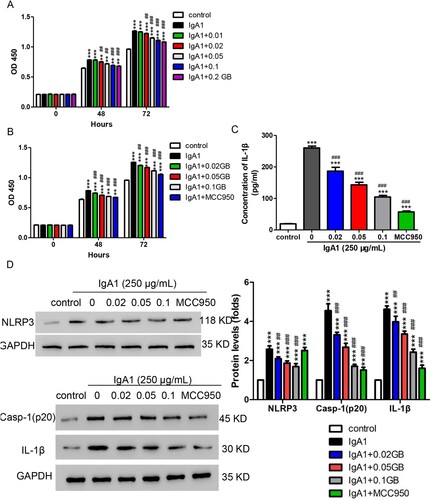
Based on the plasma concentration (0.2 mg/ml) of GB, we measured the cell toxicity of GB using different concentrations around 0.2 mg/ml and found that the concentrations lower than 0.2 mg/ml exhibited almost no inhibition of cell proliferation (Figure S1). To figure out the role of GB, 250 µg/mL of IgA1 together with various concentrations of GB (0.01, 0.02, 0.05, 0.1, and 0.2 mg/mL) was used to treat HMCs, and cell proliferation was assessed. As shown in Figure (A), IgA1 increased the cell proliferation in HMCs, but this effect was significantly alleviated by all doses of GB except 0.01 mg/mL. Based on previous results, IgA1-induced NLRP3 expression. MCC950, an NLRP3 inhibitor, was enrolled. We used IgA1 together with GB (0.02, 0.05, and 0.1 mg/mL) or MCC950 (1µM) to treat HMC and analyzed cell proliferation and the secretion of IL-1β. As shown in Figure (B), all doses of GB and MCC950 could alleviate the increase of cell proliferation induced by IgA1. Similarly, IL-1β production which is induced by IgA1 was downregulated by GB and MCC950 (Figure (C)). Furthermore, we observed that IgA1 induced the upregulation of NLRP3, which was decreased by GB and MCC950 (Figure (D)). Since the NLRP3 inflammasome regulates the activation of caspase-1 which affects the maturation and secretion of IL-1β (Swanson et al. Citation2019), we performed Western blot assay against caspase-1 and IL-1β. Our findings demonstrated that IgA1 increased the protein levels of caspase-1 and IL-1β in HMCs, but the increase was significantly inhibited by GB and MCC950 (Figure (D)). These findings indicated that GB suppressed the increase of proinflammatory cytokine induced by IgA1 via NLRP3 signaling pathway in IgAN cell model.
Figure 2. GB suppressed proinflammatory cytokine production in IgAN cell model. (A) HMCs were treated with 250 µg/mL of IgA1 and different concentrations of GB (0.01, 0.02, 0.05, 0.1, and 0.2 mg/mL). Cell proliferation was measured by CCK-8 assay. (B) HMCs were treated with 250 µg/mL of IgA1 together with different concentrations of GB (0.02, 0.05, and 0.1 mg/mL) or MCC950 (1 µM). Cell proliferation was measured by CCK-8 assay. (C) The production of IL-1β was detected by ELISA after treatment with 250 µg/mL of IgA1 together with GB (0.02, 0.05, and 0.1 mg/mL) or MCC950 (1µM). (D, E) The protein levels of caspase-1, NLRP3, and IL-1β were measured using Western blot. **P < 0.01; ***P < 0.001 vs. control; ##P < 0.01; ###P < 0.001 vs. 250 µg/mL of IgA1.
GB suppressed proinflammatory cytokine production induced by NLRP3
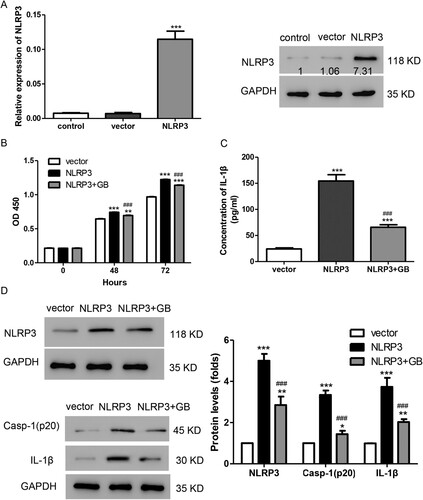
To confirm our hypothesis, NLRP3 overexpression plasmid was transfected into HMC. As shown in Figure (A), both mRNA and protein levels of NLRP3 were markedly increased after being transfected with NLRP3 overexpression plasmid. In addition, we found that transfection with NLRP3 promoted cell proliferation in HMCs. Cell proliferation in the group treated with GB and NLRP3 plasmid was lower than in the group transfected with NLRP3, but still higher than in the vector group (Figure (B)). Moreover, NLRP3 overexpression stimulated the production of IL-1β, and GB could alleviate this effect (Figure (C)). In addition, GB inhibited the increase in protein levels of NLRP3, caspase-1, and IL-1β, which was stimulated by NLRP3 overexpression (Figure (D)). Taken together, GB alleviated the effects induced by NLRP3 overexpression in HMC.
Figure 3. GB alleviated the effects induced by NLRP3 overexpression in HMCs. (A) The mRNA and protein level of NLRP3 were detected by using qPCR (left panel) and Western blot (right panel), respectively. ***P < 0.001 vs. control. (B) HMCs were treated with 0.05 mg/mL of GB together with NLRP3 overexpression plasmid. Cell proliferation was measured by CCK-8 assay. (C) The production of IL-1β was detected by ELISA. (D, E) The protein levels of caspase-1, NLRP3, and IL-1β were measured using Western blot. ***P < 0.001 vs. control; ###P < 0.001 vs. vector.
Discussion
IgAN is the most common primary renal disease for which there is no effective treatment. The aberrant IgA deposition is considered as a fundamental factor in the development of IgAN (Lai et al. Citation2016). Moreover, the high production of inflammatory factors including TNF-α and IL-1β is involved in this progress (Deng et al. Citation2019). In this study, we demonstrated that GB decoction played a protective role in IgAN cell model.
IgA has been found to induce the secretion of IL-1β through NLRP3 inflammasome in macrophages (Tsai et al. Citation2017). In this study, we found that IgA1 stimulated cell proliferation and the secretion of IL-1β in HMCs (Figure ), suggesting that treating HMCs with IgA1 could mimic the behavior of the IgAN cell model. Moreover, increased NLRP3 level was observed after IgA1 treatment in HMCs (Figure ). These results suggested a potential role for the NLRP3 inflammasome in the IgAN cell model.
GB decoction which is commonly used in TCM provides an effective treatment for IgAN in China (Shen et al. Citation2016). In the current study, we found that the increased secretion of IL-1β and protein levels of caspase-1 and IL-1β induced by IgA1 were inhibited after treatment with GB decoction (Figure ). These results suggested that GB decoction may play a protective role in IgAN. GB decoction contains nine herbs, and some of the components have been confirmed to have antioxidant and anti-inflammatory properties (Park et al. Citation2016; Shahzad et al. Citation2016). In addition, GB decoction can reduce oxidative stress resulting in the improvement of pathological injuries through regulating PPARα and L-FABP in mice with IgAN (Li et al. Citation2017). GB decoction has been found to suppress IgA deposition by changing its switch recombination of B lymphocytes in mice with IgAN (Shen and He Citation2016). Moreover, we observed that IgA1 induced the increase of NLRP3 level which was decreased by GB (Figure ). Therefore, we assumed that NLRP3 was involved in this process. However, it must be determined which ingredient is essential. In the upcoming research, we will focus on one or two monomers of GB decoction in an effort to explore new IgAN treatment options.
Clinical evidences showed that the dramatical production of proinflammatory cytokines including IL-1β is found in the patients with IgAN (Hahn et al. Citation2009). As is well known, the NLRP3 inflammasome controls the activation of caspase-1, which is required for the generation of mature IL-1β (Chen et al. Citation2011). Multiple studies indicated that the NLRP3 inflammasome is associated with a number of renal diseases including IgAN (Lorenz et al. Citation2014; Tsai et al. Citation2017). In the current study, we discovered that the production of IL-1β induced by IgA1 could be reversed by MCC950, an NLRP3 inhibitor (Figure ). Furthermore, NLRP3 overexpression was observed to stimulate cell proliferation and IL-1β production, and these effects were alleviated by GB decoction (Figure ). Consistently, NLRP3 expression induced by IgA1 deposition was reported to be associated with the accumulation of inflammation in IgA nephropathy (Peng et al. Citation2019). In addition, IgA immune complex was also confirmed to be mediated by the activation of Nlrp3 inflammasome (Yang et al. Citation2013). In conclusion, GB decoction reduced the production of IL-1β generated by IgA1 through the NLRP3 pathway, suggesting the protective function of GB in IgAN.
Supplemental Material
Download TIFF Image (1.1 MB)Acknowledgements
Xue-Jun Yang and Pei-Cheng Shen contributed to the conception and design of the work; Wei Meng, Jiao-Jiao Shen, and Ting-Yu Liang contributed to the acquisition, analysis and interpretation of data for the work; Qing Wu, Luo-Bing Wang, and Di Huang contributed to the acquisition a small part of data; Fei-Peng Xu and Jia-Yuan Bai contributed to the interpretation of the data; Wei Meng contributed to drafting the work; Wei Meng, Jiao-Jiao Shen, and Ting-Yu Liang contributed to the revision of the work; Pei-Cheng Shen contributed to the final approval of the version to be published, and that all authors agree to be accountable for all aspects of the work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available in ‘figshare’ [https://figshare.com/] at http://doi.org/10.6084/m9.figshare.16814854, http://doi.org/10.6084/m9.figshare.16814902, http://doi.org/10.6084/m9.figshare.16816087, http://doi.org/10.6084/m9.figshare.19427369.
Additional information
Funding
References
- An HJ, Nugroho A, Song BM, Park HJ. 2015. Isoeugenin, a novel nitric oxide synthase inhibitor isolated from the rhizomes of Imperata cylindrica. Molecules. 20(12):21336–21345. doi:10.3390/molecules201219767.
- Chan LY, Leung JC, Lai KN. 2004. Novel mechanisms of tubulointerstitial injury in IgA nephropathy: a new therapeutic paradigm in the prevention of progressive renal failure. Clin Exp Nephrol. 8(4):297–303. doi:10.1007/s10157-004-0324-9.
- Chen M, Wang H, Chen W, Meng G. 2011. Regulation of adaptive immunity by the NLRP3 inflammasome. Int Immunopharmacol. 11(5):549–554. doi:10.1016/j.intimp.2010.11.025.
- Coppo R, Amore A, Peruzzi L, Vergano L, Camilla R. 2010. Innate immunity and IgA nephropathy. J Nephrol. 23(6):626–632.
- Deng F, Zhang J, Li Y, Wang W, Hong D, Li G, Feng J. 2019. Hirudin ameliorates immunoglobulin A nephropathy by inhibition of fibrosis and inflammatory response. Renal Fail. 41(1):104–112. doi:10.1080/0886022X.2019.1583113.
- Hahn WH, Cho BS, Kim SD, Kim SK, Kang S. 2009. Interleukin-1 cluster gene polymorphisms in childhood IgA nephropathy. Pediatr Nephrol. 24(7):1329–1336. doi:10.1007/s00467-009-1146-5.
- Hua KF, Yang SM, Kao TY, Chang JM, Chen HL, Tsai YJ, Chen A, Yang SS, Chao LK, Ka SM. 2013. Osthole mitigates progressive IgA nephropathy by inhibiting reactive oxygen species generation and NF-kappaB/NLRP3 pathway. PLoS One. 8(10):e77794. doi:10.1371/journal.pone.0077794.
- Lai KN, Tang SC, Schena FP, Novak J, Tomino Y, Fogo AB. 2016. IgA nephropathy. Nat Rev Dis Primers. 2:16001. doi:10.1038/nrdp.2016.1.
- Li WW, Huang D, Shen PC, WU Q, SUN C, WANG XX, HE LQ. 2017. Effects of Gubentongluo formula on oxidative stress reflected by expressions of PPARα and L-FABP in mice with IgA nephropathy. J Sichuan Univ. 48(2):210–215.
- Lorenz G, Darisipudi MN, Anders HJ. 2014. Canonical and non-canonical effects of the NLRP3 inflammasome in kidney inflammation and fibrosis. Nephrol Dial Transplant. 29(1):41–48. doi:10.1093/ndt/gft332.
- Moroni G, Belingheri M, Frontini G, Tamborini F, Messa P. 2019. Immunoglobulin A nephropathy. Recurrence after renal transplantation. Front Immunol. 10:1332. doi:10.3389/fimmu.2019.01332.
- Mulay SR, Evan A, Anders HJ. 2014. Molecular mechanisms of crystal-related kidney inflammation and injury. Implications for cholesterol embolism, crystalline nephropathies and kidney stone disease. Nephrol Dial Transplant. 29(3):507–514. doi:10.1093/ndt/gft248.
- Park SJ, Kim YW, Park MK, Byun SH, Kim SC, Lee JR. 2016. Anti-inflammatory steroid from Phragmitis rhizoma modulates LPS-mediated signaling through inhibition of NF-kappaB pathway. Inflammation. 39(2):727–734. doi:10.1007/s10753-015-0299-6.
- Peng W, Pei GQ, Tang Y, Tan L, Qin W. 2019. IgA1 deposition may induce NLRP3 expression and macrophage transdifferentiation of podocyte in IgA nephropathy. J Transl Med. 17(1):406. doi:10.1186/s12967-019-02157-2.
- Reinhart D, Weik R, Kunert R. 2012. Recombinant IgA production: single step affinity purification using camelid ligands and product characterization. J Immunol Methods. 378(1–2):95–101. doi:10.1016/j.jim.2012.02.010.
- Shahzad M, Shabbir A, Wojcikowski K, Wohlmuth H, Gobe GC. 2016. The antioxidant effects of Radix Astragali (Astragalus membranaceus and related species) in protecting tissues from injury and disease. Curr Drug Targets. 17(12):1331–1340. doi:10.2174/1389450116666150907104742.
- Shao X, Li B, Cao L, Liang L, Yang J, Wang Y, Feng S, Wang C, Weng C, Shen X, et al. 2017. Evaluation of crescent formation as a predictive marker in immunoglobulin A nephropathy: a systematic review and meta-analysis. Oncotarget. 8(28):46436–46448. doi:10.18632/oncotarget.17502.
- Shen P, Shen J, Sun C, Yang X, He L. 2016. A system biology approach to understanding the molecular mechanisms of Gubentongluo decoction acting on IgA Nephropathy. BMC Complement Altern Med. 16(1):312. doi:10.1186/s12906-016-1268-9.
- Shen PC, He LQ. 2016. Effect of ‘Gubentongluo formula’ on the IgA class switch recombination of B Lymohocytes in Peyer's patches in mice with IgA nephropathy. J Sichuan Univ Med Sci Ed. 47(3):337–341.
- Shi B, Ni Z, Cao L, Zhou M, Mou S, Wang Q, Zhang M, Fang W, Yan Y, Qian J. 2012. Serum IL-18 is closely associated with renal tubulointerstitial injury and predicts renal prognosis in IgA nephropathy. Mediat Inflamm. 2012:728417. doi:10.1155/2012/728417.
- Swanson KV, Deng M, Ting JP. 2019. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 19(8):477–489. doi:10.1038/s41577-019-0165-0.
- Tsai YL, Hua KF, Chen A, Wei CW, Chen WS, Wu CY, Chu CL, Yu YL, Lo CW, Ka SM. NLRP3 inflammasome: pathogenic role and potential therapeutic target for IgA nephropathy. Sci Rep. 7:41123. doi:10.1038/srep41123.
- Tschopp J, Schroder K. 2010. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 10(3):210–215. doi:10.1038/nri2725.
- Xiong J, Wang Y, Shao N, Gao P, Tang H, Su H, Zhang C, Meng XF. 2015. The expression and significance of NLRP3 inflammasome in patients with primary glomerular diseases. Kidney Blood Press Res. 40(4):344–354. doi:10.1159/000368511.
- Yang SM, Ka SM, Hua KF, Wu TH, Chuang YP, Lin YW, Yang FL, Wu SH, Yang SS, Lin SH, et al. 2013. Antroquinonol mitigates an accelerated and progressive IgA nephropathy model in mice by activating the Nrf2 pathway and inhibiting T cells and NLRP3 inflammasome. Free Radical Biol Med. 61:285–297. doi:10.1016/j.freeradbiomed.2013.03.024.
